Abstract
Objective:
To report outcomes of pregnancies that occurred during the fingolimod clinical development program.
Methods:
Pregnancy outcomes from phase II, phase III, and phase IV clinical studies (with optional extensions) were reported by clinical trial investigators. Fingolimod exposure in utero was defined as fingolimod treatment at the time of conception or in the 6 weeks before conception.
Results:
As of October 31, 2011, 89 pregnancies were reported in completed or ongoing clinical studies, with 74 in fingolimod treatment arms. Of 66 pregnancies with in utero exposure to fingolimod, there were 28 live births, 9 spontaneous abortions, 24 elective abortions, 4 ongoing pregnancies, and 1 pregnancy with an unknown outcome (patient lost to follow-up). Two infants were born with malformations: 1 with congenital unilateral posteromedial bowing of the tibia and 1 with acrania. Elective abortions were performed for 1 case each of tetralogy of Fallot, spontaneous intrauterine death, and failure of fetal development. There were 5 cases (7.6%; 95% confidence interval 3%–17%) of abnormal fetal development in the 66 pregnancies that had in utero exposure to fingolimod. In all 5 cases, fetal exposure to the drug took place in the first trimester of pregnancy.
Conclusions:
The number of patients becoming pregnant during fingolimod therapy remains small and does not permit firm conclusions to be drawn about fetal safety of fingolimod in humans. Given the known risks of teratogenicity in animals and the present data, women of childbearing potential should use effective contraception during fingolimod therapy and for 2 months after discontinuation.
Multiple sclerosis (MS) is predominant among women of reproductive age, in whom approximately 50% of all pregnancies are unplanned.1 Fingolimod (FTY720; Gilenya, Novartis Pharma AG, Basel, Switzerland) is a sphingosine 1-phosphate receptor modulator that has been approved as a once-daily oral therapy for relapsing MS. Two phase II studies, 3 large-scale phase III studies, and their ongoing extension studies have indicated that fingolimod has a manageable safety profile and is generally well tolerated at the approved dose of 0.5 mg.2–6 As preclinical studies indicate a risk of fetal toxicity,7 fingolimod clinical study protocols require a negative pregnancy test at enrollment and that women of childbearing potential use reliable contraception throughout the studies. We report the outcomes of pregnancies that occurred in the fingolimod clinical development program despite these measures.
METHODS
Standard protocol approvals, registrations, and patient consents.
Pregnancy outcomes are reported from 9 clinical studies in patients with relapsing MS: the phase III FREEDOMS study3 (ClinicalTrials.gov number, NCT00289978 [core study] and NCT00662649 [extension]); phase III FREEDOMS II study (ClinicalTrials.gov number, NCT00355134 [core study] and NCT00774670 [extension]); phase III TRANSFORMS study with optional extension4,8 (ClinicalTrials.gov number, NCT00340834); phase II global study2,5 (ClinicalTrials.gov number, NCT00333138 [core study] and NCT00235430 [extension]); phase III open-label safety and tolerability study (ClinicalTrials.gov number, NCT01127750); phase II study in Japanese patients6 (ClinicalTrials.gov number, NCT00537082 [core study] and NCT00670449 [extension]); phase III umbrella extension study (ClinicalTrials.gov number, NCT01201356 and NCT01281657); and 2 phase IV local studies (study FTY720DFR01 and ClinicalTrials.gov number, NCT01216072). Four of these studies were placebo controlled (FREEDOMS, FREEDOMS II, and the 2 phase II studies), and 1 used an active comparator (IM interferon β-1a; TRANSFORMS). All studies had optional extensions during which all patients received fingolimod. All studies adhered to the International Conference on Harmonisation Guidelines for Good Clinical Practice and were conducted in accordance with the Declaration of Helsinki.9,10 All study participants provided written informed consent. All study protocols were approved by the independent ethics committee or institutional review board for each study site.
Study designs and analysis of pregnancy outcomes.
