Abstract
Study Objective
To assess the relationship of the 33 single nucleotide polymorphisms (SNPs) previously associated with fasting glucose in Caucasians in genome-wide association studies (GWAS) with glucose response to antihypertensive drugs shown to increase risk for hyperglycemia and diabetes.
Design
Randomized, multicenter clinical trial.
Subjects
A total of 456 Caucasian men and women with uncomplicated hypertension
Measurements and Main Results
The Pharmacogenomic Evaluation of Antihypertensives Responses study evaluated blood pressure and glucose response in uncomplicated hypertensive patients randomized to either atenolol or hydrochlorothiazide (HCTZ) monotherapy, followed by combination therapy with both agents. Association of these SNPs with atenolol- or HCTZ-induced glucose response was evaluated in 456 Caucasian patients using linear regression adjusting for age, gender, body mass index, baseline glucose, baseline insulin, and principal component for ancestry.
SNP rs340874 in the 5’ region of PROX1 gene was significantly associated with atenolol-induced glucose change (p=0.0013). Participants harboring the C allele of this SNP had greater glucose elevation after approximately 9 weeks of atenolol monotherapy (beta = +2.39 mg/dL per C allele), consistent with the direction of effect in fasting glucose GWAS that C allele is associated with higher fasting glucose.
Conclusion
These data suggest that PROX1 SNP rs340874 discovered in fasting glucose GWAS may also be a pharmacogenetic risk factor for antihypertensive - induced hyperglycemia. β-blockers and thiazides may interact with genetic risk factors to increase risk for dysglycemia and diabetes.
Keywords: pharmacogenomics, dysglycemia, adverse metabolic effects, atenolol, hydrochlorothiazide, hypertension, antihypertensives
Hypertension is the most common chronic disease for which medications are prescribed and places over one-third of Americans at substantially increased risk for stroke, coronary heart disease (including myocardial infarction), renal failure, and heart failure.1 Two of the widely used first-line therapies for hypertension, β-blockers and thiazide diuretics,2 are associated with decreased insulin sensitivity and hyperglycemia3. Short-term use of these drugs is associated with an increased incidence of impaired fasting glucose4 and in numerous clinical trials, long-term use has been shown to increase the risk of new-onset diabetes5–7.
There is considerable variability in glucose changes after exposure to β-blockers and thiazide diuretics.4 We have previously reported that reductions in blood pressure were not correlated with changes in glucose elevation after β-blocker and thiazide treatment.8 Although genetic polymorphisms likely explain a portion of this variability, the few studies that have investigated the pharmacogenomics of β-blocker and thiazide-induced glucose have largely focused on glucose response to thiazide diuretics9, 10.
Studies have shown that even for individuals with normal fasting glucose, the diabetes risk increases as fasting glucose levels increase.11, 12 In a study of more than 45,000 individuals with a mean follow-up of 81 months, each mg/dL increase in fasting glucose was associated with a 6% higher risk for diabetes (hazard ratio [HR] 1.06; 95% confidence interval [CI] 1.05–1.07)11. There is evidence that drug-associated new-onset diabetes and diabetes of other etiologies may share common mechanisms.13, 14 Antihypertensive drugs such as β-blockers and thiazide diuretics could act as an environmental trigger to accelerate the onset of diabetes.5–7 We believe that for nondiabetic individuals with genetic risk for higher fasting glucose, taking antihypertensives with glucose-increasing side effects may further increase their glucose and put them at a greater risk for developing diabetes. More specifically, we hypothesize that genetic variants associated with higher fasting glucose are also associated with glucose increases induced by β-blockers and thiazide diuretics. - To date, 36 loci have been associated with fasting glucose in genome-wide association studies (GWAS) of nondiabetic Caucasians.15, 16 To test our hypothesis, we analyzed data from the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study to assess the association of these loci with glucose response to atenolol (a β-blocker) and hydrochlorothiazide (HCTZ, a thiazide diuretic). Identifying genetic predictors of β-blocker and thiazide-induced glucose change may help guide clinicians to prescribe medications that mitigate the development of hyperglycemia and diabetes in hypertensive patients.
Research Design and Methods
PEAR Study Design and Protocol
The PEAR study (clinicaltrials.gov Identifier NCT00246519) was a randomized, multicenter clinical trial examining the role of genetic variability on blood pressure and adverse metabolic responses to HCTZ and atenolol.17 Males and females of any race between the ages of 17 and 65 with uncomplicated hypertension were recruited to participate at University of Florida (Gainesville, FL), Mayo Clinic (Rochester, MN), and Emory University (Atlanta, GA). Patients with diabetes were excluded from the study. The institutional review boards at each institution approved the protocol and each participant provided informed written consent prior to entry into the study.
Those treated at entry had an average of 4 weeks of washout of antihypertensive medications. Participants were randomized to receive either HCTZ 12.5 mg daily or atenolol 50 mg daily for 3 weeks, followed by dose titration to 25 mg and 100 mg daily respectively, for systolic blood pressure (SBP) > 120 mmHg or diastolic blood pressure (DBP) > 70 mmHg. In the atenolol treatment arm, 84% of the participants underwent dose titration to atenolol 100 mg and 99% of the participants in the HCTZ arm received titration to HCTZ 25 mg daily. Blood pressure and metabolic responses to monotherapy were assessed after 9 weeks of treatment. In the second part of the study, the alternate drug was added in those patients with SBP > 120 mmHg or DBP > 70 mmHg (> 90% for both randomization arms), with dose titration after 3 weeks and response assessment after 9 weeks, as in the first portion of the study. Participants were asked to maintain their current life style behaviors throughout the study period. The current analysis focuses on the glucose change after atenolol or HCTZ monotherapy.
