Abstract
Ulceration is an important prognostic factor in melanoma whose biologic basis is poorly understood. Here we assessed the prognostic impact of pleckstrin homology domain-interacting protein (PHIP) copy number and its relationship to ulceration. PHIP copy number was determined using fluorescence in situ hybridization (FISH) in a tissue microarray cohort of 238 melanomas. Elevated PHIP copy number was associated with significantly reduced DMFS (P = 0.01) and DSS (P = 0.009) by Kaplan-Meier analyses. PHIP FISH scores were independently predictive of DMFS (P = 0.03) and DSS (P = 0.03). Increased PHIP copy number was an independent predictor of ulceration status (P = 0.04). The combined impact of increased PHIP copy number and tumor vascularity on ulceration status was highly significant (P< 0.0001). Stable suppression of PHIP in human melanoma cells resulted in significantly reduced glycolytic activity in vitro, with lower expression of LDH5, HIF1A, and VEGF, and was accompanied by reduced microvessel density in vivo. These results provide further support for PHIP as a molecular prognostic marker of melanoma, and reveal a significant linkage between PHIP levels and ulceration. Moreover, they suggest that ulceration may be driven by increased glycolysis and angiogenesis.
Introduction
Melanoma is an important clinical problem, with an estimated 76,250 new cases and 9,180 deaths in 2012 (Siegel et al. 2012). The clinical behavior of melanoma can be unpredictable, making prognostic predictions difficult in individual patients. While tumor thickness consistently emerges as the single most significant prognostic factor determining survival, and while ulceration increases the risk of death within a given thickness range, additional factors are required to refine the prognostic assessment of melanoma patients. Beyond histological factors that are reliably associated with decreased survival, molecular markers represent the next frontier in the evaluation of melanoma outcome. Despite the identification of numerous putative molecular prognostic factors, no prognostic biomarkers are routinely used in the evaluation of melanoma patients (Gogas et al. 2009). In addition, few biomarkers have been shown to retain prognostic impact, when measured on more than one platform (i.e., measurements at the DNA versus RNA versus protein level).
Recent studies performed by our group identified a previously unknown role for the pleckstrin homology domain-interacting protein (PHIP) in melanoma progression (De Semir et al. 2012). PHIP was identified as the top gene overexpressed in metastatic versus primary melanomas by gene expression profiling analysis (Haqq et al. 2005). shRNA-mediated suppression of PHIP resulted in significant suppression of melanoma cell invasion and metastatic potential in both murine and melanoma cell lines. High levels of PHIP expression were associated with significantly reduced survival in both mouse models and human tissues. Specifically, high PHIP expression (as determined by immunohistochemical analysis) was independently predictive of DMFS and DSS. In this study, we analyze the role of PHIP copy number for its prognostic significance in primary cutaneous melanoma and explore the linkage between PHIP levels and ulceration development in melanoma.
Results
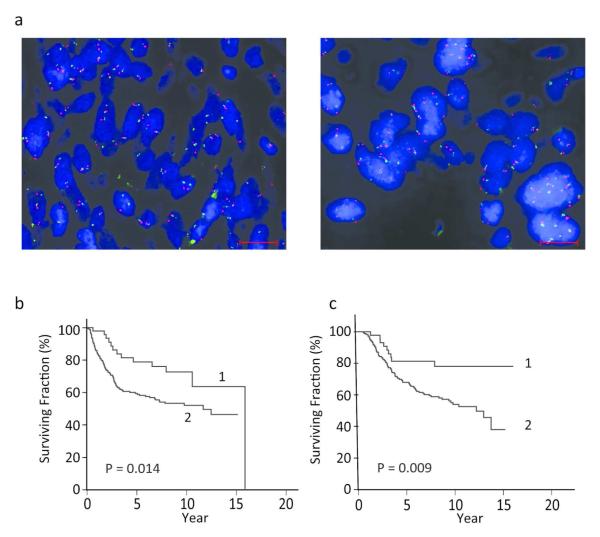
Given the recent demonstration of a significant prognostic role for PHIP protein expression and the presence of elevated PHIP copy number in melanoma (De Semir et al. 2012), we aimed to determine the prognostic impact of PHIP copy number in primary cutaneous melanoma. PHIP copy number was analyzed using FISH on a TMA cohort of 238 specimens, with known immunohistochemical analysis of PHIP protein expression, by an observer blinded to patient outcomes. Overall, 22% of the cohort possessed a mean copy number of 3 or greater. See Figure 1a for representative examples of low versus high PHIP copy number. As the ratio of PHIP and probes representing the centromere of chromosome 6 in almost all of the cases was close to 1.0 (Table S1), the primary attribute assessed was percent of cells expressing 3 or more copies of the PHIP gene, in which there was a range from 0-87%, with a mean of 33.4%. There was a significant correlation between PHIP copy number and level of expression of the PHIP protein (P < 0.0001, Figure S1).
