Abstract
Objective:
To examine the relationship between previously identified nonadherence trajectories during the first 6 months of antiepileptic drug (AED) therapy and long-term seizure-free rates (defined as ≥1 year of seizure freedom at the 4 years postdiagnosis milestone) in a cohort of children with newly diagnosed epilepsy.
Methods:
A prospective longitudinal observational study of AED adherence and seizure freedom in a consecutive cohort of 124 children (ages 2–12 years) with newly diagnosed epilepsy was conducted. The association between previously identified AED adherence trajectories (i.e., near-perfect adherence [e.g., average adherence = 96.8%] vs nonadherent) and seizure freedom for ≥1 year at the 4 years postdiagnosis milestone was determined.
Results:
Children who exhibited nonadherence to AED therapy in the first 6 months of treatment were 3.24 times more likely not to have achieved ≥1 year of seizure freedom at the 4 years postdiagnosis milestone compared to children in the near-perfect adherence group (χ2 = 5.13; p = 0.02). Specifically, at the 4 years postdiagnosis milestone, only 12% of children in the near-perfect adherence group were continuing to experience seizures compared to 31% of children in the nonadherent group.
Conclusions:
Children with epilepsy who achieved near-perfect adherence during the first 6 months of therapy experienced a higher rate of seizure freedom 4 years postdiagnosis compared with those children who demonstrated early nonadherence. This suggests that adherence intervention early in the course of treatment could play a role in improving long-term seizure freedom rates in children with epilepsy.
In our prior study, 58% of young children with epilepsy and their families exhibited nonadherence to antiepileptic drugs (AEDs) in the first 6 months of treatment.1 However, the patterns of nonadherence vary in course and level of nonadherence: mild, moderate, severe early, and severe delayed nonadherence.1 Although identifying adherence patterns was an important first step to describing adherence behaviors in pediatric epilepsy, this previous study was unable to address the relationship between short-term adherence trajectories and long-term seizure outcomes because of its short 6-month duration. Understanding the role of early adherence patterns on long-term seizure outcomes is important because adherence patterns appear to be established and stable by 6 months postdiagnosis, and these patterns likely have an impact on both short-term and long-term outcomes for children with epilepsy. The purpose of this study was to examine the relationship between nonadherence within the first 6 months of AED therapy and long-term seizure freedom at the 4 years postdiagnosis milestone. A 4 years milestone was chosen because it ensured the study would capture seizure outcome for children with either good response to AED therapy (i.e., 2 years seizure freedom with their first, second, or third AED trial) or ongoing seizures despite multiple AED trials. It was hypothesized that children with near-perfect adherence (e.g., average adherence 96.8%) in the first 6 months of AED therapy would be more likely to be seizure-free for ≥1 year at the 4 years postdiagnosis milestone compared to children who exhibited nonadherence during the first 6 months of treatment.
METHODS
Participants were recruited on the day of their epilepsy diagnosis from the New Onset Seizure Clinic at Cincinnati Children's Hospital Medical Center for a 2-year longitudinal study examining adherence, predictors of adherence, and health outcomes. The New Onset Seizure Clinic primarily treats children with idiopathic epilepsies with no major medical comorbidities. Inclusion/exclusion criteria were 1) children with newly diagnosed epilepsy, 2) age between 2 and 12 years, 3) no comorbid medical conditions requiring daily medication or major developmental disorders (e.g., autism), and 5) initiation on 1 AED. Children and their caregivers were approached for study participation by a trained research assistant. After study procedures were explained to participants, written informed consent/verbal assent was obtained.
During the first study visit, primary caregivers completed a demographics form and were given an electronic monitor to assess daily adherence to AED therapy. Study visits coincided with routine clinic appointments; the first follow-up visit was 1 month postdiagnosis and then every 3 months thereafter. At each visit, data from the electronic monitor were downloaded and medical chart reviews were conducted by trained research assistants. A final medical chart review was performed at the 4 years postdiagnosis milestone by the first author. Patients were recruited from 2006 to 2009 and final data collection (e.g., electronic medical record review) occurred in March 2013.
Standard protocol approvals, registrations, and patient consents.
Institutional Review Board approval was obtained for the prospective longitudinal adherence study.
Measures.
AED adherence.
The MEMS TrackCap (Aardex Corporation, Union City, CA) and bottle were given to all participating families on the day of diagnosis. The MEMS TrackCap is an electronic monitor that records the date and time the bottle was opened (e.g., when a pill was removed). Data from the MEMS TrackCap, which represent daily adherence, were used to identify trajectories of AED adherence in the first 6 months of therapy for children with newly diagnosed epilepsy.1 These adherence trajectory groups included near-perfect adherence, mild nonadherence, moderate nonadherence, severe delayed nonadherence, and severe early nonadherence.
Seizure outcomes.
Throughout the study, the presence or absence of seizures was determined using data in the electronic medical record. Specifically, the presence/absence of seizures was ascertained using parental report in the clinic visit or via telephone calls between clinic visits and for some seizure types (e.g., absence) EEG findings.
Background information form.
