Abstract
The capacity of organisms to sense changes in the levels of internal and external gases and to respond accordingly is central to a range of physiologic and pathophysiologic processes. Carbon dioxide, a primary product of oxidative metabolism is one such gas that can be sensed by both prokaryotic and eukaryotic cells and in response to altered levels, elicit the activation of multiple adaptive pathways. The outcomes of activating CO2-sensitive pathways in various species include increased virulence of fungal and bacterial pathogens, prey-seeking behavior in insects as well as taste perception, lung function, and the control of immunity in mammals. In this review, we discuss what is known about the mechanisms underpinning CO2 sensing across a range of species and consider the implications of this for physiology, disease progression, and the possibility of developing new therapeutics for inflammatory and infectious disease.
Keywords: Carbon dioxide (CO2), Hypercapnia, Physiological gases, Immune regulation, NF-kappaB
Introduction
The natural history of CO2
In the time since the formation of the planet approximately 4,500 million years ago, the composition of the Earth’s terrestrial atmosphere has varied both dramatically and continuously. Furthermore, fluctuations in the gaseous composition of the atmosphere have had significant implications for the evolution of both aquatic and terrestrial life whereby the rise or fall of various atmospheric gases over geological has time played a major role in shaping the nature of the planet’s biota [1]. For example, rising levels of atmospheric molecular oxygen (O2), which began approximately 2,500 million years ago as a result of the proliferation of photosynthesizing cyanobacteria (the blue-green algae of the planet’s oceans), led to eradication of the majority of life on earth during a period termed the “great oxidation event” while at the same time ultimately allowing the rise and radiation of the metazoans (multicellular organisms) through the provision of the chemical energy by which to fuel oxidative metabolism [1].
Another atmospheric gas, the levels of which have fluctuated over geologic time has heavily influenced the nature of life on earth is carbon dioxide (CO2). Since the radiation of metazoans, the level of CO2 in the atmosphere has fluctuated from around 7,000 ppm during the Cambrian period to current levels, which were just recently reported to exceed 400 ppm. Therefore, in the Earth’s current atmosphere, the background levels of atmospheric CO2, although clearly of key importance in climate determination [2], are relatively low when compared to levels reached previously over geologic time. However, it is important to note that local CO2 levels found in the microenvironments of niches such as in and around respiring organisms or decomposing matter are greatly increased relative to background atmospheric levels.
CO2 produced in cells during the citric acid cycle is a by-product of oxidative metabolism (respiration) and as such is found at significantly higher concentrations inside a respiring organism than in the external atmosphere. Because CO2 produced is expired, this leads to the formation of CO2 gradients away from a respiring organism. Indeed, use of CO2 gradients in prey-seeking or predator-avoidance behavior has been described for various insects and nematodes, respectively (discussed below). Furthermore, exposure to altered tissue levels of CO2 due to disturbed homeostasis during disease can lead to the common pathological states of hypocapnia and hypercapnia in mammals. Such conditions can lead to disrupted pH homeostasis, which in turn can result in systemic alkalosis/acidosis, organ failure, and in some cases, death. These are just selected examples of the many reasons why most organisms have evolved mechanisms to sense changes in microenvironmental CO2 levels and elicit an appropriate physiologic response.
Therefore, like other physiologic gases such as O2 and nitric oxide (NO), it is important that cells, tissues and organisms retain the ability to sense changes in CO2 and respond accordingly. Recent advances in our understanding of oxygen-sensing in cells was led by the discovery of the ubiquitous oxygen-sensing mechanisms of the hypoxia-inducible factor (HIF) pathway and the specialized oxygen-sensing mechanisms of the carotid body [3, 4]. Similarly, the discovery that soluble guanylate cyclase can act as a nitric oxide sensor in mammalian cells shed light on how this endogenous gas is sensed [5]. Less, however, is known about cellular CO2-sensing mechanism(s), particularly as it pertains to transcriptional responses to hypercapnia. In this review, we will discuss both what is known and what remains to be discovered in relation to cellular CO2 sensing.
The biology of CO2
CO2, as a primary by-product of oxidative metabolism is constantly produced during the citric acid cycle within mitochondria. The majority of CO2 leaves the cell in which it was produced through the cell membrane. The most straightforward way by which CO2 molecules are capable of traversing the cell membrane is via passive diffusion. In this process, CO2 dissolved in the lipid bilayer traverses the opposing face of the membrane. This mechanism is passive and is dependent upon the transmembrane concentration gradient of CO2. Molecular carbon dioxide is suitable for passive diffusion as it is a small non-polar molecule [6].
While it was previously believed that CO2 moves across biological membranes only by passive diffusion, recent evidence has also proposed the existence of discreet CO2 channels in biological membranes [7]. To date, aquaporins, rhesus channels and connexins have all been implicated in the selective transport of CO2 molecules. Currently there are conflicting experimental data regarding the significance of active transport in the passage of CO2 through membranes [8].
Aquaporins primarily function as water conduits within cells. Yet these membrane intrinsic proteins are also capable of associating with uncharged gases such as CO2 [9, 10]. The movement of carbon dioxide through aquaporin channels is facilitated by the formation of soluble complexes [11]. It has been demonstrated in Xenopus oocytes that injection with carbonic anhydrase (CA) enhances AQ1 (aquaporin 1) expression thus rendering the membrane more porous to CO2 [12]. Similarly, in plants the tobacco aquaporin (NtAQP1) is responsive to CO2. Upregulation of NtAQP1 increases the permeability of plant membranes to H2O and CO2 consequently augmenting leaf development [11]. In contrast, aquaporins do not appear to be of importance to CO2 transport in mammalian cells. The deletion of AQ1 in erythrocyte and lung mice models does not alter CO2 movement [13]. Therefore, it has been hypothesized that aquaporins are only of physiological relevance to carbon dioxide transfer in systems in which the difference between intracellular and extracellular CO2 levels is slight [11]. However, as stated above the relative contribution of passive diffusion to CO2 movement versus channel mediated transport of CO2 is controversial. A recent review discusses this issue in more depth and postulates that channels may be of particular importance in cells that contain a high proportion of membrane proteins, e.g. red blood cells [14].
Rhesus (Rh) proteins are highly conserved constituents of red blood cell plasma membranes. A search for a common biological role for Rh antigens in different organisms has engendered much debate [15]. Expression of the RH1 gene in the green algae Chlamydomonas reinhardtii is increased in hypercapnia (3 % CO2) in comparison to ambient conditions (0.03 % CO2) [16]. Growth of C. reinhardtii in high CO2 is hindered by repression of RH1 [15]. For these reasons it was proposed that Rhesus proteins may act as carbon dioxide channels. It has subsequently been shown that Rh antigens in aquatic species function as dual ammonia and carbon dioxide transporters [17, 18].
In vertebrates, connexin proteins accumulate to form inter neuronal channels known as gap junctions. These pathways are involved in chemoreception and exhibit sensitivity to carbon dioxide [19]. Gap junctions link neuronal cells allowing the exchange of small molecules including carbon dioxide [20]. Immunohistochemistry in rats identified the gap junction proteins Cx26 and Cx32 as being possible substrates in cellular communication [21]. Cx26 reacts to increases in CO2 by opening and closes when CO2 levels decrease. Furthermore, the release of ATP by Cx26 is reliant on the amount of carbon dioxide present [22].
Therefore, CO2 is a key by-product of oxidative metabolism, which is generated by respiring cells. CO2 is transported out of cells primarily by passive diffusion but this transport may also be facilitated by the existence of CO2 channels, which in turn can be regulated in a CO2-dependent manner. It has recently become clear, however, that rather than simply being a waste product of oxidative metabolism, CO2 can also act as a physiologic stimulus for a number of cellular signaling pathways across virtually all species. Selected examples of the mechanisms underpinning the capacity of various species to sense CO2 are outlined below.
Co2 sensing across species
Bacteria
The capacity to sense and respond to altered CO2 allows bacteria to adjust to their environment, thus increasing the likelihood of their persistence. This may be of key importance as bacteria leave the relatively low CO2 levels of the external atmosphere for the higher CO2 levels found inside most multicellular host organisms. Bacteria may upregulate virulence factors at host physiologic CO2 levels (as opposed to atmospheric CO2 levels) in order to facilitate colonization or infection. Examples of these pathways in a selected number of pathogens are given below.
