Abstract
To better understand the evolution of a key metabolic pathway, we have sequenced the trpCFBA gene cluster of the hyperthermophilic bacterium Thermotoga maritima. The genes were cloned by complementation in vivo of trp deletion strains of Escherichia coli. The new sequences, together with earlier findings, establish that the trp operon of T.maritima has the order trpE(G.D)CFBA, which might represent the ancestral organization of the tryptophan operon. Heterologous expression of the trp(G.D) and trpC genes in E.coli and N-terminal sequencing of their polypeptide products showed that their translation is initiated at the rate start codons TTG and ATC, respectively. Consequently, the N-terminus of the trp(G.D) fusion protein is 43 residues shorter than previously postulated. Amino acid composition and sequence analyses of the protein products of T.maritima trpC (indoleglycerol phosphate synthase), trpF (phosphoribosyl anthranilate isomerase) and trpA (alpha-subunit of tryptophan synthase) suggest that these thermostable (beta alpha)8-barrel proteins may be stabilized by additional salt bridges, compared with the mesostable forms. Another notable feature is the predicted lack of the N-terminal helix alpha 0 in the alpha-subunit of tryptophan synthase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Bachleitner M., Ludwig W., Stetter K. O., Schleifer K. H. Nucleotide sequence of the gene coding for the elongation factor Tu from the extremely thermophilic eubacterium Thermotoga maritima. FEMS Microbiol Lett. 1989 Jan 1;48(1):115–120. doi: 10.1016/0378-1097(89)90157-2. [DOI] [PubMed] [Google Scholar]
- Burggraf S., Olsen G. J., Stetter K. O., Woese C. R. A phylogenetic analysis of Aquifex pyrophilus. Syst Appl Microbiol. 1992 Aug;15(3):352–356. doi: 10.1016/S0723-2020(11)80207-9. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. The complete nucleotide sequence, gene organization, and genetic code of the mitochondrial genome of Paracentrotus lividus. J Biol Chem. 1989 Jul 5;264(19):10965–10975. [PubMed] [Google Scholar]
- Cheng Y. L., Kalman L. V., Kaiser D. The dsg gene of Myxococcus xanthus encodes a protein similar to translation initiation factor IF3. J Bacteriol. 1994 Mar;176(5):1427–1433. doi: 10.1128/jb.176.5.1427-1433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Darimont B., Sterner R. Sequence, assembly and evolution of a primordial ferredoxin from Thermotoga maritima. EMBO J. 1994 Apr 15;13(8):1772–1781. doi: 10.1002/j.1460-2075.1994.tb06445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. M., Cejka Z., Lupas A., Lottspeich F., Baumeister W. Isolation and cloning of Omp alpha, a coiled-coil protein spanning the periplasmic space of the ancestral eubacterium Thermotoga maritima. EMBO J. 1992 Dec;11(12):4369–4378. doi: 10.1002/j.1460-2075.1992.tb05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Hyde C. C., Ahmed S. A., Padlan E. A., Miles E. W., Davies D. R. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988 Nov 25;263(33):17857–17871. [PubMed] [Google Scholar]