An inclusion criterion for entry into all clinical studies of fingolimod in MS was that female patients of childbearing potential must have had a negative serum pregnancy test prior to entry and were required to use simultaneously 2 forms of effective contraception (either partner), unless surgically sterile, during treatment and for 3 months after discontinuation of study medication. Investigators were required to report information on pregnancies that occurred in the studies despite these protocol requirements and on their outcomes. Because fingolimod has a long elimination half-life (6–9 days), fingolimod exposure in utero was defined as fingolimod treatment at the estimated time of conception or within 6 weeks prior to conception. Duration of in utero exposure was calculated as the number of days from conception until fingolimod was expected to be no longer in circulation (6 weeks) or the pregnancy terminated because of a spontaneous or elective abortion (i.e., date of last fingolimod dose + 6 weeks or date of abortion [whichever was earlier] − date of conception + 1 day). The date of conception was estimated as 2 weeks after the last menstrual period. Confidence intervals (CIs) for the proportions of pregnancies that resulted in each outcome (live births, spontaneous abortions, elective abortions) were calculated using the Clopper–Pearson exact interval method.
RESULTS
As of October 31, 2011, 89 pregnancies were reported in the fingolimod clinical development program. Outcomes are summarized in table 1. Of the 11 pregnancies that occurred in patients who had been receiving placebo, 1 resulted in the birth of a healthy newborn, 1 resulted in a spontaneous abortion, and 9 were electively terminated. Of the 4 pregnancies that occurred in patients who had been receiving interferon β-1a, 2 resulted in the birth of a healthy newborn and 2 were electively terminated. Of the 74 pregnancies that occurred in patients in the fingolimod treatment arms, fingolimod was discontinued at least 6 weeks before the assumed date of conception in 8 patients; of these 8 pregnancies with no in utero fingolimod exposure, 7 resulted in the birth of healthy babies with no congenital abnormalities and 1 was electively terminated. The durations of fingolimod exposure in utero for the remaining 66 pregnancies are shown in the figure. In the 74 women in the fingolimod treatment arms who became pregnant, all prior pregnancies (if any) had no fingolimod exposure.
Table 1.
Pregnancy outcomes in the fingolimod clinical development program (as of October 31, 2011)
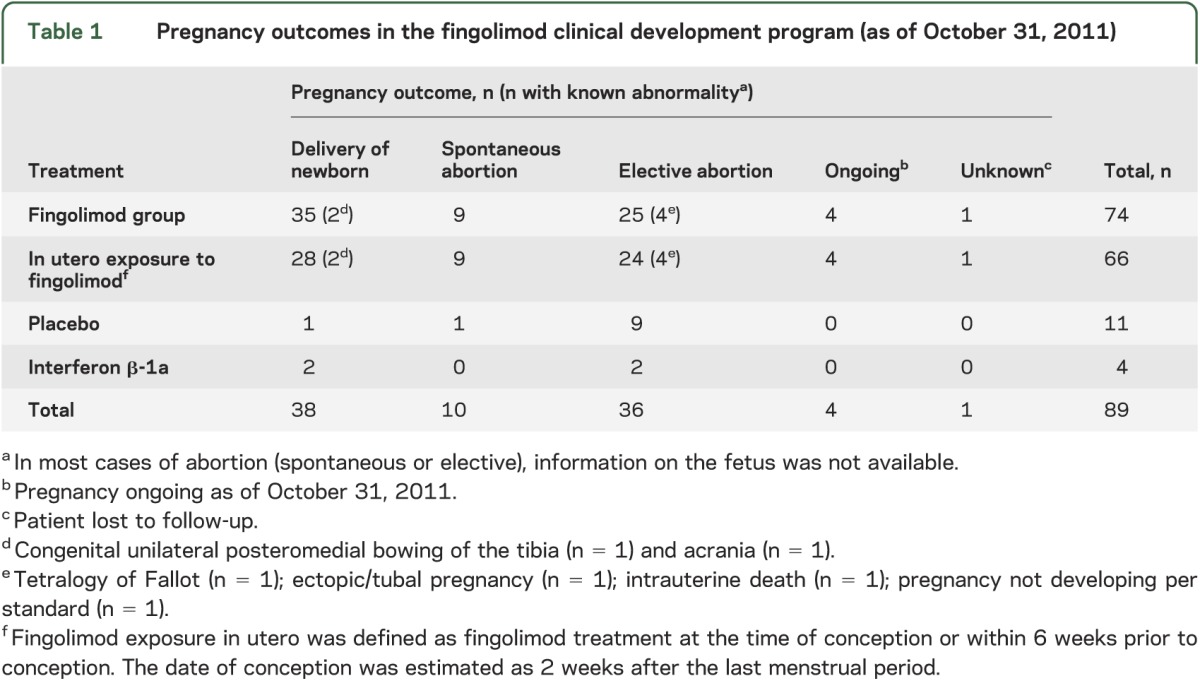
Figure. Pregnancy outcomes in relationship to estimated duration of in utero exposure to fingolimod.