Figure 1.
PHIP copy number and its correlation with DMFS and DSS in melanoma patients. (a) Dual color FISH for PHIP locus, red, and centromere of chromosome 6, green, representative of low PHIP copy number, left panel, and high copy number, right panel. Scale bar = 20 Rm. (b) Kaplan-Meier analysis of DMFS in patients with low PHIP copy number (curve 1) versus patients with high PHIP copy number (curve 2). (c) Kaplan-Meier analysis of DSS in patients with low PHIP copy number (curve 1) versus patients with high PHIP copy number (curve 2).
Initially, we analyzed the relationship between PHIP copy number and melanoma outcome using univariate analysis. High PHIP copy number was associated with decreased melanoma survival, when DMFS (P = 0.01 by log-rank test) and DSS (P= 0.009 by log-rank test) were assessed using Kaplan-Meier analysis (Figure 1b and 1c). In addition, there was a significantly increased risk of distant metastasis or death due to melanoma with the presence of high PHIP FISH scores. 45.4% of patients with high copy number had distant metastasis, when compared to 25.5% with low copy number (Fisher’s exact test, P = 0.015). 42.2% of patients with high copy number died of metastatic melanoma, when compared to 17.7% of patients with low copy number (Fisher’s exact test, P < 0.002).
Next, we examined the relationship between PHIP copy number and survival using multivariate Cox regression analysis. High PHIP FISH scores were significantly predictive of reduced DMFS (P = 0.03, Table 1) and DSS (P = 0.027, Table 2) in multivariate models that included tumor thickness, ulceration, age, gender, and tumor site. Thus, PHIP copy number was independently predictive of survival associated with melanoma, similar to the results obtained for PHIP protein expression (De Semir et al. 2012).
Table 1.
Cox regression analysis of impact of various prognostic factors on DMFS of melanoma cohort (N = 231).
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Tumor thickness | 6.96 | 1.38 | .008 |
| High PHIP copy number | 4.73 | 1.99 | .03 |
| Ulceration | 3.31 | 1.51 | .07 |
| Sex | 1.41 | 1.34 | .24 |
| Site | 0.92 | 1.24 | .34 |
| Age | 0.61 | 0.95 | .44 |
Table 2.
Cox regression analysis of impact of various prognostic factors on DSS of melanoma cohort (N = 231).
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Tumor thickness | 6.13 | 1.38 | .013 |
| High PHIP copy number | 4.87 | 2.21 | .027 |
| Ulceration | 3.63 | 1.58 | .057 |
| Sex | 1.00 | 1.30 | .32 |
| Site | 0.81 | 1.24 | .37 |
| Age | 0.01 | 0.99 | .91 |
Recently, high PHIP immunohistochemical scores were shown to correlate with ulceration status (De Semir et al. 2012). We aimed to confirm this relationship at the DNA level. High PHIP copy number was significantly associated with ulceration status when analyzed by univariate logistic regression analysis (P=0.004). In addition, high PHIP copy number was associated with a significantly increased risk of developing ulceration. Thus, ulceration was present in 45.5% of cases with high PHIP copy number, when compared with 28.8% of cases with low PHIP copy number (P = 0.013, Fisher’s exact test).