Phenotype
The phenotype of interest for this subgroup analysis was glucose change after monotherapy, calculated as glucose after 9 weeks of monotherapy minus glucose at baseline (untreated). Fasting blood samples were collected for glucose and insulin before and after completion of atenolol or HCTZ monotherapy. Plasma glucose was measured using a Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) at the central laboratory at the Mayo Clinic, Rochester, MN, using spectrophotometry by an automated enzymatic assay. Plasma insulin was measured using the Access Ultrasensitive Insulin immunoassay system (Beckman Coulter, Brea, CA). Insulin sensitivity status was calculated using the homeostatic model assessment – insulin resistance (HOMA-IR).18 All samples were tested in duplicate, and data reported are means of the duplicate samples.
Genotyping and Quality Control steps
We were able to obtain genotypes on 33 SNPs from the 36 loci associated with fasting glucose level in previous GWAS (Illumina Omni1M quad GWAS Beadchip or HumanCVD Beadchip (Illumina, San Diego, CA; Table S1). Three loci (rs16913693 in IKBKAP, rs10747083 in P2RX2 and rs2302593 in GIPR) or their proxies (SNPs with r2 > 0.8 with the index SNPs) were not covered on either platform.
Illumina Human CVD Beadchip
The Human CVD Beadchip19 was a customized gene-centric array including ~2100 genes and ~50,000 SNPs genotyped using the Infinium II Assay (Illumina, San Diego, CA). Genotypes were called using GenomeStudio software version 2011.1 and the Genotyping Module version 1.9 calling algorithm (Illumina, San Diego, CA). Participants were excluded if sample genotype call rates were below 95% and SNPs were excluded if genotype call rates were below 95%. Sample contamination was detected by checking gender mismatches using X chromosome genotype data and cryptic relatedness was estimated by pairwise identity-by-descent (IBD) analysis implemented using PLINK (http://pngu.mgh.harvard.edu/purcell/plink/). Hardy-Weinberg Equilibrium was assessed with chi-square test of one degree of freedom. After the quality control (QC) procedures, the total SNP call rate in the remaining individuals in these loci was 99.95%.
Illumina Omni1M quad GWAS chip
Human Omni1M quad GWAS chip also used Infinium II assay and genotypes were called using BeadStudio software and GenTrain2 calling algorithm (Illumina, San Diego, CA). Quality control (QC) procedures were performed as with Human CVD Beadchip data. After the QC procedures, the total SNP call rate in the remaining individuals and SNPs was 99.86%. Principal component analysis was performed using Omni1M Quad GWAS data to assess the ancestral background. Participant’s self-identified race information was confirmed with principal component analysis of the Omni1M Quad data for genetic ancestry.
Statistical Analysis
We limited our analysis in the substudy to the Caucasian patients enrolled in PEAR since SNPs were selected from GWAS in Caucasians. To exclude patients that were potentially nonfasting at either visit, we regressed glucose response against nongenetic covariates (age, gender, body mass index (BMI), baseline glucose and baseline insulin) and then standardized the residuals into a distribution with mean of 0 and standard deviation of 1. Participants with standardized residuals that were outside 4 standard deviations were excluded from analysis (n=2). In the remaining 456 Caucasian participants, associations of the 33 SNPs with glucose response after atenolol or HCTZ monotherapy were evaluated using linear regression that adjusted for significant nongenetic predictors of glucose response, including baseline glucose, baseline insulin, age, gender and BMI. The analyses also adjusted for the first principal component for ancestry, which corresponds to European ancestry in PEAR individuals. Additive mode of inheritance was assumed where the SNPs were coded as 0, 1 and 2 in the linear regression model.
To correct for multiple testing of 33 SNPs, we lowered the alpha level to 0.0015 (0.05/33) so that the type I error (false positive) of the study is less than 0.05. Therefore SNPs with p values of less than 0.0015 were considered statistically significant. For nominally significant (p<0.05) SNPs with lower minor allele frequencies, we also explored a dominant model whereby heterozygotes and minor allele homozygotes were combined and compared with the common allele homozygotes. To evaluate the overall risk and benefit of antihypertensive treatments, we also assessed the systolic blood pressure response for SNPs nominally associated with glucose response using linear regression, adjusting for baseline systolic blood pressure, age, gender and first principal component. All single SNP linear regression analyses were performed using PLINK20. Other analyses were performed in SAS version 9.3 (Cary, NC).
RESULTS
The baseline characteristics of the 456 Caucasian PEAR participants assigned to atenolol (n = 232) and HCTZ treatment (n = 224) are presented in Table 1. The participants had a mean age of 50 years, 44% were females; 43% and 44% were overweight and obese, respectively. The average baseline blood pressure was 145.0 ± 9.4 /92.9 ± 5.5 mmHg. The mean baseline glucose in both treatment groups was in the normal range, with a median of 90 mg/dL and interquartile range of 84 – 96 mg/dL.