a Pregnancy ongoing as of October 31, 2011. b Patient lost to follow-up. c Fingolimod exposure in utero was defined as fingolimod treatment at the time of conception or within 6 weeks prior to conception. In utero exposure was calculated as date of last fingolimod dose + 6 weeks or date of abortion (whichever was earlier) − date of conception + 1 day. The date of conception was estimated as 2 weeks after the last menstrual period. d All percentages are calculated as n/66.
The total fingolimod exposure for women up to 50 years of age was approximately 7,702 patient-years, with 25% (n = 1,116/4,444) of patients treated for 3 or more years. In contrast, exposure to placebo and interferon β-1a was approximately 733 (n = 437) and 210 (n = 211) patient-years, respectively.
In the 66 pregnancies in which in utero exposure to fingolimod occurred, there were 28 reported live births (42%; 95% CI 30%–55%), 9 spontaneous abortions (14%; 95% CI 6%–24%), 24 elective abortions (36%; 95% CI 25%–49%), 4 pregnancies that were ongoing (6%; 95% CI 1.7%–15%) at the time of this report, and 1 pregnancy (1.5%; 95% CI 0.04%–8%) with an unknown outcome (patient lost to follow-up). The duration of in utero fingolimod exposure in the majority of live births was estimated to be >8 weeks to ≤12 weeks (figure). The estimated duration of in utero fingolimod exposure in the majority of elective abortions was >4 weeks to ≤8 weeks, and in the majority of spontaneous abortions was >4 weeks to ≤12 weeks (figure). There were 5 pregnancies with >12 weeks of in utero exposure to fingolimod; 1 was still ongoing and 4 resulted in the birth of healthy babies with no congenital abnormalities. In most cases of abortion (elective or spontaneous), information on the fetus was not available. Follow-up data beyond birth are not available.
Overall, 37 pregnancies (excluding ongoing pregnancies, an unknown outcome [patient lost to follow-up], and the 24 elective abortions) had a known outcome. One of the elective abortions also had a known congenital abnormality of tetralogy of Fallot. Of the 37 pregnancies, 9 (24%; 95% CI 12%–41%) resulted in spontaneous abortion, 26 (70%; 95% CI 53%–84%) in delivery of healthy babies with no congenital abnormalities, and 2 (5%; 95% CI 0.7%–18%) in delivery of newborns with a congenital abnormality; the 2 congenital abnormalities that occurred were 1 female baby with congenital unilateral (right leg) posteromedial bowing of the tibia and 1 baby with acrania (absence of the cranium). The newborn with congenital unilateral posteromedial bowing of the tibia was born prematurely in the 35th week and was otherwise healthy. The mother had received fingolimod 0.5 mg for approximately 7 months before becoming pregnant. Fingolimod was discontinued 17 days after conception, yielding an estimated in utero exposure of 8.6 weeks (table 2). The case of acrania was detected in utero by ultrasound in a woman who had received fingolimod 0.5 mg for >3 years prior to becoming pregnant. Fingolimod was discontinued 3 weeks after conception, yielding an estimated in utero exposure of 9.0 weeks (table 2). The newborn was delivered prematurely by caesarean section at 7 months and died 2 days later. The patient's obstetric history included 3 healthy births, 1 ectopic pregnancy (1997), and 1 spontaneous abortion (2006). No other risk factors (including smoking, alcohol consumption, drug/substance abuse, environmental/occupational risks, infection, hypertension, diabetes, familial disorders) were identified that could have affected the outcome of the pregnancy.