Previously, we reported that ulceration was associated with increased tumor vascularity in the primary tumor, when assessed morphologically (Kashani-Sabet et al. 2002). Elevated tumor vascularity (as determined by melanomas with prominent versus all lower degrees of vascularity) was significantly associated with ulceration status. Ulceration was present in 60.3% of the cases with prominent tumor vascularity, when compared with 22.2% of cases with lower degrees of tumor vascularity (P < 0.0001, Fisher’s exact test). This served to confirm the previously reported results in this tissue microarray cohort. We then explored the combined impact of high PHIP copy number and elevated tumor vascularity on the development of ulceration. Ulceration was present in 20.2% of melanomas with both low PHIP copy number and low tumor vascularity. This increased to 38.8% of cases ulcerated with either prognostic factor elevated, and to 72.2% of melanomas ulcerated, when both factors were elevated (P < 0.0001, Chi-square test of percentage differences).
We then assessed the relationship between PHIP copy number and ulceration status, using multivariate logistic regression analysis. Presence of ulceration in melanoma is also known to increase both with increasing tumor thickness and with increasing mitotic rate (Balch et al. 2001b; Thompson et al. 2011). A multivariate logistic regression analysis was performed, including low versus high PHIP copy number, low versus high tumor vascularity, tumor thickness, and mitotic rate. All four factors were significantly predictive of the incidence of ulceration (Table 3). Thus, PHIP FISH scores were independently predictive of ulceration status, even with the inclusion of these other factors (P = 0.04).
Table 3.
Logistic regression analysis of impact of various prognostic factors on incidence of ulceration in melanoma cohort (N = 228).
| Prognostic factor | Chi-square | Odds Ratio | P value |
|---|---|---|---|
| Mitotic rate | 15.24 | 1.23 | .0001 |
| Tumor vascularity | 10.26 | 3.05 | .001 |
| Tumor thickness | 5.99 | 1.63 | .01 |
| High PHIP copy number | 4.10 | 1.96 | .04 |
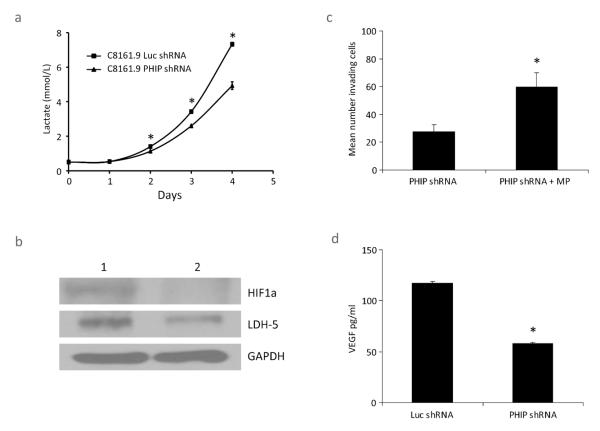
Next, we explored cellular pathways regulated by PHIP expression potentially relevant to the development of ulceration. Targeted suppression of PHIP (using an anti-PHIP shRNA) in C8161.9 human melanoma cells (with elevated PHIP copy number) was previously shown to significantly suppress melanoma cell invasiveness and metastatic potential (De Semir et al. 2012). Given that PHIP functions in the IGF1R pathway that is important in regulating glucose metabolism, we assessed whether suppression of PHIP activity resulted in altered glycolytic activity of melanoma cells. C8161.9 cells expressing the anti-PHIP shRNA produced significantly lower amounts of lactate than control cells expressing a shRNA targeting luciferase (anti-luc shRNA) (Figure 2a). This was associated with decreased expression of LDH5, the most efficient isoenzyme of lactate dehydrogenase, which catalyzes the conversion of pyruvate to lactate in the final step of glycolysis (Figure 2b). In addition, culturing anti-PHIP shRNA-expressing cells in the presence of methylpyruvate, which bypasses glycolysis and directly enters the Krebs cycle, resulted in significantly increased invasion (Figure 2c). Thus, the proinvasive role of PHIP in melanoma is mediated, at least in part, by activating the glycolytic pathway. Moreover, C8161.9 melanoma cells with reduced PHIP expression produced lower levels of VEGF, as determined by an ELISA assay of human VEGF secretion (Figure 2d). As VEGF expression is controlled by HIF1A (Forsythe et al. 1996), melanoma cells with suppressed PHIP expression were also shown to express lower levels of HIF1A by Western analysis (Figure 2b).