- Jaenicke R. Protein stability and molecular adaptation to extreme conditions. Eur J Biochem. 1991 Dec 18;202(3):715–728. doi: 10.1111/j.1432-1033.1991.tb16426.x. [DOI] [PubMed] [Google Scholar]
- Kelly C. A., Nishiyama M., Ohnishi Y., Beppu T., Birktoft J. J. Determinants of protein thermostability observed in the 1.9-A crystal structure of malate dehydrogenase from the thermophilic bacterium Thermus flavus. Biochemistry. 1993 Apr 20;32(15):3913–3922. doi: 10.1021/bi00066a010. [DOI] [PubMed] [Google Scholar]
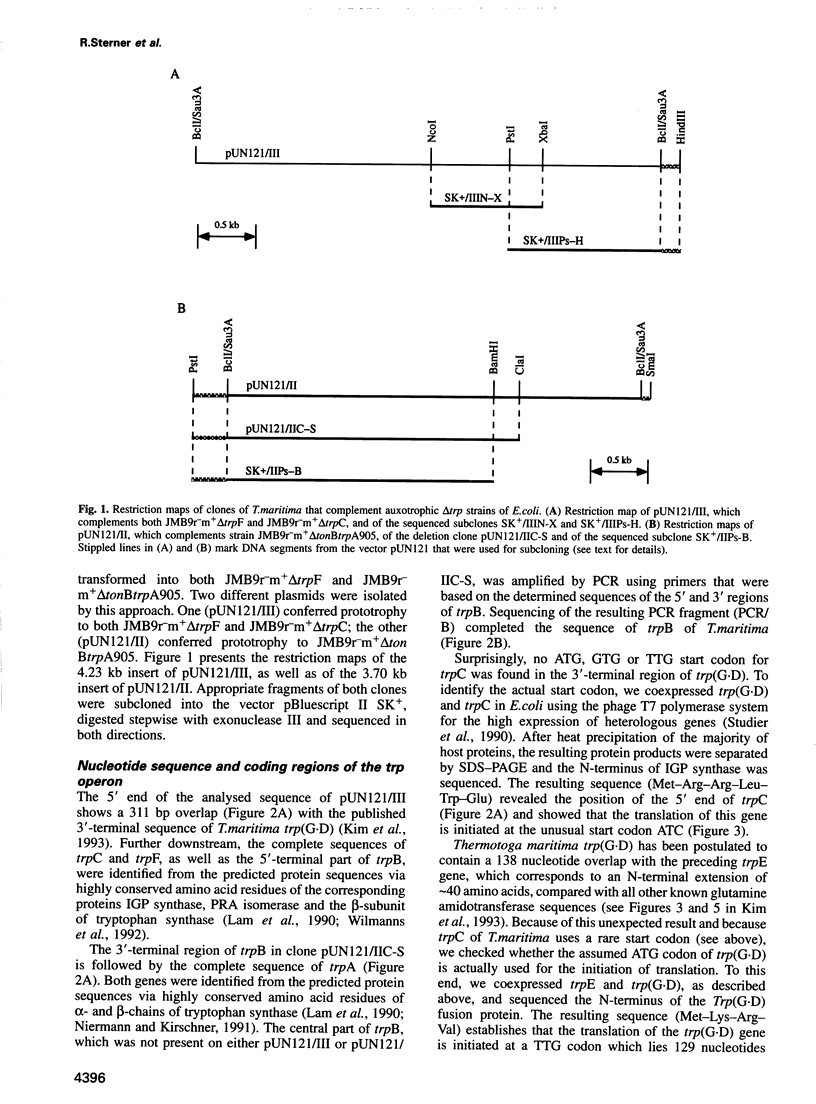
- Kim C. W., Markiewicz P., Lee J. J., Schierle C. F., Miller J. H. Studies of the hyperthermophile Thermotoga maritima by random sequencing of cDNA and genomic libraries. Identification and sequencing of the trpEG (D) operon. J Mol Biol. 1993 Jun 20;231(4):960–981. doi: 10.1006/jmbi.1993.1345. [DOI] [PubMed] [Google Scholar]
- Korndörfer I., Steipe B., Huber R., Tomschy A., Jaenicke R. The crystal structure of holo-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima at 2.5 A resolution. J Mol Biol. 1995 Mar 3;246(4):511–521. doi: 10.1006/jmbi.1994.0103. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam W. L., Cohen A., Tsouluhas D., Doolittle W. F. Genes for tryptophan biosynthesis in the archaebacterium Haloferax volcanii. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6614–6618. doi: 10.1073/pnas.87.17.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Logan S. M., Doolittle W. F. Genes for tryptophan biosynthesis in the halophilic archaebacterium Haloferax volcanii: the trpDFEG cluster. J Bacteriol. 1992 Mar;174(5):1694–1697. doi: 10.1128/jb.174.5.1694-1697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D., Dennis P. P. The organization and expression of essential transcription translation component genes in the extremely thermophilic eubacterium Thermotoga maritima. J Biol Chem. 1992 Nov 15;267(32):22787–22797. [PubMed] [Google Scholar]
- Liebl W., Gabelsberger J., Schleifer K. H. Comparative amino acid sequence analysis of Thermotoga maritima beta-glucosidase (BglA) deduced from the nucleotide sequence of the gene indicates distant relationship between beta-glucosidases of the BGA family and other families of beta-1,4-glycosyl hydrolases. Mol Gen Genet. 1994 Jan;242(1):111–115. doi: 10.1007/BF00277355. [DOI] [PubMed] [Google Scholar]
- Meile L., Stettler R., Banholzer R., Kotik M., Leisinger T. Tryptophan gene cluster of Methanobacterium thermoautotrophicum Marburg: molecular cloning and nucleotide sequence of a putative trpEGCFBAD operon. J Bacteriol. 1991 Aug;173(16):5017–5023. doi: 10.1128/jb.173.16.5017-5023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann T., Kirschner K. Improving the prediction of secondary structure of 'TIM-barrel' enzymes. Protein Eng. 1991 Feb;4(3):359–370. doi: 10.1093/protein/4.3.359. [DOI] [PubMed] [Google Scholar]
