Abstract
Aryl hydrocarbon receptor (AhR) plays an important role in the regulation of cell responses to different environmental stimuli, as well as to various endogenous ligands. Although AhR was previously implicated in the regulation of dendritic cell (DC) activation, very little is known about its potential role in the development of these cells. Here, we report our unexpected findings that AhR may regulate the differentiation of plasmacytoid DCs (pDCs). Agonist of AhR dramatically decreased the generation of pDCs in vitro, while the AhR antagonist had an opposite effect. The differentiation of conventional DCs (cDCs) was not affected. AhR knockout mice had a substantial accumulation of pDCs in peripheral lymphoid organs; whereas, no changes in cDCs were seen. Thus, this study has identified AhR as a transcription factor involved in the development of one population of DCs - pDCs.
Keywords: plasmacytoid dendritic cells, aryl hydrocarbon receptor
Introduction
Dendritic cells (DCs) are critically important for the development of immune responses to various stimuli. Several major subsets of DCs are currently recognized: conventional (cDCs) and plasmacytoid DC (pDC). These subsets although share some common progenitors, differ in many biological characteristics. In mice, pDCs are distinguished from cDCs by their expression of B220, Siglec-H and Bst21. Several transcriptional factors including E2-2, Id2, Spi-B, and Notch were previously implicated in the regulation of pDC differentiation 2, 3.
During the analysis of Notch signaling in HPC, we serendipitously found a link with nuclear receptor aryl hydrocarbon (AhR). AhR, a basic helix-loop-helix (bHLH) transcription factor, is a highly conserved protein, which is constitutively expressed in many tissues4. AhR functions as a sensor for various xenobiotics (dioxin, aromatic hydrocarbons, etc.)5. Endogenous AhR ligands including indole-3-acetic acid, prostaglandins, bilirubin, as well as ligand-independent AhR activation were reported 5. AhR is present in cytoplasm in an inactive form, bound to the heat shock protein 90 (Hsp90), immunophilin-like XAP2, and p23. After binding to ligands, AhR translocates into the nucleus; where it dissociates from the Hsp90 and forms heterodimers with the AhR nuclear transporter. Together with co-activators, this dimer binds to the xenobiotic response elements (XREs) to regulate the transcription of the target genes.
Recently, the role of AhR in the function of immune system was demonstrated 6-9. The activation of AhR increased DC activation and induced DCs with tolerogenic characteristics10-13. However, other studies showed that AhR activation lead to DC activation 14 and AhR deficiency impairs the Langerhans cells' maturation4. Here, we report our novel unexpected findings that AhR can be involved in the specific regulation of pDC but not cDCs differentiation.
Results and Discussion
Lack of AhR promotes pDC development in vivo
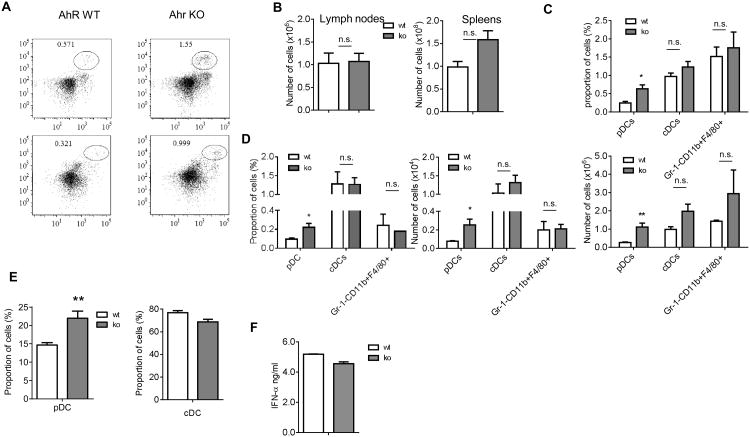
To investigate the possible role of AhR in DC differentiation, we first used AhR KO mice. pDCs were defined as CD11c+CD11b-SiglecH+, CD11c+CD11b-B220+, or CD11c+CD11b-Bst2+Ly6c+ cells (Fig. 1A and data not shown). cDCs were defined as CD11chighCD11b+I-Ab+ cells 1, 15. Gating strategy is shown in Fig. S1. The absence of AhR did not significantly affect the total number of lymph node (LN) cells or splenocytes (Fig. 1B). The proportion and absolute number of pDCs, in spleens of AhR KO, mice was higher than in wild-type (WT) mice. In contrast, no differences were found in the presence of cDCs and Gr-1-CD11b+F4/80+ macrophages (Fig. 1C). Similar results were obtained during the analysis of DC populations in LNs (Fig. 1D). AhR could affect HPC or regulate pDC development via intrinsic mechanisms or by affecting bone marrow (BM) microenvironment. To address this question, enriched HPCs, obtained from the BM of WT and AhR KO mice, were cultured for 9 days with Flt3-L, which supports the differentiation of both cDCs and pDCs. We observed a significantly higher proportion of pDC, generated from ahr-/- mice than from WT mice. No differences in the presence of cDC were found (Fig. 1E).