Figure 2.
Effects of shRNA-mediated suppression of PHIP in human melanoma cells. (a) Lactate production in C8161.9 cells expressing anti-PHIP shRNA versus control cells expressing anti-luc shRNA. Mean ± SE; N = 3. * denotes P < 0.003. (b) Western analysis of LDH5 and HIF1A in C8161.9 cells expressing anti-luc shRNA (control cells, lane 1) or anti-PHIP shRNA (lane 2). (c) Invasive capacity of C8161.9 stable transformants expressing anti-PHIP shRNA with or without the addition of methylpyruvate (MP). Mean ± SE; N = 3. * denotes P < 0.05. (d) ELISA assay of human VEGF levels produced by C8161.9 cells expressing anti-PHIP shRNA versus control cells expressing anti-luc shRNA. Mean ± SE; N = 3. * denotes P < 0.0001.
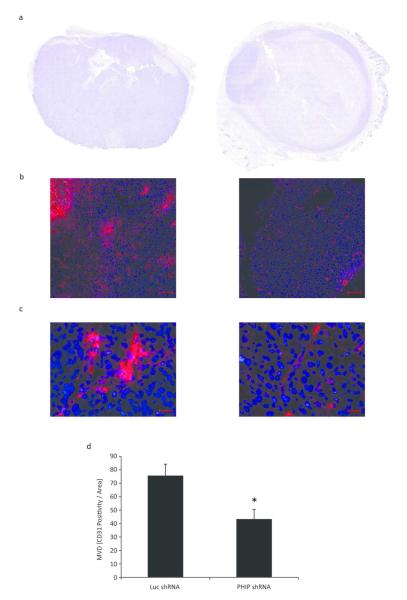
Finally, we assessed the effects of suppression of PHIP on the angiogenic potential of human melanoma cells in vivo. C8161.9 melanoma cells were inoculated subcutaneously into nude mice, and the resultant tumors examined for microvessel density using immunofluorescence of CD31 positivity. While there was no significant difference in the tumor volume between the two groups (data not shown), tumors in the anti-PHIP shRNA-expressing group (Figure 3a, right panel) exhibited large areas of necrosis when compared with the control group (Figure 3a, left panel). Analysis of CD31 expression revealed that the control tumors were characterized by large, abnormal blood vessels and hemorrhage, apparent from autofluorescence of erythrocytes and lipofuscin (Baschong et al. 2001) (Figure 3b and 3c, left panels). By contrast, the vessels in the anti-PHIP shRNA-expressing tumors were smaller, with little or no hemorrhage evident (Figure 3b and 3c, right panels). Finally, the anti-PHIP shRNA-expressing tumors had significantly reduced microvessel density when compared with the control tumors (P < 0.02 as assessed both by a T test and by a two-sample randomization test, Figure 3d). Thus, increasing PHIP expression in human melanoma cells promotes angiogenic potential.
Figure 3.
Effects of PHIP expression on the angiogenic potential of C8161.9 human melanoma cells in vivo. (a) Representative images of H&E staining of subcutaneous tumors in nude mice. The left panel shows the growth pattern of C8161.9 cells expressing anti-luc shRNA, compared with C8161.9 cells expressing anti-PHIP shRNA (right panel). (b) Composite images of immunofluorescence detection of CD31, red, and DAPI, blue as counterstain. The left panel shows the staining pattern of CD31 in subcutaneous tumor with C8161.9 cells expressing anti-luc shRNA compared with C8161.9 cells expressing anti-PHIP shRNA (right panel). Scale bar = 100 Rm. (c) Composite images of CD31 staining pattern with higher magnification for tumors with C8161.9 cells expressing anti-luc shRNA (left panel), or anti-PHIP shRNA (right panel). Scale bar = 20 Rm. (d) Quantification of microvessel density (MVD) based on CD31 immunofluorescence positivity in tumors (six per group) with C8161.9 cells expressing either anti-luc shRNA or anti-PHIP shRNA. Mean ± SD. * denotes P < 0.02.
