Abstract
Objective:
To describe a case series of adult patients in the intensive care unit in super-refractory status epilepticus (SRSE; refractory status lasting 24 hours or more despite appropriate anesthetic treatment) who received treatment with the ketogenic diet (KD).
Methods:
We performed a retrospective case review at 4 medical centers of adult patients with SRSE treated with the KD. Data collected included demographic features, clinical presentation, diagnosis, EEG data, anticonvulsant treatment, and timing and duration of the KD. Primary outcome measures were resolution of status epilepticus (SE) after initiation of KD and ability to wean from anesthetic agents.
Results:
Ten adult patients at 4 medical centers were started on the KD for SRSE. The median age was 33 years (interquartile range [IQR] 21), 4 patients (40%) were male, and 7 (70%) had encephalitis. The median duration of SE before initiation of KD was 21.5 days (IQR 28) and the median number of antiepileptic medications used before initiation of KD was 7 (IQR 7). Ninety percent of patients achieved ketosis, and SE ceased in all patients achieving ketosis in a median of 3 days (IQR 8). Three patients had minor complications of the KD including transient acidosis and hypertriglyceridemia and 2 patients ultimately died of causes unrelated to the KD.
Conclusion:
We describe treatment of critically ill adult patients with SRSE with the KD, with 90% of patients achieving resolution of SE. Prospective trials are warranted to examine the efficacy of the KD in adults with refractory SE.
Classification of evidence:
This study provides Class IV evidence that for intensive care unit patients with refractory SE, a KD leads to resolution of the SE.
Super-refractory status epilepticus (SRSE) is defined as status epilepticus (SE) that continues for at least 24 hours after initiation of general anesthetic medications, including cases in which the SE recurs with reduction or withdrawal of anesthesia.1 SRSE holds a high risk of morbidity and mortality and has been associated with conditions such as encephalitis, anoxic brain injury, and intracranial hemorrhage.2
The ketogenic diet (KD) and modified Atkins diet (MAD) are high fat, low carbohydrate, adequate protein diets designed to mimic the fasting state that have been proven as effective dietary therapies for some children with epilepsy.3,4 Small open-label studies suggest efficacy in adults as well.5 Despite their established success in children with refractory epilepsy, only a few case series from single centers describe the use of KD and MAD in adults with refractory SE (RSE) and SRSE.6–11 Given the severity of illness in patients with SRSE, there is a critical need for new therapies to halt ongoing seizure activity. We investigated KD as a treatment for critically ill adults with SRSE treated in intensive care units (ICUs).
METHODS
Standard protocol approvals, registrations, and patient consents.
The Johns Hopkins University, Mayo Clinic, Rochester, and Rush University Medical Center Institutional Review Boards approved this study.
Population.
A retrospective review of patients older than 17 years in SRSE who were treated with KD at 4 medical centers between 2010 and 2013 (Johns Hopkins Hospital; Johns Hopkins Bayview Medical Center; Mayo Clinic, Rochester; and Rush University Medical Center) was performed. SRSE was defined as SE (convulsive and/or nonconvulsive) that continued 24 hours or more after the initiation of general anesthetic medications (i.e., propofol, high-dose barbiturates, high-dose benzodiazepines, and ketamine), or recurred after wean or discontinuation of general anesthetics.
Clinical data.
Data collected included demographics, clinical diagnosis, history of seizures or SE, total hospital length of stay (LOS), ICU LOS, and Glasgow Coma Scale (GCS) score on admission and at KD initiation. EEG data collected on KD initiation, on KD wean, and at discharge were interpreted by a board-certified electroencephalographer (M.C.C., P.W.K.). Duration of SE before initiation of diet and number of antiepileptic drugs (AEDs) used before KD initiation were analyzed. Timing of KD with respect to seizure onset, KD duration, presence of serum and urine ketones, and KD side effects were also collected.
Outcome variables.
The ability to wean patients from anesthetic agents within 1 and 2 weeks of KD initiation and seizure freedom were the primary outcome measures used to demonstrate benefit. Secondary outcome measures included hospital and ICU LOS, EEG features, number of AEDs, GCS and modified Rankin Scale scores at discharge, disposition location (home, rehabilitation, nursing home, death), and modified Rankin Scale score at follow-up. Additional follow-up information gathered included seizure frequency and whether the patient was continued on the KD or transitioned to the MAD.
Data were confirmed by review of physicians' notes, laboratory results, neuroimaging studies, and other supporting data. Medians and interquartile ranges (IQRs) were calculated for all continuous variables and proportions for all categorical variables. The primary research question was whether KD led to resolution of SE in patients with SRSE in the ICU (Class IV evidence).