Figure 1. AhR KO mice show the selective accumulation of pDCs in peripheral lymphoid organs.
A. Typical example of staining the splenocytes from AhR KO and WT mice. The cells in the circle gate represent pDCs. B. The absolute number of cells in lymph nodes and spleens. Data are presented as Mean ± SEM (n=3 mice). C, D. The percentage and total cell number of different myeloid cells in spleens (C) and LNs (D) from AhR KO and control WT mice. Data are presented as Mean ± SEM (n=3 mice). *, p<0.05, **, p<0.01, compared to the WT mice. E. Enriched BM HPCs from AhR KO or WT mice were cultured with 100 ng/ml Flt3L for 9 days. The representative FACS plots from 3 independent experiments show the expression of B220 vs CD11c among live CD11b- cells. Data are presented as Mean ± SEM (left). **, p<0.01, compared to WT mice. F. 50,000 of Siglec H+ cells from 9 days' BM cultures of HPC with Flt3L were stimulated with 20ng/ml CpG ODN type A for 18 hr. Data show the production of IFN-α measured in ELISA. Data are presented as Mean ± SEM of three independent experiments.
To test their functional activity, pDCs, generated from AhR deficient HPCs, were isolated on day 9 and stimulated with CpG-A ODN. pDCs, generated from WT and AhR deficient HPCs, had an equally high level of IFN-α production (Fig. 1F); thus indicating that the population of cells, with the phenotype of pDCs accumulated in AhR deficient mice, demonstrated the functional characteristic of pDCs. These data suggested that AhR could negatively regulate the differentiation of the pDC subset of DCs without affecting cDCs.
Regulation of AhR in vitro affects pDC differentiation
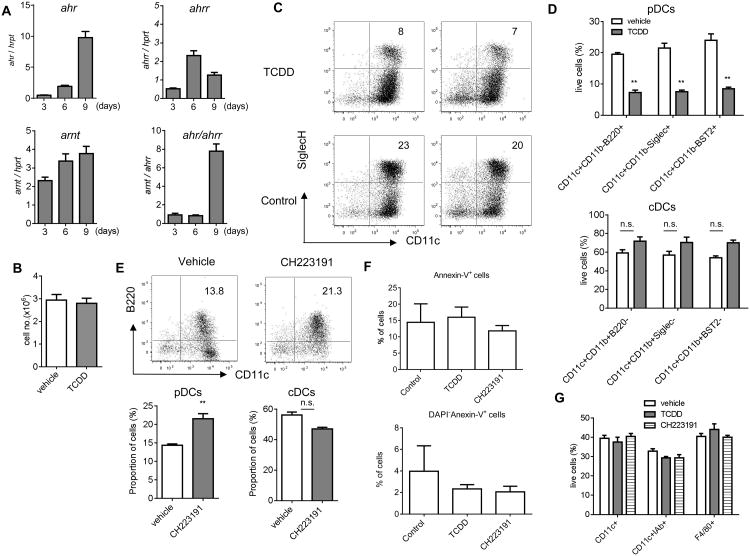
The expression of ahr was gradually increased during in vitro differentiation of DCs from bone marrow HPC in the presence of Flt3-L. The up-regulation of AhR repressor ahrr, was much smaller; and, as a result, the ahr/ahrr ratio increased substantially by day 9 (Fig. 2A). To investigate the role of AhR signaling in differentiation of pDCs further, we activated AhR with its specific ligand 2,3,7,8-tetracholrodibenzo-p-dioxin (TCDD)16 during 9-day HPC culture with FLT-3L. TCDD did not affect the total number of cells generated from HPC (Fig. 2B), but it caused a substantial decrease in the proportion of pDCs (Fig. 2C and D, top panel). In contrast, the presence of cDCs was not significantly changed (Fig. 2D, bottom panel). We used AhR antagonist CH22319117. A significant increase of pDCs was observed; whereas, no effect on cDCs was found (Fig. 2E). Side-by-side experiments with TCDD and CH223191 using the same cell markers confirmed these observations (Fig. S2). TCDD and CH223191 at selected concentration did not induce apoptosis of pDCs (Fig. 2F) or cDCs (data not shown) generated in vitro. To clarify the effect of AhR agonist on cDC development, we differentiated DCs from HPCs in the presence of GM-CSF. Under these conditions, only cDCs are generated. TCDD had no effect on cDC differentiation (Fig. 2G). Thus, it appears that the activation of AhR had an inhibitory effect on pDC differentiation in vitro, but did not affect cDCs.
Figure 2. AhR negatively regulates pDC development in vitro.