Table 1.
Baseline characteristics of Caucasian participants in PEAR.
| Characteristics* | Atenolol | HCTZ |
|---|---|---|
| (n = 232) | (n = 224) | |
| Age (mean ± SD) | 49.6 ± 9.5 | 50.1 ± 9.4 |
| Female gender (n, %) | 109 (47.0%) | 91 (40.6%) |
| BMI (kg/m2) | 30.3 ± 5.6 | 30.3 ± 4.9 |
| Waist circumference (cm) | 97.6 ± 12.7 | 98.9 ± 13.6 |
| Glucose (mg/dL) | 92.0 ± 11.6 | 92.9 ± 12.3 |
| Insulin (uU/mL) | 10.0 ± 9.9 | 9.4 ± 8.7 |
| HOMA-IR | 2.4 ± 2.8 | 2.3 ± 2.7 |
| HDL (mg/dL) | 46.7 ± 12.4 | 48.5 ± 13.9 |
| LDL (mg/dL) | 121.7 ± 29.4 | 119.7 ± 29.0 |
| Total cholesterol (mg/dL) | 196.2 ± 34.4 | 197.5 ± 34.6 |
| Triglyceride (mg/dL) | 142.7.0 ± 91.8 | 150.2 ± 101.7 |
| Systolic blood pressure (mmHg) | 145.0 ± 9.4 | 146.1 ± 9.9 |
| Diastolic blood pressure (mmHg) | 92.9 ± 5.5 | 93.6 ± 5.5 |
Numeric characteristics were presented as mean± standard deviation and categorical variables were presented as number and percentages.
Abbreviation: SD: Standard deviation; HCTZ: hydrochlorothiazide; BMI: body mass index. HOMA-IR: homeostatic model assessment-insulin resistance. HDL: high-density lipoprotein. LDL: low-density lipoprotein.
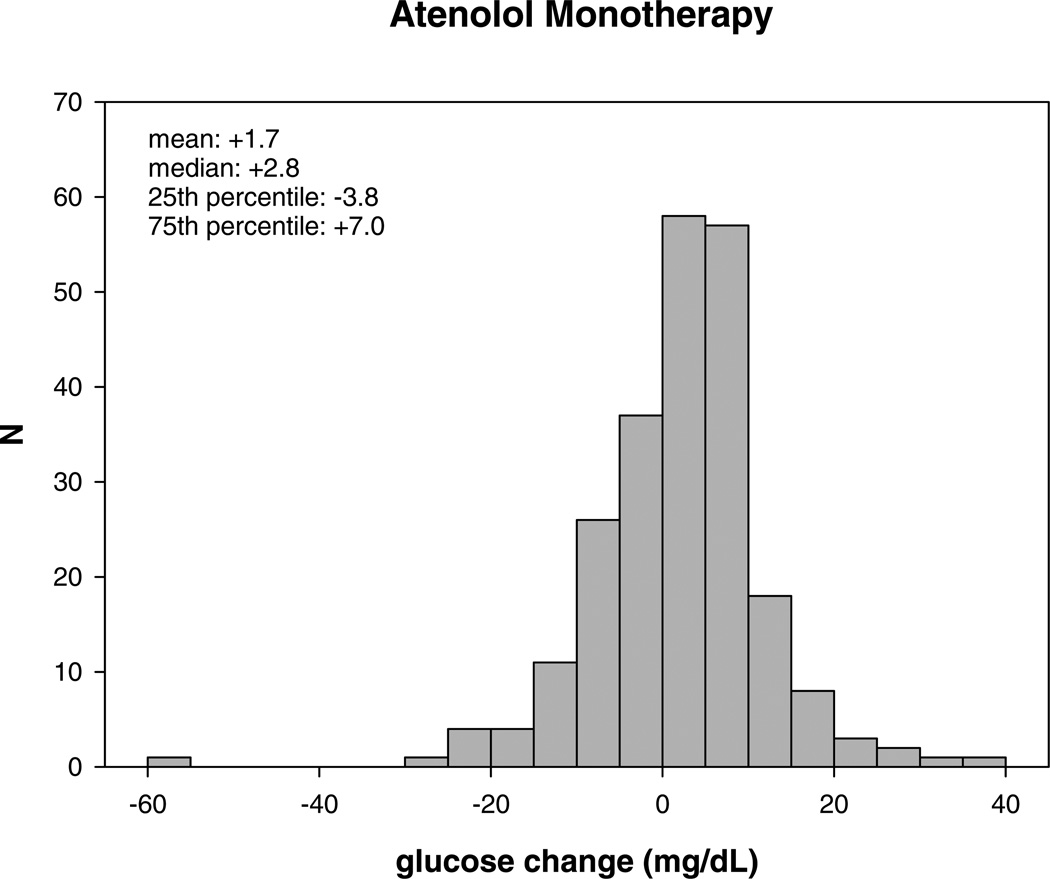
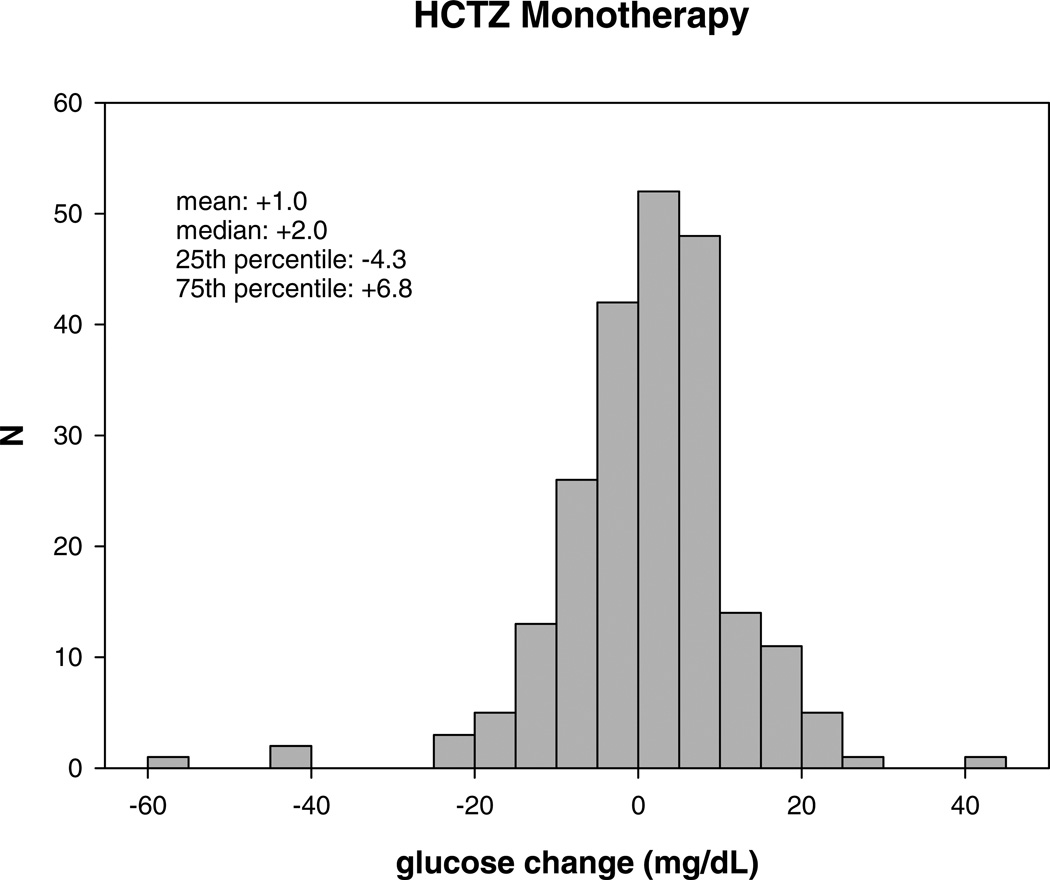
After an average of 9 weeks of atenolol monotherapy, the median glucose change was +2.8 mg/dL (interquartile range: −3.8 – +7.0 mg/dL). The median glucose change after HCTZ monotherapy was +2.0 mg/dL (interquartile range: −4.3 – +6.8 mg/dL). Figure 1 demonstrates the large interindividual variability in glucose response to atenolol and HCTZ monotherapy in Caucasian hypertensive participants.
Figure 1.
Distribution of glucose change (mg/dL) after atenolol (A) and hydrochlorothiazide monotherapy (B).
SNPs associated with atenolol-induced glucose change
Genotypes for the 33 previously identified fasting glucose GWAS SNPs were available for PEAR participants from the two genotyping platforms (Table S1). No SNPs deviated from Hardy-Weinberg Equilibrium in Caucasians.