Table 2.
Estimated duration of in utero exposure to fingolimod in pregnancies with a known abnormality
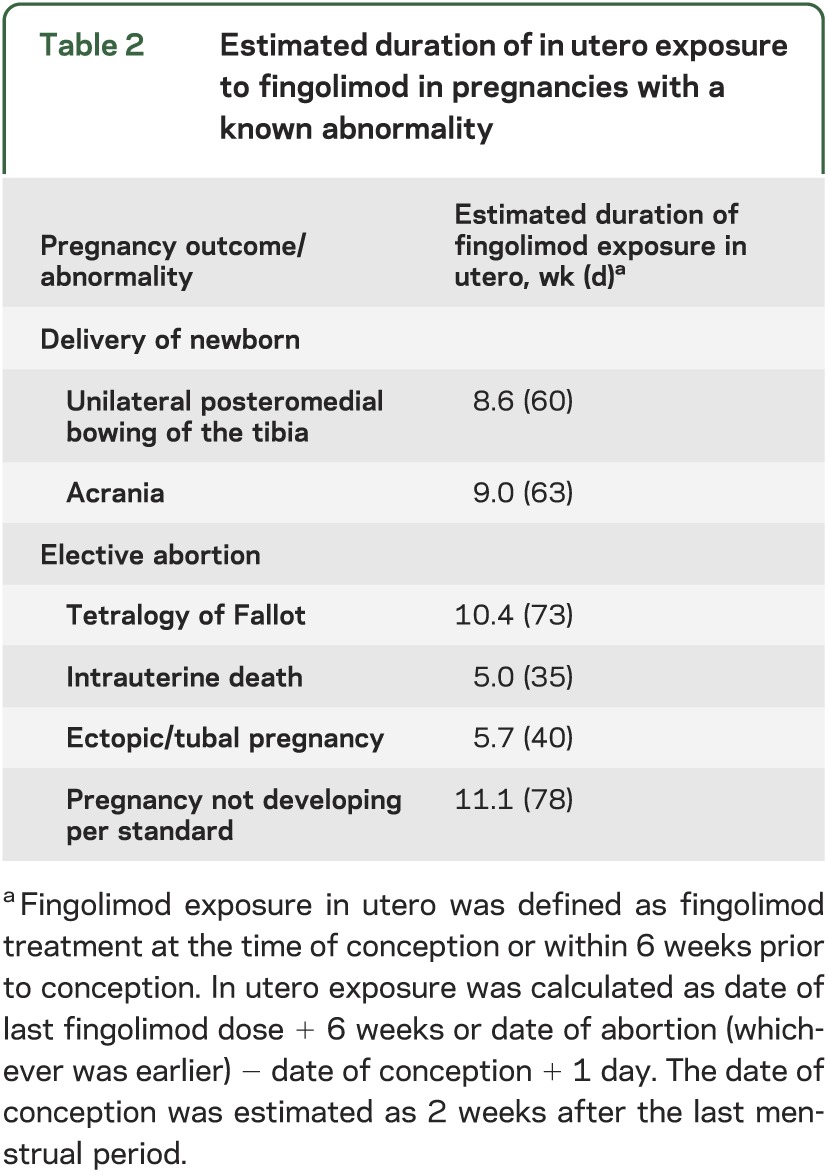
The majority of elective abortions were reported to be due to the patient's personal choice. In 4 cases, the abortion was reported to be due to an abnormality of the fetus or of the pregnancy: tetralogy of Fallot (n = 1, see above), ectopic/tubal pregnancy (n = 1), intrauterine death (n = 1), and pregnancy not developing per standard (stopped in evolution; n = 1). The duration of in utero exposure to fingolimod in these 4 elective abortions ranged from 5 to 11 weeks (table 2). The case of tetralogy of Fallot was reported in a patient who had been receiving fingolimod 1.25 mg for approximately 9 months before becoming pregnant. In the 2 months prior to detection of the pregnancy, the patient received the following concomitant medications: imacillin (for 10 days), swine flu inoculation, and duroferon (for approximately 6 weeks). The patient's obstetric history included 2 pregnancies resulting in healthy babies and 1 elective abortion. Fingolimod was discontinued approximately 1 month after conception, leading to an estimated 10.4 weeks of in utero exposure (table 2). Ultrasound at 18 weeks revealed tetralogy of Fallot (partial ventricular septal defect, overriding aorta, slight right ventricular hypertrophy, pulmonary artery stenosis) and an elective abortion was performed at week 19. Chromosomal tests of the fetus for trisomy 21 and 22q11 deletion were negative.
DISCUSSION
There is no evidence that MS increases the risk of spontaneous abortion or congenital abnormalities.11 Given that the incidence of MS peaks at about 30 years of age and is greater in women than in men,12 pregnancy issues are highly relevant and concern is raised regarding the potential impact of treatments for MS in the mother on fetal outcomes. Based on preclinical data, fingolimod has been found to have a teratogenic effect (persistent truncus arteriosus, ventricular septal defect) in rats, while studies in rabbits found excess fetal loss that precluded