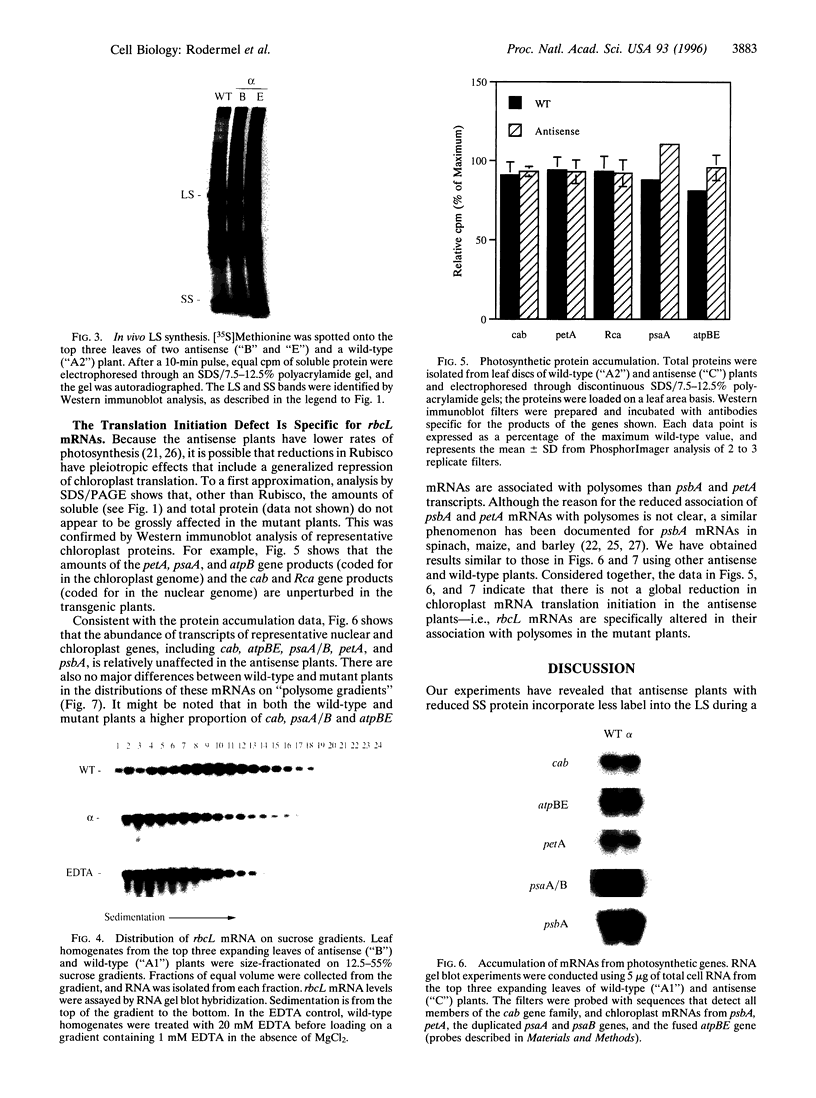
Abstract
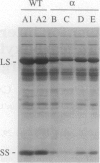
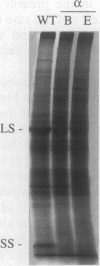
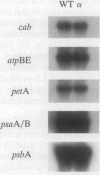
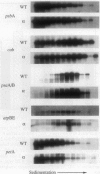
Multimeric protein complexes in chloroplasts and mitochondria are generally composed of products of both nuclear and organelle genes of the cell. A central problem of eukaryotic cell biology is to identify and understand the molecular mechanisms for integrating the production and accumulation of the products of the two separate genomes. Ribulose bisphosphate carboxylase (Rubisco) is localized in the chloroplasts of photosynthetic eukaryotic cells and is composed of small subunits (SS) and large subunits (LS) coded for by nuclear rbcS and chloroplast rbcL genes, respectively. Transgenic tobacco plants containing antisense rbcS DNA have reduced levels of rbcS mRNA, normal levels of rbcL mRNA, and coordinately reduced LS and SS proteins. Our previous experiments indicated that the rate of translation of rbcL mRNA might be reduced in some antisense plants; direct evidence is presented here. After a short-term pulse there is less labeled LS protein in the transgenic plants than in wild-type plants, indicating that LS accumulation is controlled in the mutants at the translational and/or posttranslational levels. Consistent with a primary restriction at translation, fewer rbcL mRNAs are associated with polysomes of normal size and more are free or are associated with only a few ribosomes in the antisense plants. Effects of the rbcS antisense mutation on mRNA and protein accumulation, as well as on the distribution of mRNAs on polysomes, appear to be minimal for other chloroplast and nuclear photosynthetic genes. Our results suggest that SS protein abundance specifically contributes to the regulation of LS protein accumulation at the level of rbcL translation initiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkan A. Nuclear Mutants of Maize with Defects in Chloroplast Polysome Assembly Have Altered Chloroplast RNA Metabolism. Plant Cell. 1993 Apr;5(4):389–402. doi: 10.1105/tpc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Breiding D. E., Klessig D. F. Light-mediated control of translational initiation of ribulose-1, 5-bisphosphate carboxylase in amaranth cotyledons. Plant Cell. 1990 Aug;2(8):795–803. doi: 10.1105/tpc.2.8.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Carr J. P., Klessig D. F. mRNAs encoding ribulose-1,5-bisphosphate carboxylase remain bound to polysomes but are not translated in amaranth seedlings transferred to darkness. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4190–4194. doi: 10.1073/pnas.85.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque D. P., Wildman S. G. Evidence that nuclear genes code for several chloroplast ribosomal proteins. Biochem Biophys Res Commun. 1973 Jan 23;50(2):532–537. doi: 10.1016/0006-291x(73)90872-3. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991 Dec;10(13):3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994 Dec 9;266(5191):1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Hauser C. R. Translational regulation of gene expression in chloroplasts and mitochondria. Annu Rev Genet. 1994;28:71–93. doi: 10.1146/annurev.ge.28.120194.000443. [DOI] [PubMed] [Google Scholar]