Discussion
In this manuscript, we describe a previously unreported role for PHIP copy number in the prognosis associated with melanoma. PHIP FISH scores were predictive of a significantly increased risk of both distant metastasis and death due to melanoma, and were independently predictive of DMFS and DSS. In addition, elevated PHIP FISH scores were associated with a higher incidence of ulceration and were independently predictive of ulceration status. The combined impact of PHIP copy number and tumor vascularity was additive, as cases with high levels of both factors had a significantly higher incidence of ulceration than cases with low levels of either or both factors. Finally, C8161.9 human melanoma cells with suppressed PHIP expression exhibited reduced glycolytic activity and angiogenic potential.
These results provide further support for the role of PHIP as a molecular prognostic marker for melanoma, as initially described using immunohistochemical analysis of PHIP expression. High PHIP protein expression was associated with a significantly increased risk of distant metastasis and death due to melanoma, and was independently predictive of DMFS and DSS (De Semir et al. 2012). Our analysis of PHIP copy number parallels the results obtained in human and murine melanoma cell lines, in which suppressed PHIP expression resulted in significantly reduced metastatic potential and increased survival in xenograft models.
To date, few markers have been shown to provide significant prognostic impact in melanoma when assessed on more than one platform. For example, SPP1 expression has been shown to have independent prognostic significance when assessed at the RNA and protein levels (Rangel et al. 2008; Conway et al. 2009). Our studies have demonstrated a significant prognostic role for PHIP at both the DNA and protein levels. Although the ratio of PHIP to chromosome 6 centromeric probe was close to 1.0 in the great majority of cases examined, changes in PHIP copy number, ranging from euploidy to 3 or greater copies, were highly correlated with increased PHIP expression. Additional studies of PHIP levels will be required in distinct cohorts in order to further validate its prognostic role.
Beyond an independent impact on survival, these studies confirmed a significant and independent relationship between PHIP levels and ulceration status. In addition, PHIP copy number combined dramatically with tumor vascularity to increase the incidence of ulceration in primary melanoma specimens. Ulceration is an important prognostic factor that has been incorporated into the AJCC staging classification for melanoma for over a decade (Balch et al. 2001a, 2009). More recently, ulceration has been suggested as a potential predictive marker of benefit to adjuvant interferon alpha therapy (McMasters et al. 2010; Eggermont et al. 2012). However, the biologic basis for the development of ulceration is poorly understood.
Increasing incidence of ulceration in melanoma is associated with increasing tumor thickness, mitotic rate, and tumor vascularity (Balch et al. 2001b; Thompson et al. 2011). In addition, gene expression profiling studies have identified a gene signature that correlates with ulceration (Winnepenninckx et al. 2006). However, the specific cellular pathways and mechanisms by which ulceration develops are unclear. The linkage between PHIP levels and ulceration implies a role for the IGF1R pathway in ulceration, given PHIP’s involvement in this signal transduction pathway. In addition, our studies in human melanoma cells demonstrating that downregulation of PHIP expression results in significantly reduced glycolytic activity, VEGF secretion, and microvessel density provide mechanistic insights, identifying glucose metabolism and tumor angiogenesis as pathways by which ulceration may develop in melanoma.
Tumor cell growth is promoted by increased glucose consumption, a phenomenon known as the Warburg effect (Warburg 1956). The importance of glycolysis to melanoma progression has been amply reported in melanoma cell lines (Halaban et al. 1997, 2002; Bagheri et al. 2006) and is demonstrated clinically by the utility of PET scanning in the radiologic assessment of melanoma both during initial staging (Reinhardt et al. 2006; Falk et al. 2007) and in response to targeted therapy (McArthur et al. 2012). Glycolysis in tumor cells is driven by AKT (Elstrom et al. 2004), and PHIP is a potent activator of AKT in melanoma (De Semir et al. 2012). Moreover, increased glucose metabolism can itself drive tumor angiogenesis, invasion, and metastasis (Lay et al. 2000; Tsutsumi et al. 2004). Thus, our studies on PHIP in melanoma describe a previously unreported link between PHIP, the Warburg effect, tumor angiogenesis, and ulceration development. As PHIP is activated through increased copy number in a subset of melanomas, additional markers of ulceration will need to be identified in melanomas without PHIP activation. However, the mechanistic analyses provided here should facilitate the identification of these additional markers in distinct molecular subsets of melanoma patients.