- Ostendorp R., Liebl W., Schurig H., Jaenicke R. The L-lactate dehydrogenase gene of the hyperthermophilic bacterium Thermotoga maritima cloned by complementation in Escherichia coli. Eur J Biochem. 1993 Sep 15;216(3):709–715. doi: 10.1111/j.1432-1033.1993.tb18190.x. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Priestle J. P., Grütter M. G., White J. L., Vincent M. G., Kania M., Wilson E., Jardetzky T. S., Kirschner K., Jansonius J. N. Three-dimensional structure of the bifunctional enzyme N-(5'-phosphoribosyl)anthranilate isomerase-indole-3-glycerol-phosphate synthase from Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5690–5694. doi: 10.1073/pnas.84.16.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon D., Farber G. K. The structure and evolution of alpha/beta barrel proteins. FASEB J. 1995 Apr;9(7):497–503. doi: 10.1096/fasebj.9.7.7737457. [DOI] [PubMed] [Google Scholar]
- Romero A., García P. Initiation of translation at AUC, AUA and AUU codons in Escherichia coli. FEMS Microbiol Lett. 1991 Dec 1;68(3):325–330. doi: 10.1016/0378-1097(91)90377-m. [DOI] [PubMed] [Google Scholar]
- Sanangelantoni A. M., Forlani G., Ambroselli F., Cammarano P., Tiboni O. The glnA gene of the extremely thermophilic eubacterium Thermotoga maritima: cloning, primary structure, and expression in Escherichia coli. J Gen Microbiol. 1992 Feb;138(2):383–393. doi: 10.1099/00221287-138-2-383. [DOI] [PubMed] [Google Scholar]
- Schurig H., Beaucamp N., Ostendorp R., Jaenicke R., Adler E., Knowles J. R. Phosphoglycerate kinase and triosephosphate isomerase from the hyperthermophilic bacterium Thermotoga maritima form a covalent bifunctional enzyme complex. EMBO J. 1995 Feb 1;14(3):442–451. doi: 10.1002/j.1460-2075.1995.tb07020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Role of leader peptide synthesis in tryptophanase operon expression in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):383–386. doi: 10.1128/jb.167.1.383-386.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tomschy A., Glockshuber R., Jaenicke R. Functional expression of D-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima in Escherichia coli. Authenticity and kinetic properties of the recombinant enzyme. Eur J Biochem. 1993 May 15;214(1):43–50. doi: 10.1111/j.1432-1033.1993.tb17894.x. [DOI] [PubMed] [Google Scholar]
- Tutino M. L., Scarano G., Marino G., Sannia G., Cubellis M. V. Tryptophan biosynthesis genes trpEGC in the thermoacidophilic archaebacterium Sulfolobus solfataricus. J Bacteriol. 1993 Jan;175(1):299–302. doi: 10.1128/jb.175.1.299-302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmanns M., Hyde C. C., Davies D. R., Kirschner K., Jansonius J. N. Structural conservation in parallel beta/alpha-barrel enzymes that catalyze three sequential reactions in the pathway of tryptophan biosynthesis. Biochemistry. 1991 Sep 24;30(38):9161–9169. doi: 10.1021/bi00102a006. [DOI] [PubMed] [Google Scholar]
- Wilmanns M., Priestle J. P., Niermann T., Jansonius J. N. Three-dimensional structure of the bifunctional enzyme phosphoribosylanthranilate isomerase: indoleglycerolphosphate synthase from Escherichia coli refined at 2.0 A resolution. J Mol Biol. 1992 Jan 20;223(2):477–507. doi: 10.1016/0022-2836(92)90665-7. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aubenton Carafa Y., Brody E., Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990 Dec 20;216(4):835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]