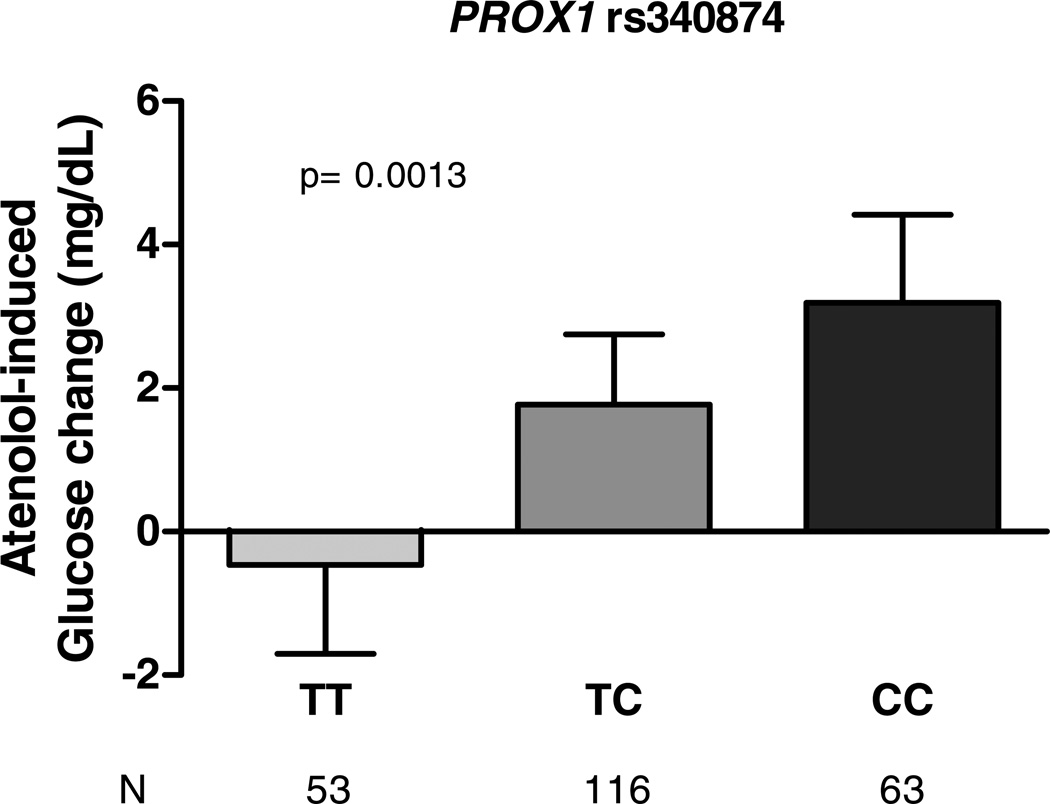
A SNP in the 5’ untranslated region of the prospero homeobox 1 gene (PROX1), rs340874, was significantly associated with glucose change after atenolol monotherapy (p=0.0013, beta = +2.4 mg/dL, Table 2). Participants with T/T, T/C and C/C genotypes had mean glucose change of −0.46, +1.77 and +3.19 mg/dL, respectively (Figure 2). The systolic blood pressure reduction was not statistically different among the three genotype groups (−12.6 mmHg for T/T, −11.1 mmHg for T/C, and −9.3 mmHg for C/C individuals, respectively, p=0.15), although the trend was such that those with the greatest glucose increase also had a smaller reduction in blood pressure.
Table 2.
Fasting glucose SNPs associated with glucose response to atenolol or HCTZ monotherapy in PEAR Caucasian hypertensive patients (with p < 0.05).
| Gene | SNP | chromosome | Location (bp) |
Alleles¶ | Glucose- increasing allele |
MAF | N | BETA | SE | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Atenolol | ||||||||||
| PROX1 | rs340874 | 1 | 212,225,879 | C/T | C | 0.48 | 232 | 2.39 | 0.73 | 0.0013* |
| ARAP1 | rs11603334 | 11 | 72,110,633 | A/G | A | 0.12 | 232 | 2.74 | 1.13 | 0.016 |
| Hydrochlorothiazide | ||||||||||
| SLC2A2 | rs11920090 | 3 | 172,200,215 | A/T | A | 0.14 | 224 | 3.09 | 1.34 | 0.022 |
Location: NCBI build 36 base pair position. MAF: minor allele frequency. Beta indicates the glucose response (in mg/dL) for each glucose increasing allele. SE: standard error. P values were linear regression p adjusted for baseline glucose, baseline insulin, age, gender, body mass index and principal components for ancestry.
Alleles were presented as major/minor alleles.
p value that reached Bonferroni-corrected significance level.
Figure 2.
Fasting glucose SNP PROX1 rs340874 associated with glucose response to atenolol monotherapy among Caucasian hypertensive patients. Error bars represent standard errors of the means.