Finally, our investigation of PHIP in melanoma tissues and cell lines suggests an intriguing link between ulceration and LDH levels. These results imply that a common tumor-promoting pathway (i.e., IGF1R), resulting in increased glycolysis, may underlie the biologic basis of two prominent prognostic markers incorporated into the AJCC staging classification, one that refines the prognosis of localized melanoma, the other the prognosis of disseminated melanoma. Elevated LDH5 expression in primary melanoma has been previously shown to be associated with reduced survival on univariate analysis, but was not independently predictive of melanoma outcome (Zhuang et al. 2010). Furthermore, LDH activity has been correlated to HIF1A expression and angiogenic potential (Koukourakis et al. 2003). However, further studies will be required in order to confirm the link between ulceration and LDH levels suggested by this analysis. In addition, high levels of angiopoietin-2 have been reported in multiple cancers, with a possible prognostic role (Bonner and Arbiser 2012), and are accompanied by upregulation of RAC signaling pathways (Felcht et al. 2012). Thus, it is possible that elevated PHIP copy number can modulate angiopoietin levels in melanoma, in part through mitrochondrial dysfunction, NADPH oxidase induction, and increased generation of reactive oxygen species (which result in angiopoietin-2 activation). In conclusion, our studies support an important role for PHIP as a molecular marker of melanoma ulceration, metastasis and survival, and provide insights into the cellular pathways through which ulceration can develop.
Materials and methods
Study population
This study used a tissue microarray cohort that was previously described according to REMARK guidelines (Rangel et al. 2006; Kashani-Sabet et al. 2009). The molecular prognostic factor analyses performed herein were approved by the appropriate ethics boards both at CPMC and UCSF.
Fluorescence in situ hybridization (FISH)
FISH was performed as previously described (Wiegant and Raap 2001; Chin et al. 2003; De Semir et al. 2012) using BAC clones RP11-767O1, RP11-484L10, RP11-217L13 and CTD-2297E14 to detect the PHIP locus and clones RP11-26M18 and RP11-136K2 to detect 6q11.1 and 6p11.1 respectively (February 2009 freeze of the UCSC Genome Browser, http://genome.ucsc.edu). BAC DNA’s were prepared with the Large-Construct kit (Qiagen, Valencia, CA) and labeled by nick translation with Alexa Fluor 488 and 594 dUTP’s (Life Technologies, Grand Island, NY) as described (Wiegant and Raap 2001). The quality and mapping of all probes were verified by hybridization to normal metaphase spreads in combination with a commercially available centromeric probe for chromosome 6 (Open Biosystems, Lafayette, CO) before tissue analysis. Hybridization on TMA’s was performed as described previously (Wiegant and Raap 2001; Chin et al. 2003). Images were taken with a Zeiss Axio Imager Z2 controlled by AxioVision software. The FISH signals were assessed and counted manually from images with several Z stack layers acquired using a Zeiss Axio Image Z2 microscope controlled by AxioVision software (Zeiss, Jena,Germany). At least 30 nuclei from each case were evaluated, and the signals were interpreted according to guidelines described previously (Bayani and Squire 2004) and recorded as 1 through 5 or greater. Signals from BAC clones detecting 6q11.1 and 6p11.1 were interpreted as the centromeric signal for chromosome 6 and the count was used as control.
Cell culture studies
C8161.9 human melanoma cells stably expressing anti-PHIP shRNA and anti-luciferase (anti-luc) shRNA were generated and propagated as described (De Semir et al. 2012). Lactate production and invasion into matrigel were assayed as reported (De Semir et al. 2012). C8161.9 cells were treated with 10 mM methylpyruvate (Sigma, St. Louis, MO.) for 48 hours before the invasion assay. VEGF secretion was measured by using an ELISA kit following the manufacturer’s instructions (DY293B, R&D Systems Inc, Minneapolis, MN). Western blotting was performed as described using antibodies targeting HIF1A (ab16066, Abcam, Minneapolis, MN), LDH5 (ab1015, Abcam) and GAPDH (MAB374, Millipore, Billerica, MA).