Many pathogenic bacteria have developed sensing mechanisms to determine the amount of carbon dioxide in their surroundings. These include Bordetella, a species of Gram negative Proteobacteria capable of infecting the human respiratory tract. In the external environment, Bordetella bronchiseptica has limited antigen expression. Yet it has been shown that this bacterium has increased cytotoxicity and adherence at a CO2 level consistent with that which exists within a mammalian host. The transcription of antigens such as adenylate cyclase toxin (ACT) and the type III secretion system (TTSS) is also elevated at 5 % CO2 [23]. ACT and TTSS induce immunological non-responsiveness in dendritic cells by interfering with MAPK signaling [24]. In this way, B. bronchiseptica responds to the internal mammalian CO2 concentration by increasing its capacity for respiratory tract colonization. Subsequent experiments with the whooping cough pathogens B. pertussis and B. parapertussis confirmed that a CO2-dependent increase in pathogenicity is a common characteristic of members of the Bordetella genus [23].
The food borne pathogen Bacillus cereus presents an unusual public health challenge by virtue of the fact that it can survive extreme conditions through sporulation. Comparative analyses of B. cereus under hypercapnic and normocapnic conditions have revealed genomic dissimilarities, which implicate carbon dioxide as a key determinant in the virulence of the bacteria. Pathogenic species display greater quantities of plasmid encoded virulence genes and S-layer protective proteins at elevated CO2. Additionally the activation of the pleiotropic virulence regulators PlCR and AtxA were found to be influenced by oxygen and carbon dioxide concentrations [25]. The AtxA regulon; necessary for capsule and toxin gene transcription was stimulated by increased CO2 [26]. Conversely, the PlCR regulon, which is associated with non-virulence related characteristics such as food supply and cell protection was more prevalent in ambient air [27]. Thus it appears that in order to adapt for colonization, B. cereus has evolved a CO2-sensing system that provokes the differential expression of its virulence factors.
The zoonotic agent responsible for Lyme disease Borrelia burgdorferi experiences dramatic oscillations in carbon dioxide availability throughout its complex life cycle. It must first transmit from the external environment to the arthropod vector and onwards to the mammalian reservoir host [28]. It has been shown that carbon dioxide can directly modulate borrelial gene expression. At 5 % CO2 the alternate sigma factor RpoS is activated and antigen synthesis is promoted. There is also increased translation of the lipoprotein genes ospC and dbpA, which alter the structure of B. burgdorferi to render adherence to the host more viable [29]. CO2 concentrations in vivo cause the borrelial pathogen to adapt for adherence and enhance its infectivity.
The production of enterotoxin by Vibrio cholerae, the etiological agent of cholera increases in association with rising carbon dioxide levels. It has been determined that a hypercapnic atmosphere of 10 % CO2 maximizes enterotoxin yield [30]. Carbonic anhydrases mediate the inter-conversion of carbon dioxide and bicarbonate in V. cholerae. Bicarbonate has been identified as the first positive effector for ToxT in cholera. ToxT is a regulator that transcriptionally induces the cholera virulence cascade. The introduction of ethoxzolamide, an antagonist of carbonic anhydrase prevents the occurrence of bicarbonate associated pathogenicity [31]. This inhibition highlights the importance of carbonic anhydrases and the conversion of CO2 to bicarbonate to V. cholerae. Therefore V. cholerae is reliant upon carbon dioxide and becomes more virulent at higher levels of CO2.
Pseudomonas aeruginosa is an environmental bacterium that like many of the bacteria described above resides in drastically different CO2 environments depending on whether it is colonizing a host or not. P. aeruginosa infection is a major clinical challenge in a hospital and immunocompromised setting. Recently three functionally active carbonic anhydrase isoforms were identified in P. aeruginosa PAO1 with the most abundant and active (psCA1) playing an important role in PAO1 survival at high CO2 [32].Thus, a better understanding of bacterial adaptation and survival across a range of CO2 environments may be of importance in the development of future antimicrobial therapies.
In summary, many bacterial pathogens demonstrate increased growth potential and virulence when exposed to the elevated CO2 levels found within mammalian hosts. Therefore, bacteria express CO2-sensing mechanisms, which allow them to adapt to the host environment and thrive therein.
Plants
Perhaps the most important biological function for carbon dioxide on our planet is its contribution to photosynthesis whereby CO2 and water (in the presence of chlorophyll and sunlight) give rise to the production of carbohydrates and oxygen (6 CO2 + 6H2O + light → C6H12O6 + 6O2). The initiation of this key biochemical reaction in photosynthetic bacteria altered the course of life and has been shaping evolution ever since. Carbon dioxide enters the leaf via small pores known as stomata where it combines with RuBP as part of the carbon fixing component of the Calvin cycle. This reaction is catalyzed by RUBISCO, (the most abundant protein on the planet) which accounts for up to 50 % of total protein mass in the leaves of certain plants [33]. The consequences of RUBISCO’s catalytic activity to fix CO2 is the production of two molecules of 3-phosphoglycerate, a metabolic intermediate that can be used for metabolic processes and the production of organic molecules such as glucose.
The stomata through which CO2 enters the leaf to participate in the photosynthetic reaction are also the portals through which H2O exits the leaf during transpiration. As a consequence the degree of stomatal opening needs to be a tightly regulated process to ensure adequate CO2 entry without excessive H2O loss, which is particularly important in hotter climates. Guard cells surround the stomatal aperture and physically regulate the degree of opening (pore size) in response to a number of environmental factors including the plant hormone abscisic acid (ASA) (which is produced in response to drought), light and carbon dioxide [34]. This response to increased CO2 is likely an evolutionary adaptation to prevent water loss at night when CO2 levels are relatively elevated and photosynthesis cannot occur due to the absence of light. The mechanisms through which elevated CO2 can cause stomatal closure have recently been elegantly investigated in Arabidopsis using genetic tools [34]. CO2 in combination with water can form carbonic acid, which in turn can be converted into HCO3 − and H+. This reaction is catalyzed by carbonic anhydrase of which there are several variants. Hu et al. [34] identified a key role for carbonic anhydrase (specifically CA1 and CA4) in mediating the CO2-dependent closure of stomata. Intriguingly, the introduction of structurally unrelated mammalian carbonic anhydrase was sufficient to restore CO2-sensitivity to stomata in CA-mutant Arabidopsis [34]. HCO3 − is proposed as the likely signal downstream of CO2 and carbonic anhydrase that results in stomatal closure via effects on anion channels in the guard cells [34, 35]. Taken together, these findings suggest that carbonic anhydrase can function as a CO2 transponder to facilitate key downstream signaling events in plants [36]. A detailed review of the molecular mechanisms underpinning CO2-dependent stomatal closure has recently been published [37]. Of note, the authors highlight the convergence of ABA and CO2-dependent signaling at the level of the gca (growth controlled by ASA) gene in the regulation of the stomatal regulatory circuit. gca mutant plants that do not respond to ABA with respect to stomatal closure were also strongly impaired in their stomatal response to CO2 [38].
In summary, CO2 is a key stimulus in plants that is responsible for the regulation of stomatal closure and as such requires an effective sensing mechanism, which is mediated by CA and integrates with signaling pathways utilized by other mediators of stoma regulation.
Fungi
The fungal kingdom encompasses a diverse array of microorganisms, which have evolved to possess complex growth strategies. During their life cycles fungi encounter a wide range of ecological conditions; including fluctuating carbon dioxide levels. Fungi have developed sensing mechanisms to determine the concentration of CO2 in their surroundings and respond effectively to this environmental cue. The elevated levels of carbon dioxide present in mammalian tissues (5 %) when compared to the external environment (0.03 %) favor the survival of some pathogenic fungi within animal hosts.
Extensive research has elucidated a complex CO2-sensing system in the model fungal organism Candida albicans. Carbon dioxide has been shown to have numerous tangible effects upon the fungus. In C. albicans, the transcription factor Flo8 functions as a carbon dioxide sensor [39]. C. albicans switches to filamentous growth in response to CO2 tension in vivo [39]. Carbon dioxide instigates this transformation in populations of C. albicans via its action as a signaling molecule [40]. The carbon dioxide induced filamentous form of C. albicans exhibits greater pathogenicity than the monocellular yeast. Elongated filaments attach to generate significant biomasses, which in turn facilitate the initial colonization of tissues and subsequent increase and dissemination of fungal infection [41]. For example, it has been demonstrated that increased concentrations of carbon dioxide at the skin surface relative to the external atmosphere exacerbate the extent of dermatological candidiasis [42]. In order to mate effectively, C. albicans undergoes a transition from a white to an opaque phenotype [40]. Physiological carbon dioxide levels preferentially select the opaque phenotype thus augmenting the fungal rate of reproduction [43].
Like C. albicans, the opportunistic human pathogen Cryptococcus neoformans also confronts dramatic variations in carbon dioxide availability during its life cycle. It has been documented that a CO2-sensing system consisting of adenylyl cyclase Cac1 and carbonic anhydrase CAN2 is integral to ensure the propagation of C. neoformans [44]. However, in areas of plentiful carbon dioxide supplies the beta-carbonic anhydrase generating CAN2 gene is no longer a prerequisite for persistence of the cryptococcal species [45]. The production of a polysaccharide capsule by C. neoformans enhances its virulence in vivo [46]. The process of capsule biosynthesis is partially reliant upon the presence of 5 % CO2 [47]. Carbon dioxide also exerts an influence upon the growth pattern of C. neoformans. The fungus is most likely to be found as a biofilm in the external environment and as planktonic cells in animal tissues. The formation of a biofilm favors the endurance of C. neoformans ex vivo [48].