An intronic SNP in ARAP1 gene (ArfGAP with RhoGAP domain, ankyrin repeat and PH domain 1), rs11603334, was nominally associated with atenolol-induced glucose change (p=0.016, beta=+2.7 mg/dL) (Table 2). Participants with the A allele had a higher glucose increase after atenolol monotherapy: +10.7, +4.6 and +0.7 mg/dL for A/A, A/G and G/G individuals, respectively (Figure S1). There was no statistically significant difference in reduction of systolic blood pressure during the same treatment period: A/A: −9.5 mmHg, A/G: −9.9 mmHg and G/G: −11.3 mmHg (p=0.62).
Two other SNPs showed a trend towards association with glucose change after atenolol monotherapy. These SNPs included rs11039149, an intronic SNP in NR1H3 (p=0.057, beta = +1.6 mg/dL) and rs4869272, an intergenic SNP between PCSK1 and MIR583 (p=0.058, beta = +1.5 mg/dL) (Table S2).
SNPs associated with HCTZ-induced glucose change
An intronic SNP rs11920090 in SLC2A2 (solute carrier family 2, member 2) gene was the only SNP nominally associated with HCTZ-induced glucose response (p=0.022, beta= +3.1 mg/dL) (Table 2). After HCTZ monotherapy, patients with A/A, A/T, and T/T genotypes of this SNP had an average glucose response of +9.1, +3.1 and +0.6 mg/dL, respectively (Figure S2). There was no significant difference in systolic blood pressure reduction among the genotypes: −9.7, −6.7 and −8.0 mmHg for A/A, A/T and T/T, respectively (p=0.46). Two other SNPs (MTNR1B rs10830963 and ADCY5 rs11708067) showed a trend towards association with glucose change after HCTZ monotherapy (Table S3).
Discussion
To our knowledge, this is the first study to test fasting glucose GWAS loci on drug-induced glucose change. Among 33 SNPs previously documented to be associated with fasting glucose levels in Caucasians, one SNP achieved statistical significance for association with glucose change after approximately 9 weeks of atenolol monotherapy. Two other SNPs were nominally associated with glucose change after exposure to atenolol or HCTZ monotherapy.
