Abstract
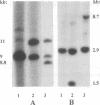
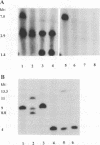
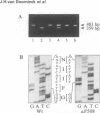
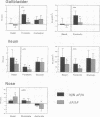
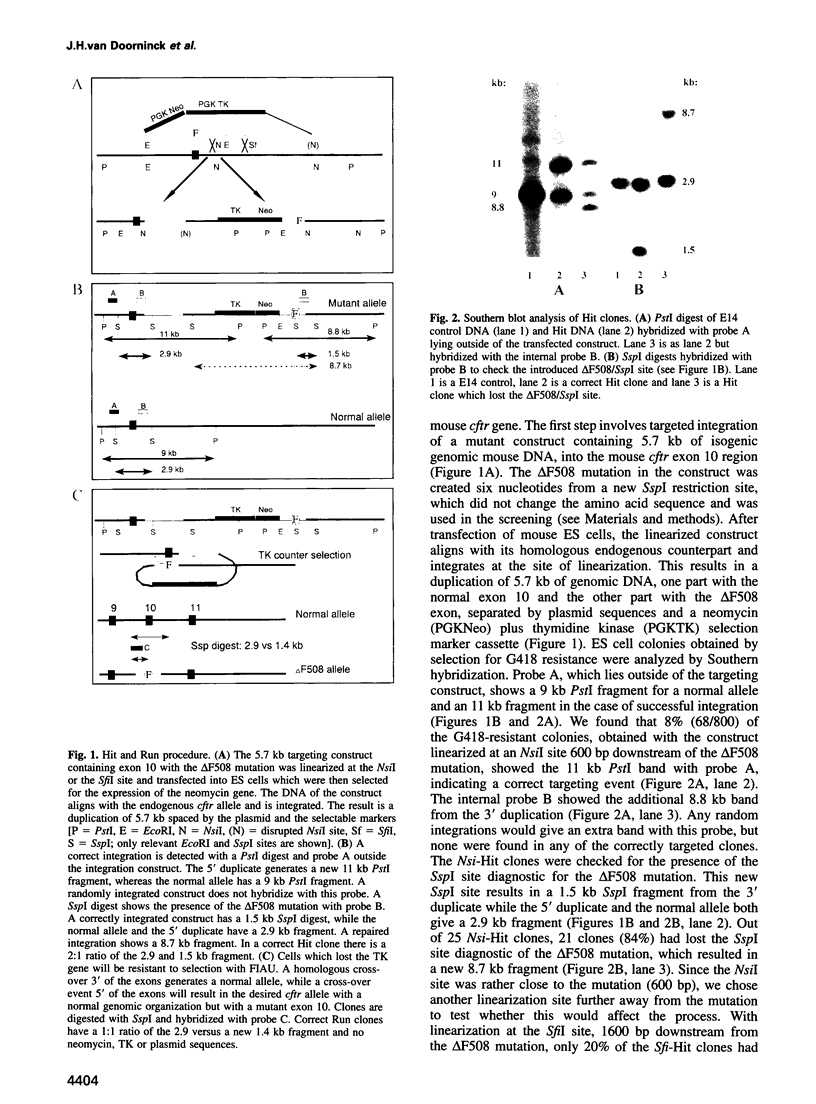
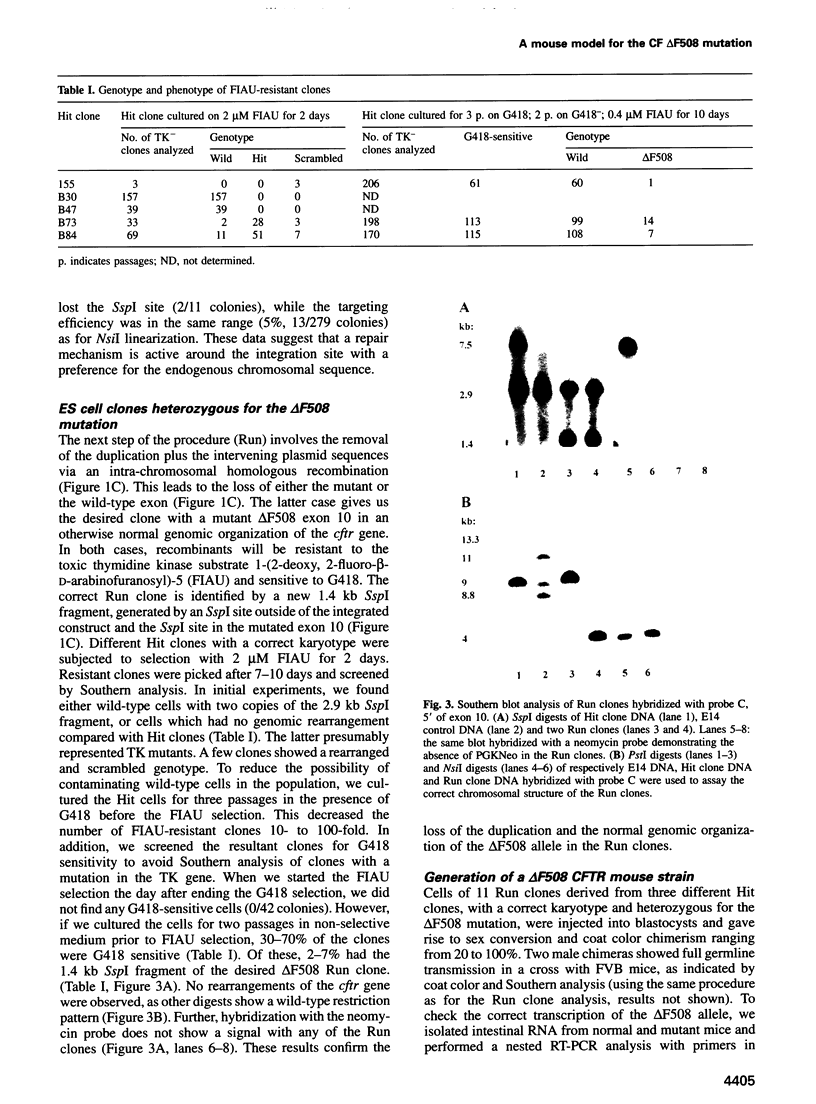
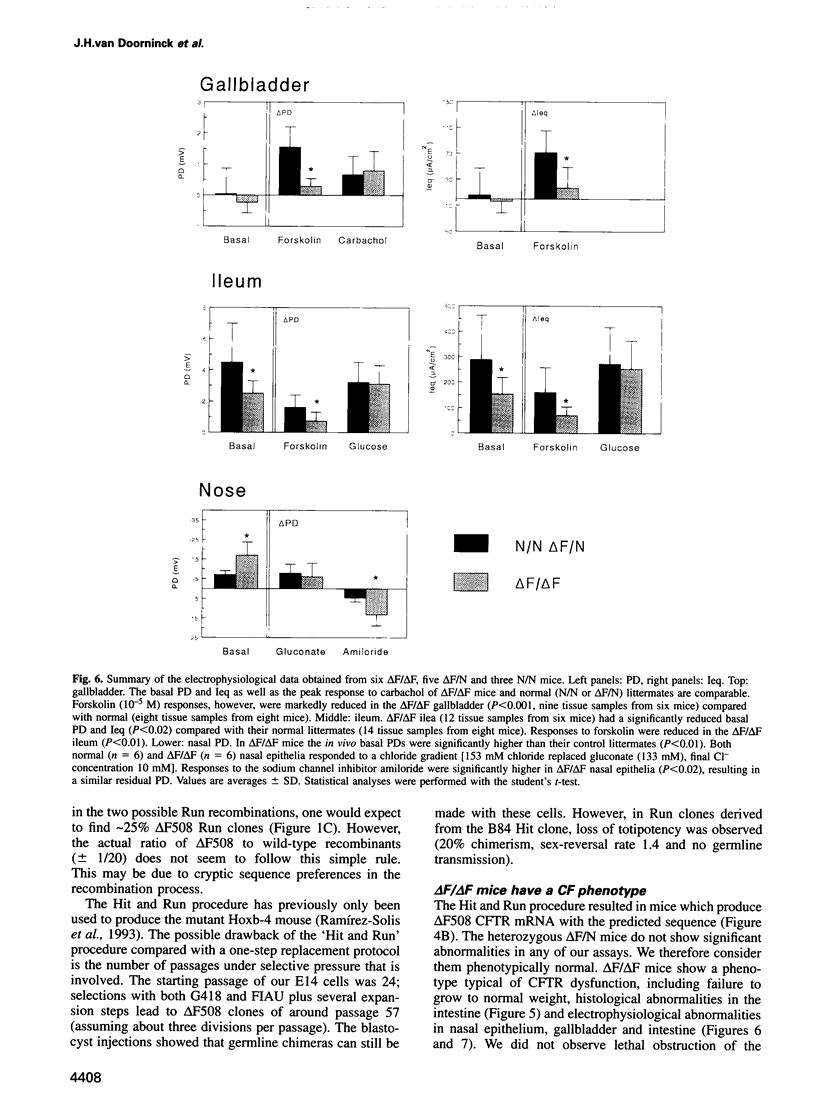
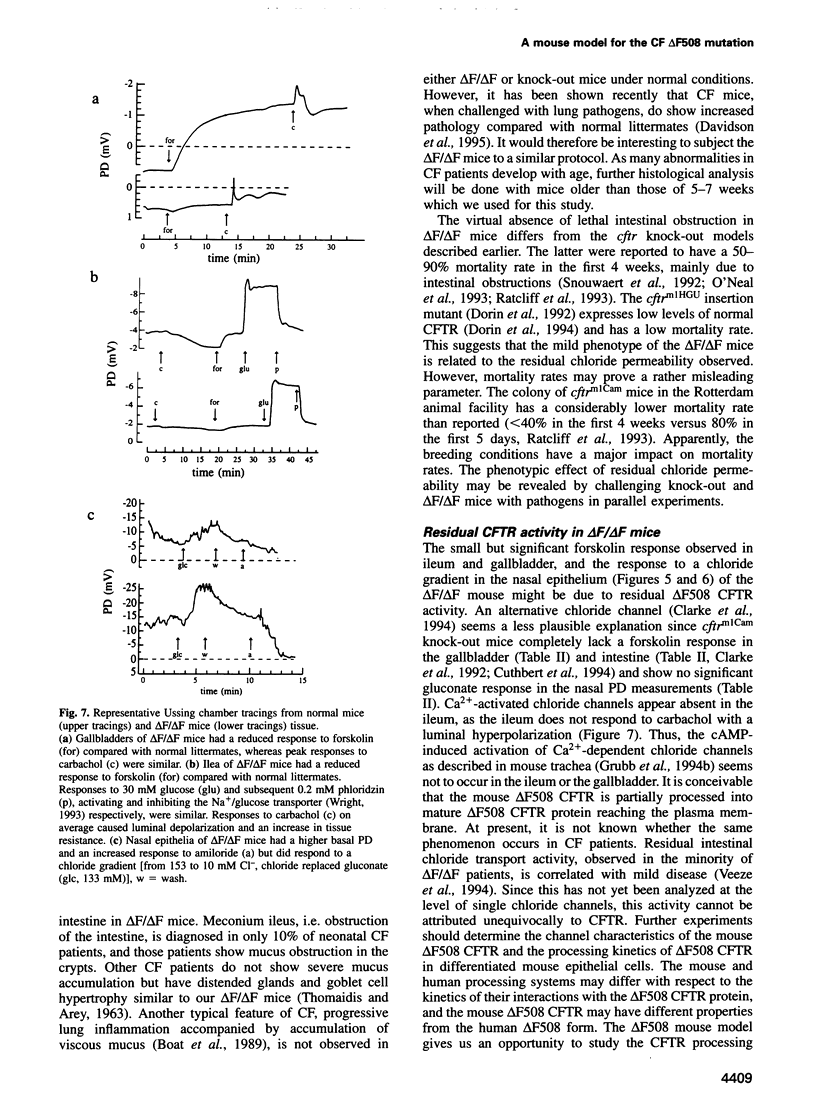
Most cystic fibrosis (CF) patients produce a mutant form (delta F508) of the cystic fibrosis transmembrane conductance regulator (CFTR), which is not properly processed in normal cells but is active as a chloride channel in several experimental systems. We used a double homologous recombination ('Hit and Run') procedure to generate a mouse model for the delta F508 mutation. Targeted embryonic stem (ES) cells (Hit clones) were found; of these either 80 or 20% of the clones had lost the delta F508 mutation, depending on the distance between the linearization site in the targeting construct and the delta F508 mutation. Correctly targeted clones underwent a second selection step resulting in ES cell clones (Run clones) heterozygous for the delta F508 mutation with an efficiency of 2-7%. Chimeric mice were generated and offspring homozygous for the delta F508 mutation showed electrophysiological abnormalities in nasal epithelium, gallbladder and in the intestine, and histological abnormalities in the intestine, typical of CF. Our data suggest that the delta F508 mice have residual delta F508 CFTR activity which would explain the mild pathology of the delta F508 mice. The delta F508 mouse may provide a useful model for the study of the processing defect of delta F508 CFTR and for the development of novel therapeutic approaches based on circumvention of the processing block.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton E. W., Currie D., Logan-Sinclair R., Warner J. O., Hodson M. E., Geddes D. M. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J. 1990 Sep;3(8):922–926. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Clarke L. L., Grubb B. R., Gabriel S. E., Smithies O., Koller B. H., Boucher R. C. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992 Aug 21;257(5073):1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- Clarke L. L., Grubb B. R., Yankaskas J. R., Cotton C. U., McKenzie A., Boucher R. C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I., Maloney P. C., Zeitlin P. L., Guggino W. B., Hyde S. C., Turley H., Gatter K. C., Harris A., Higgins C. F. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., MacVinish L. J., Hickman M. E., Ratcliff R., Colledge W. H., Evans M. J. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflugers Arch. 1994 Oct;428(5-6):508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Davidson D. J., Dorin J. R., McLachlan G., Ranaldi V., Lamb D., Doherty C., Govan J., Porteous D. J. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat Genet. 1995 Apr;9(4):351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- Deng C., Thomas K. R., Capecchi M. R. Location of crossovers during gene targeting with insertion and replacement vectors. Mol Cell Biol. 1993 Apr;13(4):2134–2140. doi: 10.1128/mcb.13.4.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Ostedgaard L. S., Cheng S. H., Smith A. E., Welsh M. J. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest. 1992 Jan;89(1):339–349. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson P., Kimber W. L., Kilanowski F. M., Stevenson B. J., Porteous D. J., Dorin J. R. High frequency gene targeting using insertional vectors. Hum Mol Genet. 1993 Aug;2(8):1299–1302. doi: 10.1093/hmg/2.8.1299. [DOI] [PubMed] [Google Scholar]