- Iwanij V., Chua N. H., Siekevitz P. Synthesis and turnover of ribulose biphosphate carboxylase and of its subunits during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1975 Mar;64(3):572–585. doi: 10.1083/jcb.64.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C. Z., Rodermel S. R. Regulation of Photosynthesis during Leaf Development in RbcS Antisense DNA Mutants of Tobacco. Plant Physiol. 1995 Jan;107(1):215–224. doi: 10.1104/pp.107.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaff P., Gruissem W. Changes in Chloroplast mRNA Stability during Leaf Development. Plant Cell. 1991 May;3(5):517–529. doi: 10.1105/tpc.3.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind M. L., Schmidt G. W. Posttranscriptional Regulation of Ribulose 1,5-bisphosphate Carboxylase Small Subunit Accumulation in Chlamydomonas reinhardtii. Plant Physiol. 1983 Jul;72(3):847–854. doi: 10.1104/pp.72.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinck M., Guilley E., Durr A., Hoff M., Pinck L., Fleck J. Complete sequence of one of the mRNAs coding for the small subunit of ribulose bisphosphate carboxylase of Nicotiana sylvestris. Biochimie. 1984 Jul-Aug;66(7-8):539–545. doi: 10.1016/0300-9084(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Qian J., Rodermel S. R. Ribulose-1,5-bisphosphate carboxylase/oxygenase activase cDNAs from Nicotiana tabacum. Plant Physiol. 1993 Jun;102(2):683–684. doi: 10.1104/pp.102.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D. Post-transcriptional steps in the expression of chloroplast genes. Annu Rev Cell Biol. 1992;8:1–28. doi: 10.1146/annurev.cb.08.110192.000245. [DOI] [PubMed] [Google Scholar]
- Rodermel S. R., Abbott M. S., Bogorad L. Nuclear-organelle interactions: nuclear antisense gene inhibits ribulose bisphosphate carboxylase enzyme levels in transformed tobacco plants. Cell. 1988 Nov 18;55(4):673–681. doi: 10.1016/0092-8674(88)90226-7. [DOI] [PubMed] [Google Scholar]
- Rodermel S. R., Bogorad L. Maize plastid photogenes: mapping and photoregulation of transcript levels during light-induced development. J Cell Biol. 1985 Feb;100(2):463–476. doi: 10.1083/jcb.100.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Spreitzer R. J., Goldschmidt-Clermont M., Rahire M., Rochaix J. D. Nonsense mutations in the Chlamydomonas chloroplast gene that codes for the large subunit of ribulosebisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5460–5464. doi: 10.1073/pnas.82.16.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]