Immunofluorescence
Groups of 6 nu/nu mice were inoculated subcutaneously with 1 million C8161.9 human melanoma cells expressing either control luc or anti-PHIP shRNA. On day 25, the mice were euthanized and the tumors were assessed for microvessel density based on CD31 positivity by immunofluorescence, which was performed using a rabbit anti-CD31 antibody (ab28364, Abcam) and a secondary antibody labeled with Alexa Fluor 594 (Life Technologies) as described previously (Jalas et al. 2011). Mosaic images were acquired with 20X magnification at a fixed exposure with a Zeiss Axio Imager Z2 microscope controlled by AxioVision software. After automatically stitching the images, the number of CD31 positive objects and area were determined using ImageJ software. The number of CD31 positive vessels was quantitated and the microvessel density reported as the total CD31 count over the entire area of the tumor.
Statistical Analysis
Statistical methods used to assess the significance of various prognostic factors on melanoma outcome were previously described (Rangel et al. 2006; Kashani-Sabet et al. 2009). The coding for clinical or pathological attributes was performed as described by the AJCC staging committee for melanoma (Balch et al. 2001b). The correlation between PHIP copy number and immunohistochemical score was tested by regression analysis using Data Desk software. For both DMFS and DSS, the definition of high PHIP FISH scores, as measured by percentage of cells harboring 3 or more copies of PHIP, was established as equal to or greater than 16%. A 16% cutoff served to maximize the average of the sensitivity and specificity in the prediction of both DMFS and DSS, analyzed separately. The same 16% cutoff was then uniformly and consistently used in all subsequent analyses of DMFS and DSS. The association between high PHIP copy number and DMFS or DSS was assessed using both Kaplan-Meier analysis and multivariate Cox regression. The association between high PHIP copy number and presence or absence of ulceration was assessed using both Fisher’s exact test and multivariate logistic regression analysis. The association between a high degree of tumor vascularity and ulceration was also assessed using both Fisher’s exact test and multivariate logistic regression. Assessment of the differential impacts of neither, either, and both of these two factors was done using both Chi-square analysis and Fisher’s exact test. In predicting ulceration status, high PHIP copy number was uniformly defined as greater than or equal to 35% of tumor cells harboring 3 or more copies of the PHIP gene. This cutoff was similarly selected as the one that maximized the average of sensitivity and specificity in predicting ulceration. All P values reported are two-sided.
Supplementary Material
Supplementary Figure 1. Dot plot presenting the significant correlation between the immunohistochemical score and the percentage of cells with three or more copies of the PHIP locus in primary melanoma (P < 0.0001, linear regression analysis).
Supplementary Table 1. FISH results as counts of signals from BAC clones mapping the PHIP locus and the flanking centromeric region of chromosome 6
Acknowledgments
This work was supported by United States Public Health Service Grants CA114337 and CA122947 (to MKS), and by the Kay Charitable Trust. JEC is supported by the E.A. Dickson Emeritus Professorship and the Cancer Research Coordinating Committee of the University of California.
Abbreviations
- PHIP
pleckstrin homology domain-interacting protein
- FISH
fluorescence in situ hybridization
- DMFS
distant metastasis-free survival
- DSS
disease-specific survival
- LDH5
Lactate dehydrogenase 5
- VEGF
vascular endothelial growth factor
- HIF1A
hypoxia inducible factor 1 alpha subunit
- TMA
tissue microarray
- shRNA
short hairpin RNA
- CD31
cluster of differentiation 31
- BAC
bacterial artificial chromosome
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- IGF1R
insulin-like growth factor 1 receptor
- RAC
RAS-related C3 botulinum toxin substrate
- TIE
receptor tyrosine kinase epithelial-specific
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
Footnotes
Conflict of Interest Mohammed Kashani-Sabet owns stock in Melanoma Diagnostics, Inc. Intellectual property covering the marker described in this study was licensed to Myriad Genetics Laboratories.