- Dorin J. R., Dickinson P., Alton E. W., Smith S. N., Geddes D. M., Stevenson B. J., Kimber W. L., Fleming S., Clarke A. R., Hooper M. L. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992 Sep 17;359(6392):211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- Dorin J. R., Stevenson B. J., Fleming S., Alton E. W., Dickinson P., Porteous D. J. Long-term survival of the exon 10 insertional cystic fibrosis mutant mouse is a consequence of low level residual wild-type Cftr gene expression. Mamm Genome. 1994 Aug;5(8):465–472. doi: 10.1007/BF00369314. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Wilkinson D. J., Smit L. S., Worrell R. T., Strong T. V., Frizzell R. A., Dawson D. C., Collins F. S. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991 Dec 20;254(5039):1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Rich D. P., Cheng S. H., Souza D. W., Paul S., Manavalan P., Anderson M. P., Welsh M. J., Smith A. E. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991 Aug;11(8):3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981 Dec 17;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ramjeesingh M., Reyes E., Jensen T., Chang X., Rommens J. M., Bear C. E. The cystic fibrosis mutation (delta F508) does not influence the chloride channel activity of CFTR. Nat Genet. 1993 Apr;3(4):311–316. doi: 10.1038/ng0493-311. [DOI] [PubMed] [Google Scholar]
- O'Neal W. K., Hasty P., McCray P. B., Jr, Casey B., Rivera-Pérez J., Welsh M. J., Beaudet A. L., Bradley A. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet. 1993 Oct;2(10):1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- Pind S., Riordan J. R., Williams D. B. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994 Apr 29;269(17):12784–12788. [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Riordan J. R. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Sferra T. J., Collins F. S. The molecular biology of cystic fibrosis. Annu Rev Med. 1993;44:133–144. doi: 10.1146/annurev.me.44.020193.001025. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Hooper M. L. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev Biol. 1987 May;121(1):1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- Snouwaert J. N., Brigman K. K., Latour A. M., Malouf N. N., Boucher R. C., Smithies O., Koller B. H. An animal model for cystic fibrosis made by gene targeting. Science. 1992 Aug 21;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- THOMAIDIS T. S., AREY J. B. THE INTESTINAL LESIONS IN CYSTIC FIBROSIS OF THE PANCREAS. J Pediatr. 1963 Sep;63:444–453. doi: 10.1016/s0022-3476(63)80434-5. [DOI] [PubMed] [Google Scholar]
- Tata F., Stanier P., Wicking C., Halford S., Kruyer H., Lench N. J., Scambler P. J., Hansen C., Braman J. C., Williamson R. Cloning the mouse homolog of the human cystic fibrosis transmembrane conductance regulator gene. Genomics. 1991 Jun;10(2):301–307. doi: 10.1016/0888-7543(91)90312-3. [DOI] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Double-strand gap repair in a mammalian gene targeting reaction. Mol Cell Biol. 1991 Sep;11(9):4389–4397. doi: 10.1128/mcb.11.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeze H. J., Halley D. J., Bijman J., de Jongste J. C., de Jonge H. R., Sinaasappel M. Determinants of mild clinical symptoms in cystic fibrosis patients. Residual chloride secretion measured in rectal biopsies in relation to the genotype. J Clin Invest. 1994 Feb;93(2):461–466. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M. The intestinal Na+/glucose cotransporter. Annu Rev Physiol. 1993;55:575–589. doi: 10.1146/annurev.ph.55.030193.003043. [DOI] [PubMed] [Google Scholar]
- Yang Y., Janich S., Cohn J. A., Wilson J. M. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]