Fungi primarily sense carbon dioxide via the carbonic anhydrase and adenylyl cyclase pathways. This subject matter has previously been expertly reviewed by [49]. HCO3 − acts as a signaling molecule and conveys the CO2 message; thus allowing fungi to respond to alterations in the carbon dioxide levels of their surroundings [50]. Induction of adenylyl cyclase by carbon dioxide in C. neoformans and C. albicans is reliant upon carbonic anhydrase activity [45]. Furthermore it has been determined experimentally that bicarbonate is capable of directly activating adenylyl cyclase in C. albicans [51]. In fungi, carbonic anhydrases are also active in sexual mating and function to ensure that there are adequate resources of bicarbonate in CO2 limiting conditions [50]. Thus, CO2 is sensed by fungi in a manner that elicits growth advantage and promotes fungal infection in host species.
Nematodes
Nematodes including Caenorhabditis elegans demonstrate an acute avoidance response following exposure to elevated levels of CO2. This is likely an evolutionarily conserved survival mechanism to control internal CO2 levels and determine attraction/avoidance from prey/decaying food sources, however, CO2 avoidance does vary between strains of C. elegans and within different species of free-living nematode [52]. The avoidance behavior is primarily governed by a mechanism involving cGMP signaling within BAG neurons (ciliated neurons in the head whose expression is regulated by the transcription factor ETS-5 [53]) via Tax-2/Tax-4. Interestingly, the CO2-avoidance is affected by both nutritional feeding patterns (solitary feeding species C. elegans are CO2-sensitive) and nutritional status (starved C. elegans have an attenuated CO2 avoidance response), which is suggestive of a mechanism that re-balances the necessity to avoid predators in times of nutrient deprivation [52]. Subsequent studies identified a receptor-type guanylate cyclase GCY-9 (which is enriched in BAG neurons) as being required for CO2-dependent C. elegans avoidance. GCY-9 is proposed as being direct CO2 or CO2 metabolite sensor but a downstream role for GCY-9 could not be excluded [54]. Intriguingly, C. elegans in the dauer phase of development display the opposite response to CO2 of adults and are in fact attracted to elevated levels of CO2 again with a requirement for BAG neurons to mediate CO2-sensitivity [55]. Taken together, this demonstrates a key role for the BAG neurons in regulating C. elegans responsiveness to CO2 irrespective of whether CO2 is mediating repulsive or attractive responses. While central to CO2 sensitivity in C. elegans, BAG neurons are not the only CO2-sensitive neurons that govern avoidance behavior in the worm. A recent study identified AFD and ASE neurons (previously characterized as being involved in temperature and salt ion detection, respectively) as being primary CO2 sensors in addition to BAG neurons (which are also involved in O2 detection) in C. elegans [56]. Interestingly, the nature of the neuronal response to CO2 in each case is unique and differs from the pattern of neuronal activation elicited by non-CO2 stimuli, e.g. temperature in AFD neurons. The authors speculate that given their sensitivity to both CO2 and O2, these signals could be integrated at a molecular level within BAG neurons [56] .
High CO2 levels are associated with gross changes in normal physiology in C. elegans. Exposure of worms to CO2 levels in excess of 9 % had marked effects on motility, fertility and lifespan. Impaired motility was associated with age-dependent deterioration of muscle organization, brood size was significantly attenuated in a CO2 dose-dependent fashion from 0.03 to 19 % CO2 but interestingly lifespan was extended in animals grown at 19 % CO2 compared to ambient air. Gene expression analysis of C. elegans exposed to 19 % CO2 over a time course revealed specific effects of hypercapnia on sub-sets of genes including those associated with 7-transmembrane domain proteins, nuclear hormone receptors, E3 Ub ligases and innate immunity [57]. Together these reports point to both acute neuronal sensing of CO2 governing attraction/repulsion and a more chronic non-neuronal sensing of CO2 affecting distinct subsets of genes governing key physiological processes in the nematode.
Insects
Many species of insect have been reported to demonstrate CO2 sensitivity, however, here, we will focus on those species where the potential mechanism underpinning the CO2 sensitivity has been described. Indeed, the nature of anatomical adaptations to CO2 sensing in insects has been comprehensively reviewed elsewhere [58]. The CO2-dependent prey-seeking behavior of female mosquito species is an area of interest with respect to developing strategies to limit the spread of malaria. Mosquitoes are attracted to exhaled CO2 from potential hosts for the purpose of obtaining a blood meal [59] and in some species CO2 can also sensitize the mosquito to detect human skin odor [60]. Interestingly, ultra-prolonged activation of CO2-sensing neurons can disorient mosquitoes [61], an effect that is being used to develop strategies to disrupt host seeking behavior.
Drosophila melanogaster demonstrates CO2-sensitive behaviour that, depending on the circumstance can be both attracted to or repulsed by increased CO2 levels in the local environment. Furthermore, elevated CO2 elicits a change in whole organism gene expression in Drosophila. Because of the genetic tools available, this organism has the best characterized insect CO2-sensing pathways. Drosophila in contrast to their mosquito counterparts are repelled by elevated CO2 levels (in walking assays). The mechanism underpinning this repulsion involves two chemosensory receptor genes Gr21a and Gr63a, which are necessary for neuronal CO2 sensing. These genes are highly conserved in insects including mosquito but are absent in certain insects that retain CO2 sensitivity, e.g. honeybee, which is suggestive of the evolution of different CO2-sensing pathways in insects [62]. The Gr21a and Gr63a receptors are thought to work in concert to mediate CO2 avoidance behavior in Drosophila [59]. CO2 is a component of Drosophila stress odorant [63] and this in part explains the aversion of Drosophila to CO2. However, it is not obvious why an insect that feeds on ripening fruit should be repulsed by CO2. A recent study has made an intriguing observation that Drosophila will actively seek a narrow CO2 plume in flight (as opposed to in a walking assay). Interfering with the Gr21a transduction pathway surprisingly had no significant effect on Drosophila CO2 tracking in flight, suggesting an alternative mechanism for CO2 sensing during flight [64]. Several signaling components have been implicated as being required for CO2 tracking in flight including octopamine (a flight modulated biogenic amine [65]), the expression of an acid receptor Ionotropic receptor 64a (Ir64a) and an olfactory co-receptor Orco [64].
In addition to the neuronally mediated responses of insects to CO2 described above, there are a number of non-neuronal effects of CO2 of interest. Elevated CO2 causes defects in developmental morphogenesis, egg laying and hatching in Drosophila [66]. These extraneuronal effects of CO2 in Drosophila occur even in the absence of the Gr63a expression. It was also shown in this study that hypercapnia down-regulates expression of multiple antimicrobial peptides, key innate immune effectors in the fly that are regulated by the NF-kappaB analogue Relish. This effect was not mediated by acidosis, nitric oxide signaling or the heat shock response. Moreover, hypercapnia caused striking increases in mortality due to bacterial infections, an effect that was also independent of Gr63a [66]. Notably, Drosophila do not express soluble adenylyl cyclases (sACs) [67], which may function in CO2 sensing in other systems (see below). Thus, the immunoregulatory effects of hypercapnia in Drosophila appear to be mediated by mechanism(s) distinct from other well-characterized neuronal and non-neuronal CO2-sensing pathways. In summary, a range of insects display the ability to sense carbon dioxide and elicit a range of responses including prey-seeking, avoidance and immune suppression.
Fish
Fish detect CO2 in their environment and are sensitive to small changes in CO2 concentrations caused by anthropogenic or natural events. CO2 sensing in fish has been comprehensively reviewed elsewhere [68]. Fish use CO2-sensitive chemoreceptors located mainly in the gill to respond to changes in ambient CO2 levels and initiate cardiorespiratory reflexes including bradycardia and hyperventilation. In this sense, the fish gill and the mammalian carotid body are remarkably similar, acting as sensing centers for both O2 and CO2 in fish and mammals, respectively. Fish have a significantly lower circulating pCO2 than air breathers with normal levels in the region of ~2–3 mmHg as compared to the ~40 mmHg in mammalian circulation [69]. As a consequence they demonstrate a more sensitive response to CO2 commensurate with their relatively lower normocapnic pCO2. Zebrafish hyperventilate at environmental CO2 levels of ~1 mmHg. It is thought that this acute sensitivity to CO2 in the fish is a reflection of the relatively higher arterial pH changes elicited in response to a small change in CO2 in fish as compared to mammals. In the zebrafish gill, neuroepithelial cells (NEC) sense CO2 (as well as O2) with a resultant inhibition of background K+ channels and subsequent depolarization at increasing CO2 levels. Piscine carbonic anhydrase is implicated in this response as pharmacological inhibition of CA with acetazolamide blunted electrophysiological indices of NEC-CO2 sensitivity. Thus, the authors propose a CO2-dependent sensing mechanism that may be sensitive to changes in intracellular acidification/pH [69]. Finally, given the acute nature of the fish response to elevated CO2 it is likely that the continued rise of anthropogenic CO2 will have marked effects on fish physiology and behavior [70] and in particular by those species that are most sensitive to changes in pCO2.