RESULTS
Clinical characteristics.
Ten adult patients in ICUs at 4 tertiary-care medical centers (2 patients at Johns Hopkins Hospital; 4 at Johns Hopkins Bayview Medical Center; 2 at Mayo Clinic, Rochester; and 2 at Rush University Medical Center) were treated with KD for SRSE. The median age was 33 years (IQR 21), 4 patients (40%) were male, and 7 (70%) were Caucasian. Six patients (60%) had a history of seizures, with 5 patients (50%) taking AEDs before admission (table 1).
Table 1.
Patient characteristics and management
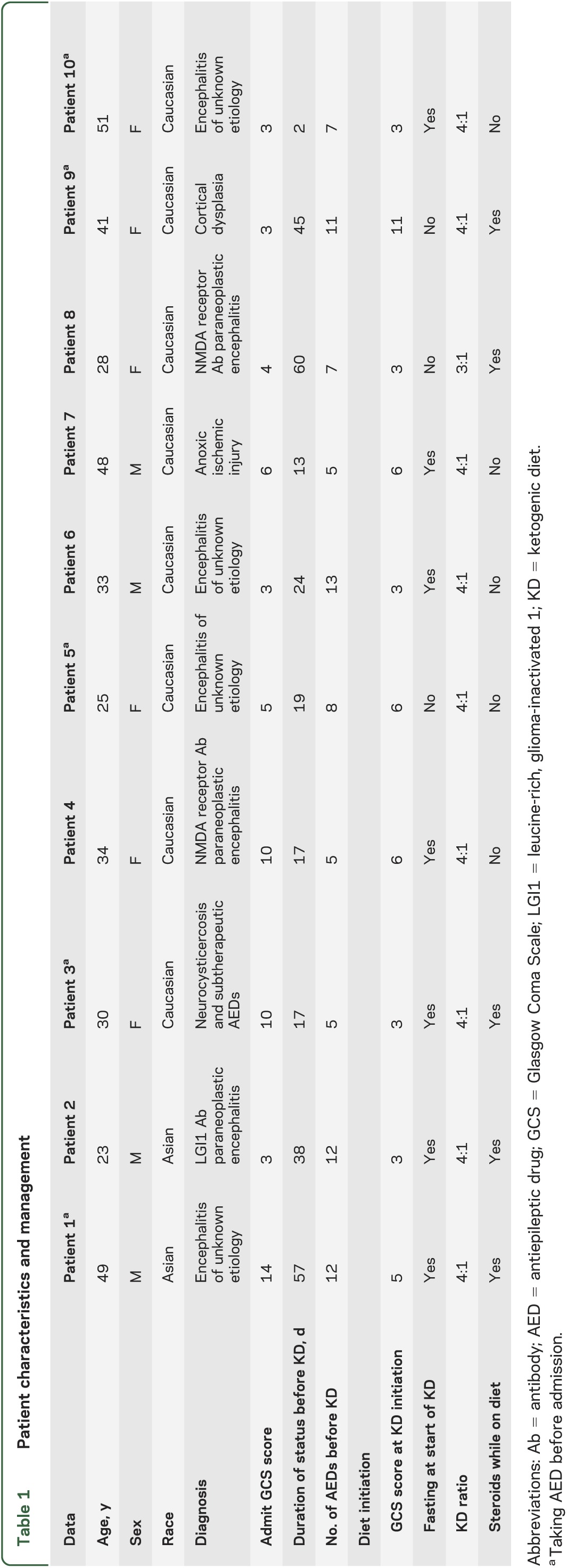
Seven patients were diagnosed with encephalitis (3 with antibody-positive paraneoplastic encephalitis and 4 with unknown etiology despite extensive serologic, molecular, and/or histopathologic analysis), one with neurocysticercosis and subtherapeutic AED levels, one with anoxic brain injury, and one with cortical dysplasia (table 1). Five patients were taking AEDs before admission for SRSE because of known diagnoses of epilepsy. Median admission GCS score was 4.5 (IQR 8), with 7 patients having a GCS score <8. Median SE duration before KD initiation was 21.5 days (IQR 28). Median number of AEDs used before KD initiation was 7 (IQR 7). All patients were in convulsive and/or nonconvulsive SE before KD initiation. Two of the 3 patients with antibody-mediated encephalitis received at least 2 courses of IV immunoglobulin and/or plasmapheresis before initiation of the KD.
Diet initiation.