Parents completed a Background Information Form including questions on child's date of birth, sex, parent education and occupation, and family composition. An occupation-based measure of socioeconomic status (SES),2,3 the Revised Duncan score,4 was calculated for each family. Scores range from 15 to 97, with higher scores representing greater occupational attainment.
Statistical analyses.
Descriptive statistics including means, frequencies, and SDs were calculated for demographic and medical variables. Six-month adherence trajectories identified in this cohort in our prior work1 were used as a grouping variable and patients were categorized as follows: 1) patients who were in the near-perfect adherence trajectory were classified as adherent, and 2) patients in the severe early, severe delayed, moderate, and mild nonadherence trajectories were classified as nonadherent.
Children who did not have any seizures for ≥1 year at their 4 years postdiagnosis milestone were considered seizure-free. This included patients who had become 2 years seizure-free, weaned from their AEDs, and then did not have any subsequent reported seizures noted in their electronic health record at the 4 years postdiagnosis mark. Children who continued to have seizures during the year prior to their 4 years postdiagnosis milestone were considered to not be seizure-free. χ2 analyses were conducted to compare the proportions of patients at the 4 years postdiagnosis mark who were ≥1 year seizure-free and those with continued seizures by their adherence classification.
RESULTS
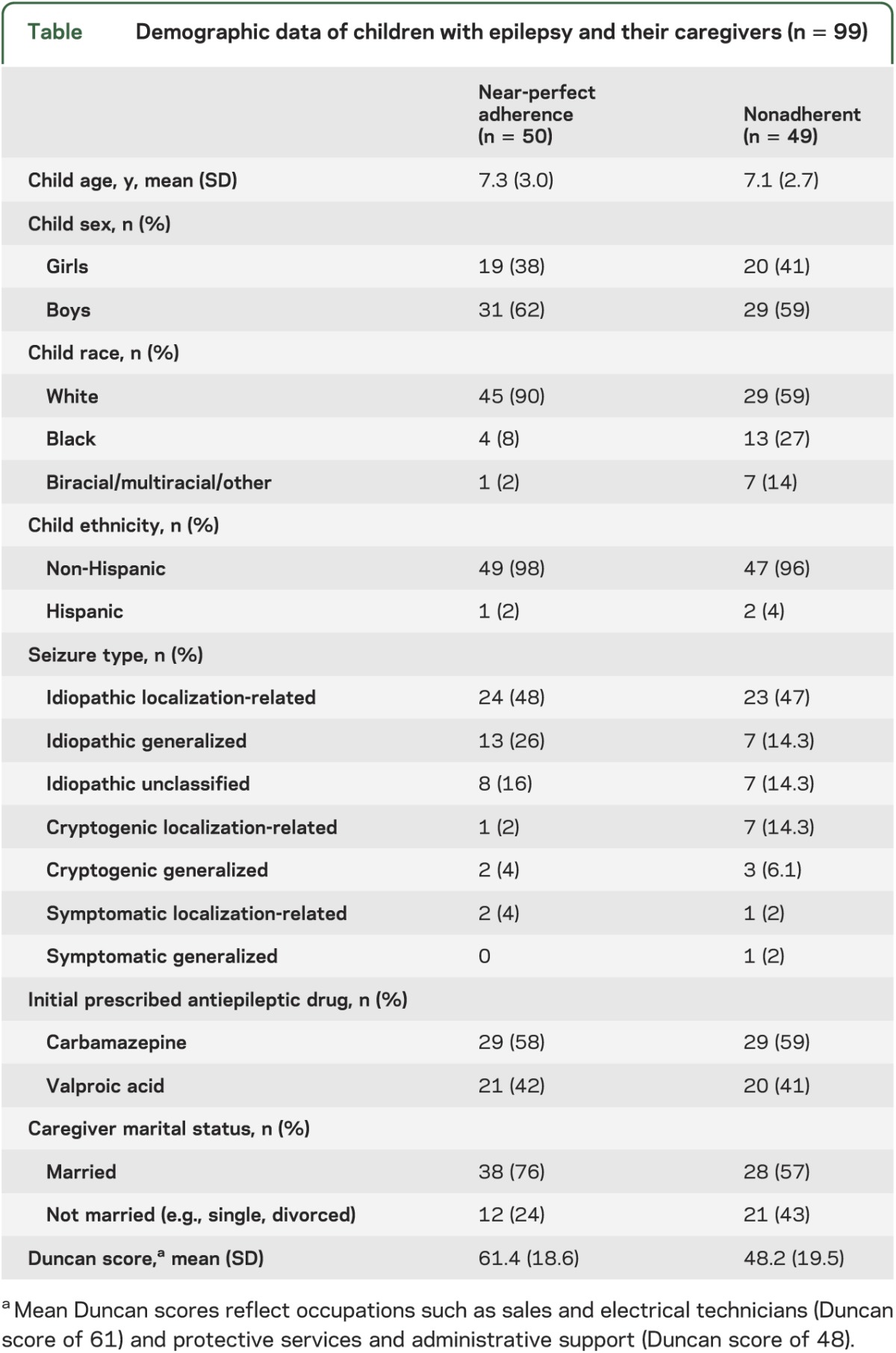
A total of 130 children with newly diagnosed epilepsy and their primary caregivers were approached for study participation. Five participants stated they were too busy, which yielded a recruitment rate of 96% (n = 125). One patient was found to have pervasive developmental disorder after consent was obtained and was removed from analyses (n = 124). Seizure outcome data at the 4 years postdiagnosis milestone were available for 80% of the sample (n = 99). Missing seizure outcome data is attributed to families moving/transferring care to another institution (n = 6) or not returning to our hospital for follow-up clinical care (n = 19). No significant differences in child age, child sex, child race/ethnicity, seizure type, or caregiver marital status were found between families with missing seizure outcome data compared to those included in the study cohort. However, children who were missing data had lower SES (t[121] = 2.78; p < 0.01) and were more likely to be in the nonadherent trajectories (χ2 = 14.8; p < 0.001). Demographic characteristics of the final sample are presented in the table.