References
- Bagheri S, Nosrati M, Li S, et al. Genes and pathways downstream of telomerase in melanoma metastasis. Proc Natl Acad Sci USA. 2006;103:11306–11. doi: 10.1073/pnas.0510085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001a;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001b;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH. Control of Autofluorescence of Archival Formaldehyde-fixed, Paraffin-embedded Tissue in Confocal Laser Scanning Microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–71. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- Bayani J, Squire JA. Fluorescence in situ Hybridization (FISH) Curr Protoc Cell Biol. 2004 doi: 10.1002/0471143030.cb2204s23. Chapter 22: Unit 22.4. [DOI] [PubMed] [Google Scholar]
- Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435–42. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin S-F, Daigo Y, Huang H-E, et al. A simple and reliable pretreatment protocol facilitates fluorescent in situ hybridisation on tissue microarrays of paraffin wax embedded tumour samples. MP, Mol Pathol. 2003;56:275–9. doi: 10.1136/mp.56.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C, Mitra A, Jewell R, et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009;15:6939–46. doi: 10.1158/1078-0432.CCR-09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont AMM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–25. doi: 10.1016/j.ejca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Falk MS, Truitt AK, Coakley FV, et al. Interpretation, accuracy and management implications of FDG PET/CT in cutaneous malignant melanoma. Nucl Med Commun. 2007;28:273–80. doi: 10.1097/MNM.0b013e3280708ecf. [DOI] [PubMed] [Google Scholar]
- Felcht M, Luck R, Schering A, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogas H, Eggermont AMM, Hauschild A, et al. Biomarkers in melanoma. Ann Oncol. 2009;20(Suppl 6):vi8–13. doi: 10.1093/annonc/mdp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Zhang Y, et al. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci USA. 1997;94:6210–5. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Patton RS, Cheng E, et al. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem. 2002;277:14821–8. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA. 2005;102:6092–7. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalas JR, Vemula S, Bezrookove V, et al. Metastatic melanoma with striking adenocarcinomatous differentiation illustrating phenotypic plasticity in melanoma. Am J Surg Pathol. 2011;35:1413–8. doi: 10.1097/PAS.0b013e31822280d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani-Sabet M, Sagebiel RW, Ferreira CMM, et al. Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–31. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- Kashani-Sabet M, Venna S, Nosrati M, et al. A multimarker prognostic assay for primary cutaneous melanoma. Clin Cancer Res. 2009;15:6987–92. doi: 10.1158/1078-0432.CCR-09-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–85. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay AJ, Jiang XM, Kisker O, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–73. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- McArthur GA, Puzanov I, Amaravadi R, et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol. 2012;30:1628–34. doi: 10.1200/JCO.2011.39.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMasters KM, Edwards MJ, Ross MI, et al. Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Ann Surg. 2010;252:460–465. doi: 10.1097/SLA.0b013e3181f20bb1. discussion 465–466. [DOI] [PubMed] [Google Scholar]
- Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–50. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- Rangel J, Torabian S, Shaikh L, et al. Prognostic significance of nuclear receptor coactivator-3 overexpression in primary cutaneous melanoma. J Clin Oncol. 2006;24:4565–9. doi: 10.1200/JCO.2006.07.3833. [DOI] [PubMed] [Google Scholar]
- Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–87. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- De Semir D, Nosrati M, Bezrookove V, et al. Pleckstrin homology domain-interacting protein (PHIP) as a marker and mediator of melanoma metastasis. Proc Natl Acad Sci USA. 2012;109:7067–72. doi: 10.1073/pnas.1119949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Soong S-J, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29:2199–205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Yanagawa T, Shimura T, et al. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10:7775–84. doi: 10.1158/1078-0432.CCR-04-1015. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wiegant J, Raap AK. Probe labeling and fluorescence in situ hybridization. Curr Protoc Cytom. 2001 doi: 10.1002/0471142956.cy0803s00. Chapter 8: Unit 8.3. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–82. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Scolyer RA, Murali R, et al. Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod Pathol. 2010;23:45–53. doi: 10.1038/modpathol.2009.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dot plot presenting the significant correlation between the immunohistochemical score and the percentage of cells with three or more copies of the PHIP locus in primary melanoma (P < 0.0001, linear regression analysis).
Supplementary Table 1. FISH results as counts of signals from BAC clones mapping the PHIP locus and the flanking centromeric region of chromosome 6