Nine patients were placed on a 4:1 KD ratio (fat to carbohydrates and protein grams) and one on a 3:1 ratio. At the time of KD initiation, EEGs showed pharmacologic burst suppression in 2 patients (after at least one prior failed attempt to wean anesthetics), electrographic seizures in 6 patients, slowing with interictal epileptiform activity in one patient, and generalized periodic epileptiform discharges in one patient. Nine patients had a GCS score <8 at initiation. Ketosis was achieved in 9 patients within a median of 3 days (IQR 5). Two patients developed hypertriglyceridemia and one had transient acidosis that resolved without interrupting dietary treatment. The median length of time on the KD was 17.5 days (IQR 15) (tables 1 and 2).
Table 2.
Patient outcomes and follow-up
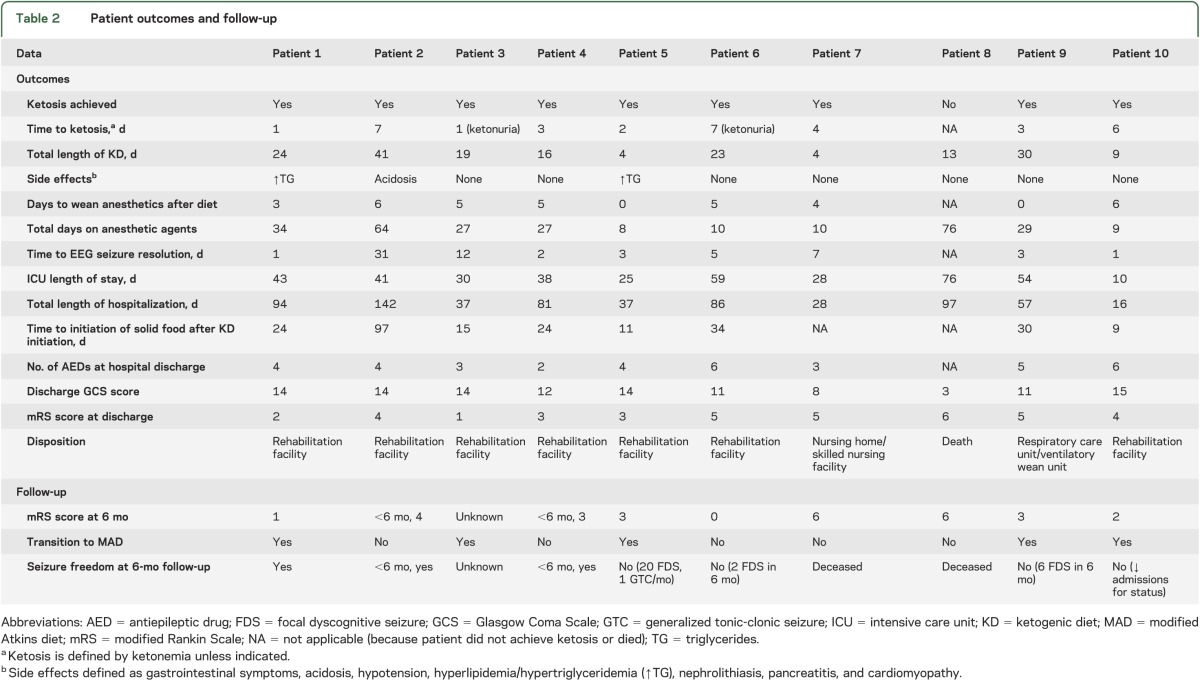
Outcome after KD initiation.
Nine patients (90%) had resolution of SE within a median of 3 days (IQR 8) after KD initiation. Seven patients had clinical and/or electrographic seizure resolution within 1 week of diet initiation, and 9 within 1 month. The median days on anesthetic agents after KD initiation was 5 (IQR 4). On discharge, 6 patients had a GCS score ≥12 (including 4 with known epilepsy taking AEDs before admission). The median number of AEDs prescribed at discharge was 4 (IQR 2.5).
Two patients ultimately died; one died after cardiac arrest in the setting of persistent seizures and exhaustion of therapeutic options, and the family of a second patient withdrew care because of persistent coma, despite seizure control. Five patients transitioned to MAD (table 2). Of these, 2 continue to have intermittent seizures on MAD with decreased frequency compared with baseline. One patient has been seizure-free and was weaned off MAD after 6 months, without recurrence. One patient stopped MAD and continues to have intermittent seizures with AED adjustments. One was lost to follow-up. Three patients have been off KD since discharge (2 are seizure-free, and one has intermittent seizures).
DISCUSSION
We describe 10 adult patients who were treated with KD for SRSE and found that a majority of these patients (90%) had resolution of SE within days after diet initiation. Although we describe a small cohort of patients, our study suggests that dietary therapy may have a role in treating adult patients with SRSE.