Mammals
Mammals exhibit a host of responses to alterations in CO2 levels, including modulation of ventilation, alveolar fluid re-absorption, olfactory and gustatory responses, cell proliferation, muscle function, inflammation and innate immune responses. These effects with be dealt with in separate sections below:
Breathing
A number of mechanisms have evolved to sense changes in arterial pO2 and pCO2 and elicit alterations in the rate and depth of breathing. Central chemosensation of carbon dioxide by neurons located within the brainstem is the pre-dominant mechanism through which changes in arterial pCO2 are detected and affect breathing [71]. The contribution of the carotid body in modulating CO2-dependent respiratory control is relatively smaller than the brainstem in general but appears to have increasing importance at lower levels of hypercapnia [72]. The importance of the carotid body in sensing changes in arterial pO2 has been expertly reviewed elsewhere [4]. There is significant ongoing research into the mechanisms of central chemoreception of CO2 and this topic has been also been reviewed elsewhere [71, 73]. A current challenge in the field is to consolidate new data suggesting increasing numbers of CO2-sensitive areas (within the brain) as well as several molecular detectors for CO2 with pre-existing theories on CO2 sensing [73]. Furthermore, there is emerging evidence for cross-talk between central and peripheral chemoreceptors whereby the carotid body can fine tune the sensitivity of central chemoreceptors under conditions of hypercapnia [74]. Intriguingly, recent work challenges the long-held view that CO2-sensitive central chemoreceptors are essential to maintain the drive for rhythmic breathing [75]. In mice that conditionally express a mutant PHOX2B gene associated with central hypoventilation syndrome in humans, resulting in loss of the murine central chemoreceptor locus, Ramanantsoa et al. [76] report that respiratory rhythm and normal gas exchange are maintained. This maintenance of respiratory rhythm and normal gas exchange is due to compensation by O2-sensitive peripheral chemoreceptors and occurs even in the absence of the murine central chemoreceptor locus.
Lung epithelial function
One of the most extensively investigated molecular signaling events in response to CO2 is in the assessment of alveolar epithelial function and endocytosis of the Na,K-ATPase. The Na,K-ATPase plays a key role in the active transport of Na+ and K+ across membranes and epithelia, thus maintaining cellular ion homeostasis and in alveolar epithelial cells. Exposure of alveolar epithelial cells to high levels of CO2 is associated with impaired alveolar fluid reabsorption [77]. Impaired fluid reabsorption is a consequence of CO2-dependent Na,K-ATPase endocytosis from the cell plasma membrane. The mechanism governing this response involves sequential CO2-induced activation of AMP kinase (AMPK) by Ca2+/calmodulin-dependent protein kinase kinase-beta, and PKC-ζ phosphorylation, resulting in the endocytosis of the Na,K ATPase [78]. More recently ERK [79], JNK [80] and PKA1a [81] have also been identified as playing a role in Na,K-ATPase downregulation and thus epithelial dysfunction. However, carbonic anhydrase II, which is expressed in the alveolar epithelial cells and is important in CO2 metabolism, does not play a role in regulation of alveolar fluid reabsorption [82]. Interestingly, the contribution of JNK to CO2-dependent signaling is evolutionarily conserved, with RNAi targeted to Drosophila JNK preventing CO2-dependent downregulation of Na,K ATPase in fly S2 cells [80]. With respect to the role of PKA1a, a novel pathway was proposed whereby hypercapnia via a CO2/HCO3 −-sensitive sAC increases the production of cAMP, activates PKA1a and α-adducin, culminating in Na,K ATPase endocytosis in epithelial cells [81].
Smell
CO2 is odorless to humans but is keenly sensed by rodents [83]. The reasons why rodents retained the ability to smell CO2 is likely associated with an advantage to detect food sources and predators. The mechanism whereby mice can smell CO2 is via a subset of olfactory sensory neurons that use bicarbonate (downstream of carbonic anhydrase II) to produce cGMP via direct activation of the intracellular cyclase domain of guanyl cyclase-D [84]. This mechanism of CO2 detection is similar to the way bicarbonate can act as on sACs in other systems.
Taste
The discovery of the pleasing taste of soda water by Joseph Priestley in the 1760s paved the way for the advent of carbonated beverages, production of which has grown into an enormous industry. Carbonation elicits both somatosensory and chemosensory responses in mammals that include activation of taste receptors [85] although the exact reasons why such a sensing mechanism may have evolved is not clear. Using electrophysiological techniques in mice with genetically impaired taste sub-type receptor cells, the authors found that selective ablation of sour sensing cells abolished the ability to taste carbonation. A carbonic anhydrase Car4 was found to be selectively expressed in sour taste cells and mice deficient in Car4 had a significantly blunted response to CO2. An extracellular increase in proton production downstream of carbonic anhydrase is the proposed messenger given that bicarbonate alone was not able to stimulate taste receptor cells [36, 85].
Cell proliferation
In a manner independent of acidosis or hypoxia, hypercapnia has been shown to inhibit proliferation of fibroblasts and alveolar epithelial cells [86]. This is due to mitochondrial dysfunction, resulting from induction by CO2 of miR-183, which in turn down-regulates the TCA cycle enzyme, isocitrate dehydrogenase-2 (IDH2). By inhibiting cell proliferation in this manner, hypercapnia might interfere with tissue homeostasis and inhibit tissue regeneration and wound repair. In a separate model, exposure of pulmonary epithelial cells to hypercapnia/acidosis impaired epithelial wound repair through an NF-kappaB-dependent mechanism [87].
Muscle function
Mice exposed to high CO2 demonstrate myocyte degradation and muscle wasting. The mechanisms underpinning this effect involve activation of the energy sensor AMPK and upregulation of the ubiquitin ligase MuRF1, resulting in proteasomal degradation of muscle cells [88]. Interestingly, the detrimental effects of elevated CO2 on muscle appear to be conserved across species as high levels of CO2 caused slower locomotion in C. elegans, which was associated with and probably due to disturbed muscle morphology [57].
Inflammation and innate immunity
The effects of alterations in the level of CO2 on mammalian inflammatory and immune responses have been explored in vitro and in vivo. Seeking to understand the basis of reduced peritoneal inflammation associated with laparoscopic surgery when CO2 was used for abdominal insufflation, West et al. [89] observed that culture of peritoneal macrophages in 80 % CO2 inhibited lipopolysaccharide (LPS)-induced secretion of tumor necrosis factor (TNF) and interleukin (IL)-1beta. Subsequently, hypercapnia at lower CO2 concentrations (10–20 %) was found to inhibit LPS-stimulated release of TNF by rat alveolar macrophages [90]. It was then shown that hypercapnia inhibited IL-6 and TNF mRNA and protein expression in human and mouse macrophage cell lines, as well as alveolar macrophages from both species; the effect of elevated CO2 was rapid, reversible, noncytotoxic, selective, and independent of extracellular and intracellular acidosis, nitric oxide signaling, and heat shock or hypoxia-inducible gene expression [91].
NF-kappaB is a family of transcription factors that play a key role in the regulation of innate immunity and inflammation [92]. Recent studies into the effects of hypercapnia on NF-kappaB signaling have provided evidence that this pathway may represent a hub of key importance in the hypercapnia-induced signaling response. Hypercapnia inhibited endotoxin-stimulated NF-kappaB RelA nuclear translocation and DNA binding in pulmonary artery endothelial cells [93], although this was not the case in human macrophages [91]. It has also been reported that elevated CO2 was associated with increased pulmonary inflammation in an NF-kappaB-dependent manner [94]. As discussed above, hypercapnic acidosis suppressed wound healing in A549 lung cells via suppression of NF-kappaB signaling [87] and in vivo NF-kappaB activation was reduced in a rat hepatic ischemia reperfusion injury model examining the effect of therapeutic hypercapnia [95]. Consistent with the concept that the NF-kappaB signaling pathway represents an important hub of CO2 sensitivity, it was demonstrated that elevated CO2 levels in cultured cells also significantly impacts upon non-canonical NF-kappaB family members through the regulation of IKKα and RelB signaling [96–98]. In terms of regulating IKKα signaling, it was found that in response to hypercapnia, IKKα rapidly and reversibly translocates to the nucleus in a manner independent of the known components of the cellular oxygen-sensing pathway, intra- or extra-cellular pH or pathways associated with acute CO2-sensing in lower species [97]. Furthermore, hypercapnia induces cleavage and nuclear translocation of RelB, a second key component of the non-canonical NF-kappaB pathway [96]. The net effect of these hypercapnia-induced events is a modulation of LPS or cytokine-induced NF-kappaB activity. This effect on NF-kappaB signaling may at least in part, underpin the anti-inflammatory and immunomodulatory effects of hypercapnia [98]. Furthermore, these studies implicate the existence of a CO2-sensing mechanism in mammalian cells that is independent of changes in intracellular or extracellular pH and which links changes in extracellular CO2 with transcriptional events. A comparable ubiquitous system that is responsible for the sensing of microenvironmental oxygen levels has been well described in mammalian cells [3]. This system utilizes the oxygen-dependence of a family of cellular hydroxylases to regulate the hydroxylation and subsequent stability of a transcription factor termed the HIF [99, 100].