assessment of teratogenicity.7 In rats, the highest no-effect dose was less than the recommended human dose of 0.5 mg/day on a body surface area (mg/m2) basis; in rabbits, the no-effect dose was approximately 20 times the recommended human dose on an mg/m2 basis.13 Furthermore, the receptor family modulated by fingolimod (sphingosine 1-phosphate receptor), particularly S1P1, is known to be involved in vascular formation during embryogenesis.14,15 For these reasons, strict contraception was mandated during exposure to fingolimod in female patients participating in the clinical trial program and continues to be mandatory in the post-approval setting.7,13,16 However, a number of pregnancies did occur in female patients receiving fingolimod despite these precautions. A formal drug–drug interaction study of fingolimod and oral contraceptive medication did not show evidence of any interaction that could have resulted in less effective contraception.17 Therefore, the occurrence of pregnancies likely reflects failure to observe preventive measures rigorously. A total of 89 pregnancies were reported (74 fingolimod, 11 placebo, and 4 interferon β-1a). The total fingolimod exposure for women up to 50 years of age was 10-fold that of placebo involving 3-fold as many patients.
Of the 66 pregnancies in which in utero exposure to fingolimod occurred, 24 were electively terminated, 1 was lost to follow-up, and 4 were ongoing, leaving 37 pregnancies. Of these, three-quarters resulted in live births and 9 (24%; 95% CI 12%–41%) resulted in spontaneous abortion. In the general population, spontaneous abortion is reported to occur in 15%–20% of known pregnancies,18 and major birth defects to occur in 4%–8% of pregnancies.19 The rate of spontaneous abortion with fingolimod may slightly exceed expected rates, but the number of cases is relatively small and CIs overlap expected rates; our estimate is also calculated conservatively, given that we include only the 37 pregnancies with potential to go to term (i.e., those that resulted in live births or spontaneous abortions) in the denominator.