The effect sizes of these SNPs on the atenolol or HCTZ-associated glucose change were in the range of 2–3 mg/dL per allele, which is approximately 10 times greater than the effect sizes observed in the fasting glucose GWAS.15, 16. This is consistent with numerous studies indicating that pharmacogenetic effect sizes are larger than disease genetic effect sizes.21–24
PROX1 encodes prospero homeobox 1, which is an early specific marker for developing liver and pancreas in foregut endoderm. In an in-vitro study, prox1 was found to be a novel co-repressor of hepatocyte nuclear factor 4α (HNF4 α) that may play a key role in the regulation of bile acid synthesis and gluconeogenesis in the liver.25 HNF4A, which is the gene that encodes HNF4 α, is responsible for a type of diabetes called maturity-onset diabetes of the young (MODY).26 A genetically heterogeneous monogenic form of noninsulin-dependent diabetes mellitus, MODY is characterized by onset usually before age 25 and often in adolescence or childhood and by autosomal dominant inheritance. In GWAS studies and meta-analyses of fasting glucose homeostasis and risk of type 2 diabetes, Caucasians carrying the PROX1 rs340874 C allele had higher fasting glucose level (beta = +0.013 mmol/L or +0.23 mg/dL per allele, p=6.6*10−6) and were at higher risk of developing type 2 diabetes (odds ratio [OR] 1.07, p=7.2*10−10).16 In our study, hypertensive Caucasians harboring the C allele of this SNP had greater glucose elevation after ~9 weeks of atenolol monotherapy (beta = +2.39 mg/dL per allele) and a trend for less pronounced blood pressure reduction. If replicated, these data suggest that hypertensive Caucasians with the C allele of this SNP should avoid treatment with atenolol where possible, given that even modest elevation in glucose could increase the long-term risk for diabetes (6% higher risk of diabetes for each mg/dL increase),11 the long-term nature of antihypertensive therapy and the high allele frequency of this SNP (48% minor allele frequency).
Two SNPs achieved nominal p values of < 0.05 but did not meet the requirement for statistical significance after considering multiple comparisons. With both ARAP1 rs11603334, associated with the atenolol-induced glucose response, and SLC2A2 rs11920090, associated with the HCTZ-induced glucose response, the direction of effects appeared to be opposite of the fasting glucose GWAS effects. In the prior fasting glucose GWAS, individuals with the G allele of ARAP1 rs11603334 had higher fasting glucose levels (beta = 0.019 mmol/L or 0.34 mg/dL per allele).15 In this study, however, patients with the G allele had a smaller glucose increase after atenolol monotherapy. In fasting glucose GWAS, the T allele of SLC2A2 rs11920090 was associated with higher glucose levels, with a beta of +0.02 mmol/L or +0.36 mg/dL16. In this study, however, the T allele was associated with a smaller increase in glucose after HCTZ monotherapy. The associations of these two SNPs were not consistent with our original hypothesis. One possible explanation is that the patients may have other variants (for which we did not test) that might influence the glucose response in the opposite direction from these two SNPs. For this study, we evaluated only the associations of single SNPs (previously identified in fasting glucose-associated GWAS) with glucose change to antihypertensive treatment. It is our long-term goal to evaluate combined effect of multiple variants in a model to predict which patient population would benefit from antihypertensive treatment without adverse metabolic side effects, including increased glucose.
The mechanisms by which atenolol and HCTZ increase glucose are not well understood. This study identifies one SNP (PROX1 rs340874 C allele) that is associated with higher glucose increase after short term exposure (approximately 9 weeks) to atenolol monotherapy; this same SNP was also previously associated with higher fasting glucose levels in GWAS.16
These data suggest that administration of atenolol in individuals already predisposed to higher fasting glucose by their PROX1 genotype might provoke an exaggerated glucose elevation. Due to the long-term nature of antihypertensive therapy and the wide use of β-blockers, it is imperative to further investigate the underlying mechanisms of the adverse metabolic effects associated with these agents. We anticipate that studies of larger sample size will enable us to find additional genetic variants. Hence, our goal is to identify multiple genetic variants that could be considered in a risk model for increased glucose when considering the initiation of antihypertensive treatment.
This study is not without some limitations. The PEAR study was designed primarily as a blood pressure response study. For blood pressure lowering, 9 weeks of treatment is sufficient to observe maximal blood lowering. Regarding the glucose response, data from the large hypertension study ALLHAT27 provides evidence that glucose continues to rise over the long term, particularly in patients without diabetes when antihypertensive treatment begins. Importantly, fasting glucose and the incidence of new onset diabetes continued to rise over the entire 5-year study period. This suggests that that duration of exposure is very important regarding the dysglycemic effect and is and likely more important than dose, which is titrated to blood pressure response early in therapy. Therefore, the PEAR study likely underestimated the glucose increase caused by the two antihypertensives.
Conclusions
These data suggest that variants discovered in fasting glucose GWAS provide pharmacogenetic risk factors for atenolol or HCTZ-induced hyperglycemia. These drugs may interact with genetic risk factors to increase the risk for dysglycemia and diabetes. Functional studies and replication of these associations are needed. After validation in other independent populations, the clinical goal would be to identify a priori those hypertensive individuals who are likely to have high risk for elevated glucose after treatment with atenolol or HCTZ and provide alternative treatment options.