The effects of hypercapnia have also been studied in rodent models of inflammatory lung injury. Hypercapnia attenuated acute lung injury induced by mechanical ventilation with high tidal volumes in rabbits [101, 102] and by endotoxin in rats [103]. On the other hand, the effects of hypercapnia in mechanically ventilated rats with E. coli lung infection were variable, depending on experimental conditions [104–108]. In one of these studies [105], rats were allowed to breathe spontaneously following infection, then mechanically ventilated at the end of the protocol; in this case, hypercapnia worsened lung injury, decreased bacterial clearance from the lungs, and inhibited the neutrophil phagocytic capacity. More recently, it was shown that hypercapnia increased the mortality of Pseudomonas pneumonia in spontaneously breathing mice, an effect associated with dysregulated expression of lung cytokines and chemokines, impaired bacterial phagocytosis and reactive oxygen species generation by lung neutrophils, and an increased burden of bacteria in the lungs, liver and spleen [109]. Interestingly, in the latter study, hypercapnia did not worsen lung injury associated with pneumonia, suggesting that the increase in mortality may have been due to systemic sepsis and extrapulmonary organ dysfunction, rather than respiratory failure per se. Taken together, these studies indicate that while hypercapnia can ameliorate lung injury triggered by noninfectious inflammatory insults, by suppressing innate immune function, it worsens the outcome of bacterial lung infections in spontaneously breathing animals.
In summary, elevated carbon dioxide modulates mammalian inflammatory and innate immune responses in vitro and in vivo. This may be of benefit in situations where inflammation is triggered by a sterile insult, but would be deleterious in the setting of infection due to host immunosuppression. This, in combination with the ability of elevated CO2 to enhance bacterial and fungal virulence and survival, suggests that hypercapnia may predispose to or worsen outcomes of infections in humans. Understanding the molecular signaling pathways involved will be of key importance in the identification of new approaches to control infection and inflammation in the clinical setting.
Candidate molecular CO2 sensors
Carbonic anhydrase
Given the prevalence of carbonic anhydrase enzymes in the sensing of CO2 across a number of species this family of enzymes will be discussed in more detail below. Carbonic anhydrases are zinc-containing metalloenzymes that mediate the reversible hydration of carbon dioxide [110]. CO2 + H2O ↔ HCO3 − + H+. This process is extremely rapid with rate constants in the region of 105–106 per second. The reversible hydration of CO2 in the absence of a catalyst is relatively slow [111]. Five distinct classes of carbonic anhydrases exist within nature (α, β, γ, δ and ζ). α CAs are found in mammals and the three main classes of CA α, β, and γ are structurally dissimilar suggestive of independent evolution [112]. The contribution of carbonic anhydrase enzymes to CO2 sensing is summarized in the table below (Table 1). Given that carbonic anhydrases facilitate a reaction that would otherwise occur more slowly, it is something of a philosophical question as to whether they should be defined as true CO2 sensors or rather transducers of CO2. Similarly, given that the downstream effects of carbonic anhydrase activity are elicited independently by both bicarbonate [84] and changes in pH (protons)[82, 85] and can be mimicked by a structurally unrelated CA isoform from another species [34] suggests that carbonic anhydrases are transducing a change in CO2 rather than sensing it directly.
Table 1.
Carbonic anhydrases as carbon dioxide sensors
| Species | Carbonic anhydrase isoform | Evidence | Downstream effector | References |
|---|---|---|---|---|
| Fungi: Cryptococcus neoformans | Can2 | Genetic | Bicarbonate | [44] |
| Fish | Pharmacological: acetazolamide | Protons | [69] | |
| Plants | CA1, CA4, unrelated mammalian CA | Genetic | Bicarbonate | [34] |
| Bacteria: Vibrio cholerae | Pharmacological: ethoxzolamide | Bicarbonate | [31] | |
| Mammals: mouse smell | CAII | Genetic | Bicarbonate | [84] |
| Mammals: mouse taste | Car4 | Genetic | Protons | [85] |
Adenylyl cyclase pathway
The second messenger 3′,5′-cyclic adenosine monophosphate (cAMP) is a key signaling molecule in biology affecting a range of processes including sensitivity to carbon dioxide. The enzyme responsible for cAMP production is adenylyl cyclase (AC) and catalyses the cyclization of ATP to produce cAMP [81, 113]. cAMP-dependent signaling is prominent in animals and lower eukaryotes, as well as having a proposed role in plant signaling systems [114]. AC and cAMP signaling has been reviewed elsewhere [115], and this section will focus exclusively on the components of the pathway that are related to carbon dioxide signaling.
Transmembrane adenylyl cyclases (tmACs)
tmACs as the name suggests have transmembrane spanning domains and are sensitive to G-proteins. Normally these enzymes are activated secondary to ligand- GPCR activation, e.g. parathyroid hormone (PTH) activation of the PTH receptor with the ligand’s signal being transduced into the cell and influencing a transmembrane AC. Downstream of tmAC activation cAMP can act as a second messenger to activate effector proteins including protein kinase A (PKA), cAMP response element binding protein (CREB), phosphodiesterase domains and cyclic nucleotide-gated ion channels. Recently it has be shown that cAMP production that is induced in this classical way via PTH activation of PTHR is suppressed by elevated CO2 and results in activation of the sodium-proton exchanger isoform 3 (NHE3). The authors ascribe this effect to CO2-dependent alterations in Ca2+ signaling downstream of IP3R activation [116]. The specific mechanism through which CO2 modulates Ca2+ release through the IP3 receptor is yet to be defined.
There is, however, evidence that tmACs are also directly CO2 sensitive as opposed to acting downstream of PTH as exemplified above. A mammalian recombinant G-protein-activated AC was found to be activated specifically in response to CO2 and not HCO3 −. These in vitro experiments were carried out under conditions of disequilibrium that exploits the fact that the predominant form of inorganic carbon (Ci) that exists in the assay is the form in which it is added, i.e. CO2 or HCO3 − when the temperature is low (approximately 0 °C). The activity of a mammalian tmAC and a related tmAC from Mycobacterium tuberculosis was stimulated by CO2 resulting in downstream CREB phosphorylation. Radiolabeled CO2 was also used to demonstrate CO2 binding to the protein, however, the precise site of incorporation is yet to be described [117].
Soluble adenylyl cyclases
sACs are distributed within the cytoplasm and in specific organelles and are more closely related to cyanobacterial ACs than to tmACs. In contrast to tmACs, which are activated by G proteins, sACs are not and instead are activated by intracellular signals including bicarbonate, calcium and ATP [118]. Recently sACs have been identified to be directly stimulated by HCO3 −, which is a key step in sperm cell maturation [119]. This response was independent of intracellular pH and evident in vitro and in vivo. Experiments designed to examine what species of Ci are responsible for eliciting sAC activation demonstrated that both CO2 and HCO3 − stimulated sACT under condition of Ci disequilibrium [117].
Taken together, there is significant evidence for ACs and the cAMP signaling pathway as being important for CO2 sensing with both sACs demonstrating sensitivity to HCO3 − and CO2 [81, 113] and a specific mammalian tmAC demonstrating selective sensitivity to CO2 [117]. Furthermore, the evidence for direct incorporation of CO2 into a CO2-sensitive tmAC is a key finding in the search for molecular insight into the effects of CO2 signaling and sensing.
Clinical implications
Hypercapnia has long been recognized as a marker of poor prognosis in patients with chronic obstructive pulmonary disease [120–123]. More recent studies have identified hypercapnia as an independent risk factor for mortality in patients hospitalized with community-acquired pneumonia [124, 125] and cystic fibrosis patients awaiting lung transplantation [126]. Despite these strong associations, clinicians have generally viewed hypercapnia in chronic lung disorders solely as a marker of advanced disease, without considering the possibility of a causal link between elevated CO2 and adverse clinical outcomes.