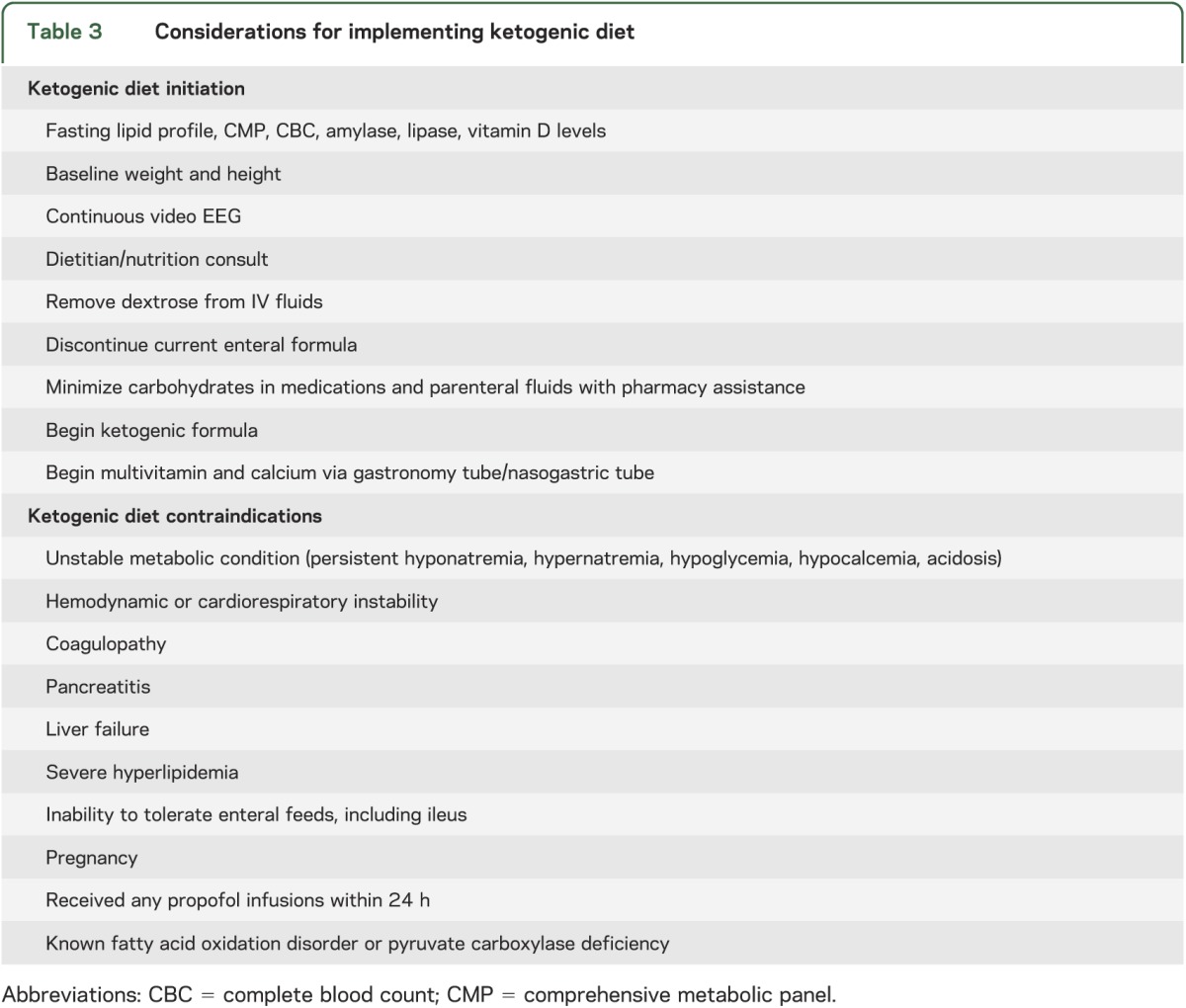
The use of KD to control SRSE has been previously reported in case reports and small case series.6–11 One case in the present series was previously reported in the literature.7 Wusthoff et al.10 reported the largest number of adult patients (2) treated with KD for RSE to date. These cases and our current findings suggest that KD is a safe and feasible treatment for critically ill adult patients with RSE and SRSE when implemented appropriately (table 3). An ongoing prospective multicenter trial using a standardized KD protocol will provide further data on the safety and efficacy of KD in critically ill patients with RSE and SRSE.
Table 3.