Supplementary Material
Acknowledgements
We acknowledge and thank the valuable contributions of the PEAR study participants, support staff, and study physicians: R. Whit Curry, Karen Hall, Frederic Rabari-Oskoui, Dan Rubin, and Siegfried Schmidt.
Funding sources
PEAR was supported by the National Institute of Health Pharmacogenetics Research Network grant U01-GM074492 and the National Center for Advancing Translational Sciences under the award number UL1 TR000064 (University of Florida); UL1 TR000454 (Emory University) and UL1 TR000135 (Mayo Clinic) and funds from the Mayo Foundation. This research was also supported by K23 HL091120 (A.L.B.).
Footnotes
Author contributions:
Y.G performed the analysis and wrote the manuscript; CWM reviewed/edited manuscript; ALB reviewed/edited manuscript; JRO contributed to the analysis of the data; JHK reviewed/edited manuscript; STT designed and conducted the study, ABC designed and conducted the study, JGG designed and conducted the study; KRB designed and conducted the study; EB designed and conducted the study; JAJ designed and conducted the study and reviewed/edited manuscript; RAC-D designed and conducted the study and reviewed/edited manuscript.
Conflict of Interest Disclosures
YG, CWM, JRO, JHK and KRB declare no conflicts of interest. RMC, ALB, ABC, JGG, EB, STT and JAJ received funding from NIH. RMC also received funding from Women’s Health Initiative. JGG received funding from Janssen Pharmaceuticals, Inc., has Speaker’s Bureau appointment from Boehringer-Ingelheim and is a consultant for Forest Pharmaceuticals and Boehringer-Ingelheim. EB received honoraria from the Foundation of Rome.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Subcommittee obotAHASCaSS. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991;14:203–209. doi: 10.2337/diacare.14.3.203. [DOI] [PubMed] [Google Scholar]
- 4.Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, Gong Y, Hall K, Parekh V, Chapman AB, Boerwinkle E, Johnson JA. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2010;55:61–68. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: A network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 6.Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117:2706–2715. doi: 10.1161/CIRCULATIONAHA.107.695007. discussion 2715. [DOI] [PubMed] [Google Scholar]
- 7.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J, Group ACR. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: A report from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM, Gong Y, Turner ST, Cooper-DeHoff RM, Beitelshees AL, Chapman AB, Boerwinkle E, Bailey K, Johnson JA, Gums JG. Blood pressure responses and metabolic effects of hydrochlorothiazide and atenolol. Am J Hypertens. 2012;25:359–365. doi: 10.1038/ajh.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitland-van der Zee AH, Turner ST, Schwartz GL, Chapman AB, Klungel OH, Boerwinkle E. Demographic, environmental, and genetic predictors of metabolic side effects of hydrochlorothiazide treatment in hypertensive subjects. Am J Hypertens. 2005;18:1077–1083. doi: 10.1016/j.amjhyper.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Karnes JH, McDonough CW, Gong Y, Vo TT, Langaee TY, Chapman AB, Gums JG, Beitelshees AL, Bailey KR, Del-Aguila JL, Boerwinkle EA, Pepine CJ, Turner ST, Johnson JA, Cooper-Dehoff RM. Association of kcnj1 variation with change in fasting glucose and new onset diabetes during hctz treatment. Pharmacogenomics J. 2012 doi: 10.1038/tpj.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519–524. doi: 10.1016/j.amjmed.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A, Group IDR. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper-DeHoff RM, Hou W, Baillie R, Beitelshees AL, Gong Y, Chapman AB, Gums JG, Turner ST, Boyle SH, Zhu H, Wikoff WR, Fiehn O, Frye RF, Kaddurah-Daouk R, Johnson JA. Pharmacometabolomics reveals a novel signature for predicting beta blocker associated impaired fasting glucose. Circulation. 2012;126:A11088. [Google Scholar]
- 15.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Mägi R, Strawbridge RJ, Rehnberg E, Gustafsson S, Kanoni S, Rasmussen-Torvik LJ, Yengo L, Lecoeur C, Shungin D, Sanna S, Sidore C, Johnson PC, Jukema JW, Johnson T, Mahajan A, Verweij N, Thorleifsson G, Hottenga JJ, Shah S, Smith AV, Sennblad B, Gieger C, Salo P, Perola M, Timpson NJ, Evans DM, Pourcain BS, Wu Y, Andrews JS, Hui J, Bielak LF, Zhao W, Horikoshi M, Navarro P, Isaacs A, O'Connell JR, Stirrups K, Vitart V, Hayward C, Esko T, Mihailov E, Fraser RM, Fall T, Voight BF, Raychaudhuri S, Chen H, Lindgren CM, Morris AP, Rayner NW, Robertson N, Rybin D, Liu CT, Beckmann JS, Willems SM, Chines PS, Jackson AU, Kang HM, Stringham HM, Song K, Tanaka T, Peden JF, Goel