Within the realm of acute pulmonary disease, over 20 years ago animal studies suggested that mechanical ventilation with high tidal volumes was injurious to the lung, and that ventilation with lower volumes had beneficial effects [127, 128]. It was subsequently confirmed that mechanical ventilation with low tidal volumes decreased mortality in humans with acute respiratory distress syndrome [129, 130]. In these studies, some patients ventilated with low tidal volumes developed hypercapnia, however, there was no difference in mortality in hypercapnic patients randomized to low tidal volume [130]. This observation, combined with some of the aforementioned animal studies [101–103] was interpreted that hypercapnia was not harmful, or even that elevated CO2 levels might account for some of the benefit of low tidal volume ventilation. Terms such as “permissive” and “therapeutic” hypercapnia were coined to reflect the idea that high levels of CO2 might have salutary effects in acute lung injury and sepsis [131]. However, none of the clinical studies on which this concept was based actually tested whether hypercapnia per se had beneficial effects. On the other hand, a recent report on a cohort of more than 14,000 mechanically ventilated patients from 40 countries found strong associations between hypercapnia (pCO2 >50 mmHg) and multiple adverse clinical outcomes, including pneumonia, sepsis and most importantly mortality (Nin et al., Hypercapnia is associated with worse outcome of mechanically ventilated patients, in review).
As reviewed in detail above, recent studies demonstrate that hypercapnia inhibits alveolar fluid clearance, cell proliferation, muscle function, innate immune responses and host defense. Hypercapnic suppression of these essential physiologic functions and protective responses likely underlies, at least in part, the negative impacts of elevated CO2 in patients with severe acute and chronic lung disease. Further studies are needed to define which of these (or other) effects of hypercapnia actually leads to adverse outcomes clinically. Pending such studies, debate will continue regarding potential benefits and harms of hypercapnia, and whether the concept of “permissive” or “therapeutic” hypercapnia should continue to hold sway or be abandoned. This topic was recently discussed in a cross-talk feature, in which two groups of researchers laid out the arguments for and against the use of “permissive” hypercapnia in the treatment of ARDS. Of note, however, both groups of authors sounded words of caution with respect to potential detrimental effects of hypercapnia, particularly in the context of infection [132–135].
Conclusions and perspectives
It is now clear that carbon dioxide, like other physiologic gases such as nitric oxide and molecular oxygen is sensed by cells in a manner which involves the activation of an adaptive response. The signaling mechanisms involved include CA and AC but there are also likely other, as yet unidentified CO2 sensors within cells. The consequences of activation of CO2-sensitive pathways are diverse across species and are summarized in Fig. 1. From a disease progression point of view, the immunosuppressive effects of hypercapnia combined with the promotion of virulence and survival in fungal and bacterial pathogens (see Fig. 2) implicates tissue hypercapnia as a potential contributor to poor patient prognosis in infectious pathologies. It is of interest to consider that the relatively low pO2 and high pCO2 found within the mammalian body (in comparison with the external environment) are reminiscent of the ancient atmospheres that existed when prokaryotes were the dominant life forms on the planet. This may explain the successful survival of certain infectious agents and microbiota within the internal environment of the mammalian body due to the existence of levels of carbon dioxide at which they are more likely to thrive. Furthermore, the survival and virulence enhancing effects for pathogens as well as the host immune suppressing effects may represent potential new therapeutic targets in combating acute and chronic infectious diseases.
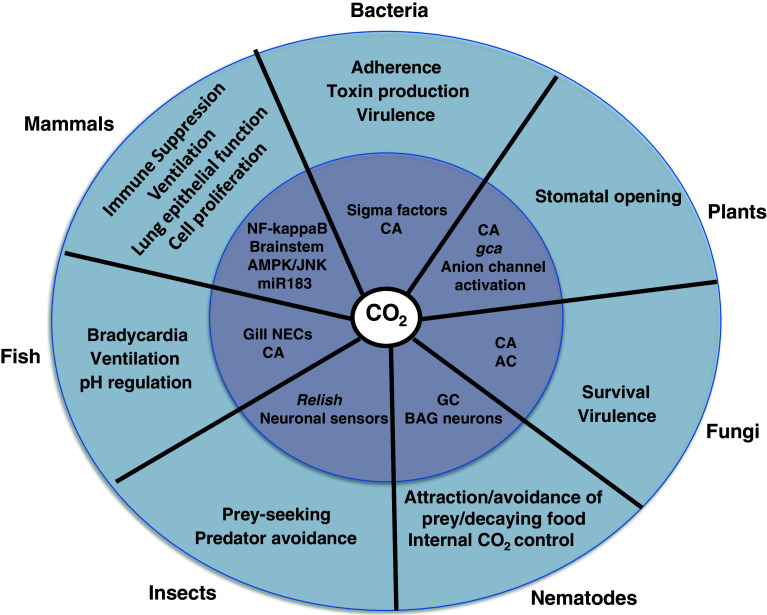
Fig. 1.
Schematic summarizing some of the key outcomes for CO2 sensing across species (outer circle) and examples of the mechanisms underpinning these effects (inner circle). AC adenylyl cyclase, CA carbonic anhydrase, GC guanylate cyclase, gca growth controlled by abscisic acid, NECs neuroepithelial cells, miR microRNA, NF-kappaB nuclear factor kappa B
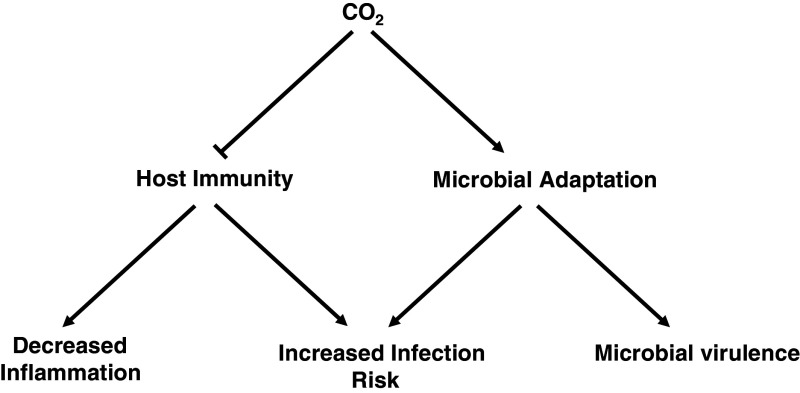
Fig. 2.
Key outcomes of CO2 signaling with respect to inflammation, infection, and microbial virulence. Elevated CO2 leads to suppression of host immunity and enhances microbial adaptation leading to a state where an increased risk of infection is favored by environmental conditions
Acknowledgments
C.T. Taylor, E.P. Cummins and A.C. Selfridge are supported by a Science Foundation Ireland (SFI) P.I award to C.T. Taylor. P.H. Sporn (HL-72891) and J.I. Sznajder (HL-85534, HL-48129 and HL 71643) are supported as indicated.