Of the 2 cases of malformation that occurred among the live births, 1 infant was born with congenital unilateral posteromedial bowing of the tibia following in utero exposure to fingolimod. The developmental etiology of this disorder is unknown, but most authors believe the occurrence is secondary to mechanical factors including abnormal fetal positioning.20–22 It is, therefore, considered unlikely that this localized lesion resulted from exposure to fingolimod during embryogenesis. The typical natural history of the bowing is spontaneous resolution, especially during the first 6 months, but some patients require surgical intervention to correct residual bowing, limb-length inequality, or ankle deformity.21,23 The other malformation among the live births was a case of acrania, a rare (∼1/10,000 births in the general population) neural tube defect with various etiologies (including chromosomal abnormalities, single gene mutations, and maternal exposure to teratogens).24,25
In addition, 3 of the elective abortions were carried out because of fetal developmental abnormality: 1 major congenital malformation (tetralogy of Fallot; ∼1/2,500 births in the general population), 1 spontaneous intrauterine death (cause unknown), and 1 failure of fetal development (further information not available). The majority of other elective abortions resulted from patients' personal reasons and occurred during the first trimester; information on the fetus was unknown in most cases.
Pooling fetal abnormalities from those with elective abortions and those with live births, there were 5 (7.6%; 95% CI 3%–17%) cases of abnormal fetal development in the 66 pregnancies that had in utero exposure to fingolimod. In all 5 cases, fetal exposure to the drug took place in the first trimester of pregnancy.
One of these abnormalities, acrania, is particularly rare, and the relationship to drug must be considered as possible even though the case was isolated. It has been hypothesized that neural tube defects, of which acrania is a severe form, may have a vascular basis.26 In animal studies, neural tube closure was severely disturbed in mouse embryos that lacked either S1P1 or the 2 enzymes that are required to convert sphingosine to sphingosine 1-phosphate.27
The number of patients in the fingolimod treatment arms who became pregnant after discontinuing fingolimod was small (n = 8), thus precluding any meaningful comparison being made between this group and those who became pregnant while exposed to fingolimod.
Previous investigations of pregnancy outcomes following in utero exposure to MS disease-modifying drugs generally report no increase in the rates of spontaneous abortions or congenital malformations. In a recent study, 425 pregnancies had in utero exposure to interferon β with 324 healthy births, 5 congenital abnormalities (4 live births, 1 stillbirth), and 49 spontaneous abortions.28 A similar pattern was observed in an earlier study in which 41 pregnancies were exposed in utero to interferon β, resulting in 20 healthy offspring, 1 congenital anomaly (hydrocephalus), 1 fetal death, and 8 spontaneous abortions.29 Two further studies in which in utero exposure to interferon β occurred (n = 88, n = 69) reported no significant drug-related congenital defects.30,31 In contrast, 1 small study of 23 pregnancies in patients treated with interferon β reported a higher rate (39.1%) of spontaneous abortions and stillbirths in those with in utero exposure than in healthy controls (5%).32 Two investigations into the implications of exposure to glatiramer acetate during pregnancy (n = 11, n = 9) reported no drug-related pregnancy complications,33,34 whereas 1 (n = 31) reported 2 malformations (club feet and atrioventricular canal).31 A recent study of 35 pregnancies in which in utero exposure to natalizumab occurred reported 28 healthy infants, 1 case of hexadactyly, and 5 spontaneous abortions.35
All labels worldwide for fingolimod state that women of childbearing potential should be advised to take effective contraceptive measures during fingolimod therapy and, given the half-life of the compound, for at least 2 months after stopping therapy.7,13,16 Fingolimod is included in the US Food and Drug Administration's pregnancy category C (definition: animal reproduction studies have shown an adverse effect on the fetus, there are no adequate and well-controlled studies in pregnant women, and the benefits of treatment in pregnant women may be acceptable despite its potential risks).13 A multinational pregnancy exposure registry has been set up, which is a prospective observational study aimed at enrolling pregnant women exposed to fingolimod worldwide in order to collect prospective data on pregnancy outcomes (for further information or enrollment in the registry, contact gpr@outcome.com; +1-877-598-7237 [toll-free, North America]; +800-688-266-37 [toll-free outside North America]).