Considerations for implementing ketogenic diet
A major limitation of the current study is its retrospective nature. Many patients received adjunctive treatment with multiple AEDs, surgical intervention, IV steroids, plasmapheresis, and IV immunoglobulin while on the KD. For patients with antibody-mediated encephalitis, we were unable to gather detailed information regarding the duration and timing of immune-mediated therapies in relation to the initiation of the KD. While we recorded AEDs used through each patient's clinical course, the specific order and duration given and serum levels were not obtained. We therefore cannot exclude the possibility that other interventions led to clinical improvement and seizure freedom. Also, protocols for KD initiation and monitoring varied. We lacked detailed follow-up information on one patient. Finally, 5 patients discontinued dietary treatment, preventing comment on long-term efficacy of continued dietary therapy in these individuals.
Our case series suggests that the KD is safe and feasible in ICU patients and may have a role in refractory cases. It remains to be shown whether early intervention with KD and transition to the MAD improves morbidity and mortality in this critically ill population.
GLOSSARY
- AED
antiepileptic drug
- GCS
Glasgow Coma Scale
- ICU
intensive care unit
- IQR
interquartile range
- KD
ketogenic diet
- LOS
length of stay
- MAD
modified Atkins diet
- RSE
refractory status epilepticus
- SE
status epilepticus
- SRSE
super-refractory status epilepticus
AUTHOR CONTRIBUTIONS
Kiran Thakur, MD, and John Probasco, MD: design, data collection, analysis, and writing of the manuscript. Sara Hocker, MD: design, data collection, and writing of the manuscript. Kelly Roehl, MS, RD: data collection and writing of the manuscript. Bobbie Henry, RD, and Eric Kossoff, MD: design, data analysis, and review of the manuscript. Peter Kaplan, MB, FRCP: design, analysis, writing and review of the manuscript. Romergryko Geocadin, MD, and Adam Hartman, MD: design, analysis, and writing of the manuscript. Arun Venkatesan, MD, PhD: design, analysis, writing and review of the manuscript. Mackenzie Cervenka, MD: design, data collection, analysis, writing and review of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
K. Thakur, J. Probasco, S. Hocker, and K. Roehl report no disclosures. B. Henry: grants from Johns Hopkins Institute for Clinical and Translational Research, funded in part by NIH grants (UL1 TR 000424-06 NCATS), NIH Roadmap for Medical Research, and Nutricia. E. Kossoff: grant from Nutricia; consulting to Atkins Nutritionals, Inc. P. Kaplan: data safety monitoring board for UCB lacosamide TRENdS trial—Duke University. A. Hartman: research support from NIH (NINDS) and Johns Hopkins University School of Medicine; receives income from his clinical practice and reading EEGs (21% effort); and has provided expert opinion in medicolegal cases. R. Geocadin: NIH grants (supported in part by 5R01HL071568 and RO1 NS074425). A. Venkatesan: grants from NIH, HHMI, Maryland Stem Cell Research Foundation, National Multiple Sclerosis Society; data-safety monitoring for Bristol-Myers Squibb and GlaxoSmithKline. M. Cervenka: grants from Johns Hopkins University School of Medicine Clinician Scientist Award, Nutricia, and NIH (NINDS R01NS075020). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Shorvon S, Ferlisi M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain 2012;135:2314–2328 [DOI] [PubMed] [Google Scholar]
- 2.Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA 2013;70:72–77 [DOI] [PubMed] [Google Scholar]
- 3.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Proc 1921;2:307–308 [Google Scholar]
- 4.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia 2006;47:421–424 [DOI] [PubMed] [Google Scholar]
- 5.Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults: a review. Epilepsia 2011;52:1941–1948 [DOI] [PubMed] [Google Scholar]
- 6.Bodenant M, Moreau C, Sejourne C, et al. Interest of the ketogenic diet in a refractory status epilepticus in adults. Rev Neurol 2008;164:194–199 [DOI] [PubMed] [Google Scholar]
- 7.Cervenka MC, Hartman AL, Venkatesan A, Geocadin RG, Kossoff EH. The ketogenic diet for medically and surgically refractory status epilepticus in the neurocritical care unit. Neurocrit Care 2011;15:519–524 [DOI] [PubMed] [Google Scholar]
- 8.Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia 2010;51:2033–2037 [DOI] [PubMed] [Google Scholar]
- 9.Nam SH, Lee BL, Lee CG, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia 2011;52:e181–e184 [DOI] [PubMed] [Google Scholar]
- 10.Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia 2010;51:1083–1085 [DOI] [PubMed] [Google Scholar]
- 11.Strzelczyk A, Reif PS, Bauer S, et al. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure 2013;22:581–583 [DOI] [PubMed] [Google Scholar]