Table.
Demographic data of children with epilepsy and their caregivers (n = 99)
Children who exhibited AED nonadherence during the first 6 months of therapy were 3.24 times more likely not to have achieved ≥1 year of seizure freedom at the 4 years postdiagnosis milestone compared to children in the near-perfect adherence group (χ2 = 5.13; p = 0.02). Specifically, at the 4 years postdiagnosis milestone, only 12% (n = 6 of 50) of children in the near-perfect adherence group were continuing to experience seizures compared to 31% (n = 15 of 49) of children in the nonadherent group.
DISCUSSION
The current study examined the relationship between AED adherence in the first 6 months of treatment for children with newly diagnosed epilepsy and long-term seizure outcomes. Seizure freedom for ≥1 year at the 4 years postdiagnosis milestone was considered a successful long-term outcome. In contrast, ongoing seizures at the 4 years postdiagnosis milestone was considered a poor long-term outcome and most likely a sign of medically resistant (intractable) epilepsy.
Children with epilepsy who demonstrated near-perfect adherence during their first 6 months of AED therapy exhibited a higher seizure freedom rate than children with early AED nonadherence. Specifically, children with early nonadherence were 3 times more likely to continue having seizures at the year 4 postdiagnosis milestone. Overall, one-third of children who exhibited early nonadherence continued having seizures compared to only 12% of children who demonstrated near-perfect adherence. These results suggest that early adherence may be critical to seizure outcomes in children with newly diagnosed epilepsy. Evidence-based treatments,5,6 including family-based and behavioral treatment, have been developed for children with chronic conditions who demonstrate nonadherence. These treatments need to be available to and used by children with epilepsy. This suggests that adherence intervention early in the course of treatment could play a role in improving long-term seizure freedom rates in children with epilepsy. Limitations of the current study include the cohort's restricted age range (2–12 years), and children had no developmental or medical comorbidities at diagnosis, limiting generalizability. Future studies should expand to adolescents and include those with comorbidities that appear at disease onset and document those that develop during the course of treatment.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the research assistants, undergraduate and graduate students, predoctoral interns, and postdoctoral fellows (Cincinnati Children's Hospital Medical Center) for recruiting study patients and data collection; and the New Onset Seizure Team at Cincinnati Children's Hospital for their support of the study.
GLOSSARY
- AED
antiepileptic drug
- SES
socioeconomic status
Footnotes
Editorial, page 652
AUTHOR CONTRIBUTIONS
Dr. Avani Modi and Dr. Joseph Rausch had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Modi and Glauser. Acquisition of data: Dr. Modi. Analysis and interpretation of data: Drs. Modi, Glauser, and Rausch. Drafting of the manuscript: Drs. Modi, Glauser, and Rausch. Critical revision of the manuscript for important intellectual content: Drs. Modi, Glauser, and Rausch. Statistical analysis: Drs. Rausch and Modi. Obtained funding: Dr. Modi. Administrative, technical, or material support: Dr. Modi. Study supervision: Drs. Modi and Glauser.
STUDY FUNDING
Funded by a grant from the NIH awarded to Dr. Modi (K23HD057333: Novel Adherence Measurement and Intervention in Children with New-Onset Epilepsy). The study sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
DISCLOSURE
A. Modi has been a consultant for Aprecia Pharmaceuticals Inc. T. Glauser is an advisor to or a speaker for companies with interests in antiepileptic drugs, including ucb Pharma, Eisai, Supernus, Sunovion, Upsher-Smith, Lundbeck, and Questcor. J. Rausch reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Modi AC, Rausch JR, Glauser TA. Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA 2011;305:1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakao K, Treas J. The 1989 Socioeconomic Index of Occupations: Construction From the 1989 Occupational Prestige Scores. Chicago: University of Chicago, National Opinion Research Center; 1992 [Google Scholar]
- 3.Hauser RM. Measuring socioeconomic status in studies of child development. Child Dev 1994;65:1541–1545 [DOI] [PubMed] [Google Scholar]
- 4.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res 1981;10:364–395 [Google Scholar]
- 5.Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. J Pediatr Psychol 2010;35:368–382 [DOI] [PubMed] [Google Scholar]
- 6.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol 2008;33:590–611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