References
- 1.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 2010;25(5):272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 2.Monastersky R. Global carbon dioxide levels near worrisome milestone. Nature. 2013;497(7447):13–14. doi: 10.1038/497013a. [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.López-Barneo J, et al. Carotid body oxygen sensing. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2008;32(5):1386–1398. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- 5.Poulos T. Soluble guanylate cyclase. Curr Opin Struct Biol. 2006;16(6):736–743. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GM (2000) The cell: a molecular approach, 2nd edn. In: Transport of small molecules. Sinauer Associates, Sunderland
- 7.Perry SF, et al. Do zebrafish Rh proteins act as dual ammonia-CO2 channels? J Exp Zool A Ecol Genet Physiol. 2010;313(9):618–621. doi: 10.1002/jez.631. [DOI] [PubMed] [Google Scholar]
- 8.Missner A, et al. Carbon dioxide transport through membranes. J Biol Chem. 2008;283(37):25340–25347. doi: 10.1074/jbc.M800096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hachez C, Chaumont F. Aquaporins: a family of highly regulated multifunctional channels. Adv Exp Med Biol. 2010;679:1–17. doi: 10.1007/978-1-4419-6315-4_1. [DOI] [PubMed] [Google Scholar]
- 10.Musa-Aziz R, et al. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA. 2009;106(13):5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uehlein N, et al. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425(6959):734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 12.Nakhoul NL, et al. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274(2 Pt 1):C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, et al. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J Biol Chem. 2000;275(4):2686–2692. doi: 10.1074/jbc.275.4.2686. [DOI] [PubMed] [Google Scholar]
- 14.Boron W, et al. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem Eur J Chem Phys Phys Chem. 2011;12(5):1017–1019. doi: 10.1002/cphc.201100034. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan A, Lieman-Hurwitz J, Tchernov D. Resolving the biological role of the Rhesus (Rh) proteins of red blood cells with the aid of a green alga. Proc Natl Acad Sci USA. 2004;101(20):7497–7498. doi: 10.1073/pnas.0402527101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soupene E, et al. Rhesus expression in a green alga is regulated by CO(2) Proc Natl Acad Sci USA. 2002;99(11):7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright PA, Wood CM. A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol. 2009;212(Pt 15):2303–2312. doi: 10.1242/jeb.023085. [DOI] [PubMed] [Google Scholar]
- 18.Kustu S, Inwood W. Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol. 2006;13(1–2):103–110. doi: 10.1016/j.tracli.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang XG, Peracchia C. Positive charges of the initial C-terminus domain of Cx32 inhibit gap junction gating sensitivity to CO2 . Biophys J. 1997;73(2):798–806. doi: 10.1016/S0006-3495(97)78112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean JB, et al. Role of gap junctions in CO(2) chemoreception and respiratory control. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L665–L670. doi: 10.1152/ajplung.00142.2002. [DOI] [PubMed] [Google Scholar]
- 21.Solomon IC, et al. Localization of connexin26 and connexin32 in putative CO(2)-chemosensitive brainstem regions in rat. Respir Physiol. 2001;129(1–2):101–121. doi: 10.1016/s0034-5687(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 22.Huckstepp RT, et al. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010;588(Pt 20):3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hester SE, et al. Identification of a CO2 responsive regulon in Bordetella . PLoS ONE. 2012;7(10):e47635. doi: 10.1371/journal.pone.0047635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner JA, et al. Bordetella type III secretion and adenylate cyclase toxin synergize to drive dendritic cells into a semimature state. J Immunol. 2004;173(3):1934–1940. doi: 10.4049/jimmunol.173.3.1934. [DOI] [PubMed] [Google Scholar]
- 25.Passalacqua KD, et al. Comparative transcriptional profiling of Bacillus cereus sensu lato strains during growth in CO2-bicarbonate and aerobic atmospheres. PLoS ONE. 2009;4(3):e4904. doi: 10.1371/journal.pone.0004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bongiorni C, et al. Dual promoters control expression of the Bacillus anthracis virulence factor AtxA. J Bacteriol. 2008;190(19):6483–6492. doi: 10.1128/JB.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohar M, et al. The PlcR virulence regulon of Bacillus cereus . PLoS ONE. 2008;3(7):e2793. doi: 10.1371/journal.pone.0002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore RD, Jr, Mbow ML, Stevenson B. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis . Microbes Infect. 2001;3(10):799–808. doi: 10.1016/s1286-4579(01)01435-6. [DOI] [PubMed] [Google Scholar]
- 29.Hyde JA, Trzeciakowski JP, Skare JT. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol. 2007;189(2):437–445. doi: 10.1128/JB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimamura T, Watanabe S, Sasaki S. Enhancement of enterotoxin production by carbon dioxide in Vibrio cholerae . Infect Immun. 1985;49(2):455–456. doi: 10.1128/iai.49.2.455-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abuaita BH, Withey JH. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun. 2009;77(9):4111–4120. doi: 10.1128/IAI.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotlikar S et al (2013) Three functional β-carbonic anhydrases in P. aeruginosa PAO1. Role in survival in ambient air. Microbiology (Reading, England) (in press) [DOI] [PMC free article] [PubMed]
- 33.Feller U, Anders I, Mae T. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot. 2008;59(7):1615–1624. doi: 10.1093/jxb/erm242. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol. 2010;12(1):87–93. doi: 10.1038/ncb2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue S, et al. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011;30(8):1645–1658. doi: 10.1038/emboj.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frommer WB. Biochemistry. CO2mmon sense. Science. 2010;327(5963):275–276. doi: 10.1126/science.1186022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim T-H, et al. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young J, et al. CO(2) signaling in guard cells: calcium sensitivity response modulation, a Ca(2+)-independent phase, and CO(2) insensitivity of the gca2 mutant. Proc Natl Acad Sci USA. 2006;103(19):7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du H, et al. The transcription factor Flo8 mediates CO2 sensing in the human fungal pathogen Candida albicans . Mol Biol Cell. 2012;23(14):2692–2701. doi: 10.1091/mbc.E12-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall RA, et al. CO(2) acts as a signalling molecule in populations of the fungal pathogen Candida albicans . PLoS Pathog. 2010;6(11):e1001193. doi: 10.1371/journal.ppat.1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadosh D, Johnson AD. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans . Mol Cell Biol. 2001;21(7):2496–2505. doi: 10.1128/MCB.21.7.2496-2505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen AM, King RD. Occlusion, carbon dioxide, and fungal skin infections. Lancet. 1978;1(8060):360–362. doi: 10.1016/s0140-6736(78)91084-x. [DOI] [PubMed] [Google Scholar]
- 43.Huang G, et al. CO(2) regulates white-to-opaque switching in Candida albicans . Curr Biol. 2009;19(4):330–334. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mogensen EG, et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell. 2006;5(1):103–111. doi: 10.1128/EC.5.1.103-111.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahn YS, et al. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol. 2005;15(22):2013–2020. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 46.Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2) Infect Immun. 2003;71(11):6155–6164. doi: 10.1128/IAI.71.11.6155-6164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravi S, et al. Biofilm formation by Cryptococcus neoformans under distinct environmental conditions. Mycopathologia. 2009;167(6):307–314. doi: 10.1007/s11046-008-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahn YS, Muhlschlegel FA. CO2 sensing in fungi and beyond. Curr Opin Microbiol. 2006;9(6):572–578. doi: 10.1016/j.mib.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Elleuche S, Poggeler S. Carbonic anhydrases in fungi. Microbiology. 2010;156(Pt 1):23–29. doi: 10.1099/mic.0.032581-0. [DOI] [PubMed] [Google Scholar]
- 51.Klengel T, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15(22):2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2008;105(23):8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guillermin ML, Castelletto ML, Hallem EA. Differentiation of carbon dioxide-sensing neurons in Caenorhabditis elegans requires the ETS-5 transcription factor. Genetics. 2011;189(4):1327–1339. doi: 10.1534/genetics.111.133835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallem E, et al. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans . Proc Natl Acad Sci USA. 2011;108(1):254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hallem E, et al. A sensory code for host seeking in parasitic nematodes. Curr Biol. 2011;21(5):377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bretscher A, et al. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69(6):1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharabi K, et al. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2009;106(10):4024–4029. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stange G, Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc Res Tech. 1999;47(6):416–427. doi: 10.1002/(SICI)1097-0029(19991215)47:6<416::AID-JEMT5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 59.Jones WD, et al. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila . Nature. 2007;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 60.Dekker T, Geier M, Cardé R. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 2005;208(Pt 15):2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- 61.Turner S, et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474(7349):87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suh G, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila . Nature. 2004;431(7010):854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 64.Wasserman S, Salomon A, Frye M. Drosophila tracks carbon dioxide in flight. Curr Biol. 2013;23(Pt 4):301–306. doi: 10.1016/j.cub.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suver M, Mamiya A, Dickinson M. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila . Curr Biol. 2012;22(24):2294–2302. doi: 10.1016/j.cub.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 66.Helenius IT, et al. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc Natl Acad Sci USA. 2009;106(44):18710–18715. doi: 10.1073/pnas.0905925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roelofs J, Van Haastert P. Deducing the origin of soluble adenylyl cyclase, a gene lost in multiple lineages. Mol Biol Evol. 2002;19(12):2239–2246. doi: 10.1093/oxfordjournals.molbev.a004047. [DOI] [PubMed] [Google Scholar]