A, Hicks AA, An P, Müller-Nurasyid M, Franco-Cereceda A, Folkersen L, Marullo L, Jansen H, Oldehinkel AJ, Bruinenberg M, Pankow JS, North KE, Forouhi NG, Loos RJ, Edkins S, Varga TV, Hallmans G, Oksa H, Antonella M, Nagaraja R, Trompet S, Ford I, Bakker SJ, Kong A, Kumari M, Gigante B, Herder C, Munroe PB, Caulfield M, Antti J, Mangino M, Small K, Miljkovic I, Liu Y, Atalay M, Kiess W, James AL, Rivadeneira F, Uitterlinden AG, Palmer CN, Doney AS, Willemsen G, Smit JH, Campbell S, Polasek O, Bonnycastle LL, Hercberg S, Dimitriou M, Bolton JL, Fowkes GR, Kovacs P, Lindström J, Zemunik T, Bandinelli S, Wild SH, Basart HV, Rathmann W, Grallert H, Maerz W, Kleber ME, Boehm BO, Peters A, Pramstaller PP, Province MA, Borecki IB, Hastie ND, Rudan I, Campbell H, Watkins H, Farrall M, Stumvoll M, Ferrucci L, Waterworth DM, Bergman RN, Collins FS, Tuomilehto J, Watanabe RM, de Geus EJ, Penninx BW, Hofman A, Oostra BA, Psaty BM, Vollenweider P, Wilson JF, Wright AF, Hovingh GK, Metspalu A, Uusitupa M, Magnusson PK, Kyvik KO, Kaprio J, Price JF, Dedoussis GV, Deloukas P, Meneton P, Lind L, Boehnke M, Shuldiner AR, van Duijn CM, Morris AD, Toenjes A, Peyser PA, Beilby JP, Körner A, Kuusisto J, Laakso M, Bornstein SR, Schwarz PE, Lakka TA, Rauramaa R, Adair LS, Smith GD, Spector TD, Illig T, de Faire U, Hamsten A, Gudnason V, Kivimaki M, Hingorani A, Keinanen-Kiukaanniemi SM, Saaristo TE, Boomsma DI, Stefansson K, van der Harst P, Dupuis J, Pedersen NL, Sattar N, Harris TB, Cucca F, Ripatti S, Salomaa V, Mohlke KL, Balkau B, Froguel P, Pouta A, Jarvelin MR, Wareham NJ, Bouatia-Naji N, McCarthy MI, Franks PW, Meigs JB, Teslovich TM, Florez JC, Langenberg C, Ingelsson E, Prokopenko I, Barroso I. Consortium DGRaM-aD. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I, Consortium D, Consortium G, Consortium GB. Consortium AHoboP, investigators M. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JA, Boerwinkle E, Zineh I, Chapman AB, Bailey K, Cooper-DeHoff RM, Gums J, Curry RW, Gong Y, Beitelshees AL, Schwartz G, Turner ST. Pharmacogenomics of antihypertensive drugs: Rationale and design of the pharmacogenomic evaluation of antihypertensive responses (pear) study. Am Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace TM, Levy JC, Matthews DR. Use and abuse of homa modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 19.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, Galver L, Barrett JC, Grant SF, Farlow DN, Chandrupatla HR, Hansen M, Ajmal S, Papanicolaou GJ, Guo Y, Li M, Derohannessian S, de Bakker PI, Bailey SD, Montpetit A, Edmondson AC, Taylor K, Gai X, Wang SS, Fornage M, Shaikh T, Groop L, Boehnke M, Hall AS, Hattersley AT, Frackelton E, Patterson N, Chiang CW, Kim CE, Fabsitz RR, Ouwehand W, Price AL, Munroe P, Caulfield M, Drake T, Boerwinkle E, Reich D, Whitehead AS, Cappola TP, Samani NJ, Lusis AJ, Schadt E, Wilson JG, Koenig W, McCarthy MI, Kathiresan S, Gabriel SB, Hakonarson H, Anand SS, Reilly M, Engert JC, Nickerson DA, Rader DJ, Hirschhorn JN, Fitzgerald GA. Concept, design and implementation of a cardiovascular gene-centric 50 k snp array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11:241–246. doi: 10.1038/nrg2751. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y, McDonough CW, Wang Z, Hou W, Cooper-DeHoff RM, Langaee TY, Beitelshees AL, Chapman AB, Gums JG, Bailey KR, Boerwinkle E, Turner ST, Johnson JA. Hypertension susceptibility loci and blood pressure response to antihypertensives: Results from the pharmacogenomic evaluation of antihypertensive responses study. Circ Cardiovasc Genet. 2012;5:686–691. doi: 10.1161/CIRCGENETICS.112.964080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del-Aguila JL, Beitelshees AL, Cooper-Dehoff RM, Chapman AB, Gums JG, Bailey K, Gong Y, Turner ST, Johnson JA, Boerwinkle E. Genome-wide association analyses suggest nell1 influences adverse metabolic response to hctz in african americans. Pharmacogenomics J. 2013 doi: 10.1038/tpj.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner ST, Bailey KR, Schwartz GL, Chapman AB, Chai HS, Boerwinkle E. Genomic association analysis identifies multiple loci influencing antihypertensive response to an angiotensin ii receptor blocker. Hypertension. 2012;59:1204–1211. doi: 10.1161/HYP.0b013e31825b30f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song KH, Li T, Chiang JY. A prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4alpha that regulates the cholesterol 7alpha-hydroxylase gene. J Biol Chem. 2006;281:10081–10088. doi: 10.1074/jbc.M513420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (mody1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 27.Trial AOaCftACRGTAaL-LTtPHA. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.