As the number of patients becoming pregnant during fingolimod therapy remains low, firm conclusions about the safety of fingolimod during pregnancy are not possible. However, the 5 cases of abnormal fetal development seen among 66 pregnancies with in utero fingolimod exposure, together with preclinical data showing teratogenicity, indicate a potential risk of treatment-related developmental abnormalities. It is therefore strongly recommended to patients and physicians that pregnancy be avoided by effective contraception during treatment with fingolimod in female patients of childbearing potential. Furthermore, the occurrence of more than 80 pregnancies in the fingolimod clinical development program despite the stipulation of double contraceptive measures highlights an educational need for both physicians and patients. A fingolimod pregnancy registry has been established to record data on pregnancy outcomes in women exposed to fingolimod should they inadvertently become pregnant.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients who participated in the study, the study site personnel, and Oxford PharmaGenesis Ltd. (funded by Novartis Pharma AG) for facilitating author discussion, collating author comments, and formatting the manuscript according to journal requirements.
GLOSSARY
- CI
confidence interval
- MS
multiple sclerosis
Footnotes
Editorial, page 654
AUTHOR CONTRIBUTIONS
All authors were involved in analysis and interpretation of the data and drafting and revising the manuscript for content. G. Karlsson, G. Francis, G. Koren, W. Collins, and J.A. Cohen were involved in study design and concept. G. Karlsson, X. Zhang, J.A. Cohen, and W. Collins were involved in acquisition of data. G. Karlsson and G. Francis were involved in statistical analysis. G. Francis, X. Zhang, and J.A. Cohen were involved in study supervision or coordination.
STUDY FUNDING
Supported by Novartis Pharma AG, Basel, Switzerland.
DISCLOSURE
G. Karlsson is an employee of Novartis. G. Francis is an employee of Novartis. G. Koren received consulting fees from Duchesnay, Novartis, and Bayer. P. Heining is an employee of Novartis. X. Zhang is an employee of Novartis. J. Cohen has received reimbursement of travel costs and fees for participation in study steering committees from Novartis. He has received consultancy fees from Biogen Idec, Elan, Five Prime Therapeutics, Eli Lilly, Novartis, Teva, and Vaccinex. J.A. Cohen has also received payment for speaker services from Novartis and Biogen Idec. L. Kappos has received research support to his institution for board membership, consultancy or speaking, or grants, in the last 3 years, from Actelion, Advancell, Allozyne, Bayer, Bayhill, Biogen Idec, BioMarin, CSL Behring, Eli Lilly, European Union, Genmab, GeNeuro, Gianni Rubatto Foundation, Glenmark, Merck Serono, MediciNova, Mitsubishi Pharma, Novartis, Novartis Research Foundation, Novo Nordisk, Peptimmune, Roche, Roche Research Foundation, Santhera, Sanofi Aventis, Swiss MS Society, Swiss National Research Foundation, Teva, UCB, and Wyeth. W. Collins is an employee of Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006;38:90–96 [DOI] [PubMed] [Google Scholar]
- 2.Comi G, O'Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010;16:197–207 [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401 [DOI] [PubMed] [Google Scholar]
- 4.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415 [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124–1140 [DOI] [PubMed] [Google Scholar]
- 6.Saida T, Kikuchi S, Itoyama Y, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult Scler 2012;18:1269–1277 [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency Gilenya: EPAR: product information (updated July 2012). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed January 25, 2013
- 8.Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011;10:520–529 [DOI] [PubMed] [Google Scholar]
- 9.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed January 25, 2013
- 10.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf
- 11.Mueller BA, Zhang J, Critchlow CW. Birth outcomes and need for hospitalization after delivery among women with multiple sclerosis. Am J Obstet Gynecol 2002;186:446–452 [DOI] [PubMed] [Google Scholar]
- 12.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532 [DOI] [PubMed] [Google Scholar]
- 13.Novartis Pharmaceuticals Corporation Gilenya US prescribing information (revised May 2012). Available at: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf. Accessed January 25, 2013
- 14.Brinkmann V. Sphingosine. 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 2007;115:84–105 [DOI] [PubMed] [Google Scholar]
- 15.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 2009;78:743–768 [DOI] [PubMed] [Google Scholar]
- 16.Therapeutic Goods Administration Australian public assessment report: fingolimod (March 30, 2011). Available at: http://www.tga.gov.au/auspar/auspar-fingolimod-110329-gilenya.htm. Accessed January 25, 2013
- 17.Collins W, Francis G, Koren G, et al. Lack of interaction between fingolimod (FTY720) and oral contraceptives, and pregnancy experience in the clinical program of fingolimod in multiple sclerosis. Presented at the American Academy of Neurology 63rd Annual Meeting; April 9–16, 2011; Honolulu, Hawaii
- 18.Vorvick LJ. MedlinePlus: miscarriage [online]. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/001488.htm. Accessed January 25, 2013
- 19.Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998). Arch Gynecol Obstet 2002;266:163–167 [DOI] [PubMed] [Google Scholar]
- 20.De Maio F, Corsi A, Roggini M, Riminucci M, Bianco P, Ippolito E. Congenital unilateral posteromedial bowing of the tibia and fibula: insights regarding pathogenesis from prenatal pathology: a case report. J Bone Joint Surg Am 2005;87:1601–1605 [DOI] [PubMed] [Google Scholar]
- 21.Johnston CE. Disorders of the leg. In: Herring JA, ed. Tachdjian's Pediatric Orthopaedics, 4th ed Philadelphia: Elsevier; 2007 [Google Scholar]
- 22.Kim HW, Park HW. The pediatric leg and knee. In: Weinstein SL, Buckwalter JA, eds. Turek's Orthopaedics: Principles and Their Application, 6th ed Philadelphia: Lippincott Williams & Wilkins; 2005:575–588 [Google Scholar]
- 23.Shah HH, Doddabasappa SN, Joseph B. Congenital posteromedial bowing of the tibia: a retrospective analysis of growth abnormalities in the leg. J Pediatr Orthop B 2009;18:120–128 [DOI] [PubMed] [Google Scholar]
- 24.Drugan A, Weissman A, Evans MI. Screening for neural tube defects. Clin Perinatol 2001;28:279–287, vii [DOI] [PubMed] [Google Scholar]
- 25.Gorgal R, Ramalho C, Brandao O, Matias A, Montenegro N. Revisiting acrania: same phenotype, different aetiologies. Fetal Diagn Ther 2011;29:166–170 [DOI] [PubMed] [Google Scholar]
- 26.Stevenson RE, Kelly JC, Aylsworth AS, Phelan MC. Vascular basis for neural tube defects: a hypothesis. Pediatrics 1987;80:102–106 [PubMed] [Google Scholar]
- 27.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate: modifiers of lymphocyte migration. N Engl J Med 2006;355:1088–1091 [DOI] [PubMed] [Google Scholar]
- 28.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler 2011;17:423–430 [DOI] [PubMed] [Google Scholar]
- 29.Sandberg-Wollheim M, Frank D, Goodwin TM, et al. Pregnancy outcomes during treatment with interferon beta-1a in patients with multiple sclerosis. Neurology 2005;65:802–806 [DOI] [PubMed] [Google Scholar]
- 30.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology 2010;75:1794–1802 [DOI] [PubMed] [Google Scholar]
- 31.Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler 2009;15:1037–1042 [DOI] [PubMed] [Google Scholar]
- 32.Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G. The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology 2005;65:807–811 [DOI] [PubMed] [Google Scholar]
- 33.Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs 2010;24:969–976 [DOI] [PubMed] [Google Scholar]
- 34.Salminen HJ, Leggett H, Boggild M. Glatiramer acetate exposure in pregnancy: preliminary safety and birth outcomes. J Neurol 2010;257:2020–2023 [DOI] [PubMed] [Google Scholar]
- 35.Hellwig K, Haghikia A, Gold R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult Scler 2011;17:958–963 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.