- 68.Perry S, Abdallah S. Mechanisms and consequences of carbon dioxide sensing in fish. Respir Physiol Neurobiol. 2012;184(3):309–315. doi: 10.1016/j.resp.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Qin Z, Lewis J, Perry S. Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2 . J Physiol. 2010;588(Pt 5):861–872. doi: 10.1113/jphysiol.2009.184739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munday P, McCormick M, Nilsson G. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J Exp Biol. 2012;215(Pt 22):3865–3873. doi: 10.1242/jeb.074765. [DOI] [PubMed] [Google Scholar]
- 71.Huckstepp R, Dale N. Redefining the components of central CO2 chemosensitivity—towards a better understanding of mechanism. J Physiol. 2011;589(Pt 23):5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forster H, et al. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol. 2008;605:322–326. doi: 10.1007/978-0-387-73693-8_56. [DOI] [PubMed] [Google Scholar]
- 73.Nattie E, Forster H. Special issue on central chemoreception. Foreword. Respir Physiol Neurobiol. 2010;173(3):193–194. doi: 10.1016/j.resp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Blain G, et al. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588(Pt 13):2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haldane J, Priestley J. The regulation of the lung-ventilation. J Physiol. 1905;32(3–4):225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramanantsoa N, et al. Breathing without CO(2) chemosensitivity in conditional Phox2b mutants. J Neurosci Off J Soc Neurosci. 2011;31(36):12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Briva A, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS One. 2007;2(Pt 11):e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vadász I, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Investig. 2008;118(2):752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welch L, et al. Extracellular signal-regulated kinase (ERK) participates in the hypercapnia-induced Na,K-ATPase downregulation. FEBS Lett. 2010;584(18):3985–3989. doi: 10.1016/j.febslet.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vadász I, et al. Evolutionary conserved role of c-Jun-N-terminal kinase in CO2-induced epithelial dysfunction. PLoS One. 2012;7(10):e46696. doi: 10.1371/journal.pone.0046696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lecuona E, et al. PKA Iα regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO2 levels. Am J Respir Cell Mol Biol. 2013;48(Pt 5):626–634. doi: 10.1165/rcmb.2012-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, et al. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. Am J Respir Cell Mol Biol. 2008;38(1):32–37. doi: 10.1165/rcmb.2007-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu J, et al. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317(5840):953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 84.Sun L, et al. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106(6):2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandrashekar J, et al. The taste of carbonation. Science (N Y) 2009;326(5951):443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vohwinkel CU, et al. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem. 2012;286(Pt 43):37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Toole D, et al. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB-dependent mechanism. Thorax. 2009;64(11):976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- 88.Jaitovich ADL, Welch L, Gusorava GA, Sznajder JI. Role of AMP-activated protein kinase (AMPK) in hypercapnia-induced muscle atrophy. Am J Respir Crit Care Med. 2012;185:A2013. [Google Scholar]
- 89.West MA, et al. Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide: relevance to laparoscopic surgery. Ann Surg. 1997;226(2):179–190. doi: 10.1097/00000658-199708000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lang CJ, et al. Effect of CO2 on LPS-induced cytokine responses in rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L96–L103. doi: 10.1152/ajplung.00394.2004. [DOI] [PubMed] [Google Scholar]
- 91.Wang N, et al. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24(7):2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeshita K, et al. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]B activation. Am J Respir Cell Mol Biol. 2003;29(1):124–132. doi: 10.1165/rcmb.2002-0126OC. [DOI] [PubMed] [Google Scholar]
- 94.Abolhassani M, et al. Carbon dioxide inhalation causes pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2009;296(4):L657–L665. doi: 10.1152/ajplung.90460.2008. [DOI] [PubMed] [Google Scholar]
- 95.Li AM, et al. Effects of therapeutic hypercapnia on inflammation and apoptosis after hepatic ischemia–reperfusion injury in rats. Chin Med J (Engl) 2010;123(16):2254–2258. [PubMed] [Google Scholar]
- 96.Oliver KM, et al. Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J Biol Chem. 2012;287(17):14004–14011. doi: 10.1074/jbc.M112.347971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cummins EP, et al. NF-kappaB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol. 2010;185(7):4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- 98.Taylor CT, Cummins EP. Regulation of gene expression by carbon dioxide. J Physiol. 2011;589(Pt 4):797–803. doi: 10.1113/jphysiol.2010.201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greer SN, et al. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Broccard AF, et al. Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med. 2001;164(5):802–806. doi: 10.1164/ajrccm.164.5.2007060. [DOI] [PubMed] [Google Scholar]
- 102.Sinclair SE, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166(3):403–408. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- 103.Laffey JG, et al. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 2004;169(1):46–56. doi: 10.1164/rccm.200205-394OC. [DOI] [PubMed] [Google Scholar]
- 104.O’Croinin DF, et al. Hypercapnic acidosis does not modulate the severity of bacterial pneumonia-induced lung injury. Crit Care Med. 2005;33(11):2606–2612. doi: 10.1097/01.ccm.0000186761.41090.c6. [DOI] [PubMed] [Google Scholar]
- 105.O’Croinin DF, et al. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Crit Care Med. 2008;36(7):2128–2135. doi: 10.1097/CCM.0b013e31817d1b59. [DOI] [PubMed] [Google Scholar]
- 106.Chonghaile MN, et al. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology. 2008;109(5):837–848. doi: 10.1097/ALN.0b013e3181895fb7. [DOI] [PubMed] [Google Scholar]
- 107.Nichol AD, et al. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med. 2009;37(11):2953–2961. doi: 10.1097/CCM.0b013e3181b028ce. [DOI] [PubMed] [Google Scholar]
- 108.Ni Chonghaile M, et al. Hypercapnic acidosis attenuates severe acute bacterial pneumonia-induced lung injury by a neutrophil-independent mechanism. Crit Care Med. 2008;36(12):3135–3144. doi: 10.1097/CCM.0b013e31818f0d13. [DOI] [PubMed] [Google Scholar]
- 109.Gates KL et al (2013) Hypercapnia impairs lung neutrophil function and increases mortality in murine Pseudomonas Pneumonia. Am J Respir Cell Mol Biol (in press) [DOI] [PMC free article] [PubMed]
- 110.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74(1):1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 111.Lindskog S, Coleman J. The catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci USA. 1973;70(9):2505–2508. doi: 10.1073/pnas.70.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aggarwal M, et al. Structural annotation of human carbonic anhydrases. J Enzyme Inhib Med Chem. 2013;28(2):267–277. doi: 10.3109/14756366.2012.737323. [DOI] [PubMed] [Google Scholar]
- 113.Kamenetsky M, et al. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362(4):623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gehring C. Adenyl cyclases and cAMP in plant signaling—past and present. Cell Commun Signal. 2010;8:15. doi: 10.1186/1478-811X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012;4(Pt 12):a011148. doi: 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cook Z, Gray M, Cann M. Elevated carbon dioxide blunts mammalian cAMP signaling dependent on inositol 1,4,5-triphosphate receptor-mediated Ca2+ release. J Biol Chem. 2012;287(31):26291–26301. doi: 10.1074/jbc.M112.349191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Townsend P, et al. Stimulation of mammalian G-protein-responsive adenylyl cyclases by carbon dioxide. J Biol Chem. 2009;284(2):784–791. doi: 10.1074/jbc.M807239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buck J, Levin L. Physiological sensing of carbon dioxide/bicarbonate/pH via cyclic nucleotide signaling. Sensors (Basel, Switzerland) 2011;11(2):2112–2128. doi: 10.3390/s110202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science (N Y) 2000;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 120.Moser KM, Shibel EM, Beamon AJ. Acute respiratory failure in obstructive lung disease. Long-term survival after treatment in an intensive care unit. JAMA. 1973;225(7):705–707. [PubMed] [Google Scholar]
- 121.Martin TR, Lewis SW, Albert RK. The prognosis of patients with chronic obstructive pulmonary disease after hospitalization for acute respiratory failure. Chest. 1982;82(3):310–314. doi: 10.1378/chest.82.3.310. [DOI] [PubMed] [Google Scholar]
- 122.Goel A, Pinckney RG, Littenberg B. APACHE II predicts long-term survival in COPD patients admitted to a general medical ward. J Gen Intern Med. 2003;18(10):824–830. doi: 10.1046/j.1525-1497.2003.20615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 124.Sin DD, Man SF, Marrie TJ. Arterial carbon dioxide tension on admission as a marker of in-hospital mortality in community-acquired pneumonia. Am J Med. 2005;118(2):145–150. doi: 10.1016/j.amjmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 125.Laserna E, et al. Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community-acquired pneumonia. Chest. 2012;142(5):1193–1199. doi: 10.1378/chest.12-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belkin RA, et al. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173(6):659–666. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dreyfuss D, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 128.Corbridge TC, et al. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis. 1990;142(2):311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 129.Amato MB, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 130.ARDSnet Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 131.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill—too little of a good thing? Lancet. 1999;354(9186):1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 132.Curley GF, Laffey JG, Kavanagh BP. CrossTalk proposal: there is added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol. 2013;591(Pt 11):2763–2765. doi: 10.1113/jphysiol.2013.252601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Curley GF, Laffey JG, Kavanagh BP. Rebuttal from Gerard F. Curley, John G. Laffey and Brian P. Kavanagh. J Physiol. 2013;591(Pt 11):2771–2772. doi: 10.1113/jphysiol.2013.255638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beitler JR, Hubmayr RD, Malhotra A. CrossTalk opposing view: there is not added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol. 2013;591(Pt 11):2767–2769. doi: 10.1113/jphysiol.2013.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beitler JR, Hubmayr RD, Malhotra A. Rebuttal from Jeremy R. Beitler, Rolf D. Hubmayr and Atul Malhotra. J Physiol. 2013;591(Pt 11):2773. doi: 10.1113/jphysiol.2013.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]