A. The relative mRNA levels of ahr, ahrr, arnt in HPC culture with Flt3L on days 3, 6, and 9. The ratio of ahr to ahrr was present. Data are presented as Mean ± SEM of three independent experiments. B-D. HPCs were cultured with 100 ng/ml Flt3L for 9 days in the presence of 10nM TCDD or vehicle. B. The total number of cells generated from HPC; C. Typical FACS plots of cells gated on CD11b- cells from three independent experiments. The percentage of pDCs from the all live cells is shown. D. The proportions of pDCs (top panel) and cDCs (bottom panel) using different combination of antibodies. Data are presented as Mean ± SEM (n=3 replicates). E. HPCs were cultured with Flt3L in the presence of 1μM CH223191 or vehicle for 9 days. Left panel - representative FACS plots of cells gated on CD11b- cells from 3 independent experiments. The percentage of pDCs from all live cells is shown. Right panel – proportion of cells. Data are presented as Mean ± SEM. F. Apoptosis was measured using staining with DAPI and Annexin-V within the population of pDCs generated from BM progenitors in the presence of TCDD and CH223191 as described in Fig. 2D,E. Mean and SEM are shown (n=3). G. HPCs cultured with 20ng/ml GM-CSF for 5 days in the presence of 10nM TCDD, 1μM CH223191, or vehicle. Data are presented as Mean ± SEM from 3 independent experiments.
Thus, this is a first report demonstrating a possible role of AhR in the regulation of pDC development. Although AhR was previously implicated in the regulation of DC activation, very little is known about its potential role in the differentiation of these cells. Previous study suggested a re-distribution of DCs after the treatment of mice with TCDD18. Our data demonstrated that AhR may selectively inhibit differentiation of pDCs but have no effect on cDC differentiation. The mechanism of this effect is not clear and need further investigation.
Methods
Mice
All mouse experiments were approved by the University of South Florida Institutional Animal Care and Use Committee. Female C57BL/6 mice (age 6–8 weeks) were obtained from the National Cancer Institute; C57BL/6-Ahrtm1.1(AHR)Arte mice from Taconic (Hudson, NY). All mice were housed in pathogen-free units of the vivarium in University of South Florida.
Regents
F4/80 (Cl:A3-1) antibody, were purchased from Serotec (Raleigh, NC); CD11c (N418), Bst2 (129c), and Siglec H (eBio440c) from Ebioscience (San Diego, CA); and antiphycoerythrin (anti-PE) microbeads from Miltenyi Biotec (Auburn, CA). All other antibodies were purchased from BD (Franklin Lakes, NJ). The recombinant GM-CSF and IL-4 are from Research Diagnostics (Flanders, NJ), LPS from Sigma (St Louis, MO), Flt3-ligand (Flt3-L) from R&D systems (Minneapolis, MN), CpG ODN type A (1585) and CpG ODN type B (1668) from Invivogen (San Diego, CA). TCDD and CH 223191 were purchased from Cambridge Isotope Laboratories Inc. (Andover, MA) and TOCRIS (Minneapolis, MN), respectively.
Generation of DCs and ELISA
HPCs were enriched with a lineage depletion kit (Miltenyi) and cultured with 100 ng/ml Flt3-L for 9 days, or with 20 ng/ml GM-CSF for 5 days. pDCs were purified with siglec-H-PE antibody, followed by anti-PE microbeads (Miltenyi) (purity>95%). 50,000 cells were stimulated with 20ng/ml CpG-A ODN 16 hr. IFN-α was measured in supernatants using mouse IFN-α ELISA kit (Ebioscience).
Real-time PCR
mRNA was extracted with a Qiagen RNeasy kit (Valencia, CA) and reverse-transcribed into cDNA with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems; Grand Island, NY). qRT-PCR were performed with SYBR®green using the following primers: ahr (forward: 5′-AGG GCC AAG AGC TTC TTT GAT-3′; reverse:5′- CTGGTCCTGGCCTCCATTT-3′); arnt (forward: 5′-AAC CAG ACA AGC TAA CCA TCT TAC G-3′; reverse: 5′-ATC AAA TGT TTC AGT TCC TGA TCA GT-3′); ahrr (forward: 5′-TCCCCGTGCAGGAAGGA-3′; reverse:5′- TGC CGA TGC ATA AAA GAT CAT C-3′); hprt severed as an interior control (forward: 5′-TTC CTC GAG ATG TGA TGA AGG A-3′; and reverse: 5′-CCA GCA GGT CAG CAA AGA ATT-3′).
Statistical methods
The data were analyzed with a two-tailed Student t-test. P values < 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
This study was supported by NIH grant CA CA141438 to DIG
Footnotes
Authors declare no conflicts of interest. H.L. – performed experiments, analyzed the results and wrote the paper; I.R. – performed experiments; D.I.G. – designed the experiments, analyzed the results and wrote the paper.
References
- 1.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–54. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–16. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–17. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 5.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proceedings of the Japan Academy; 2010; pp. 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 8.Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100:143–8. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34:2907–18. doi: 10.1002/eji.200425071. [DOI] [PubMed] [Google Scholar]
- 10.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 11.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. 2007;178:778–87. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz J, Carson WE., 3rd Review of S100A9 biology and its role in cancer. Biochim Biophys Acta. 2013;1835:100–9. doi: 10.1016/j.bbcan.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaraj S, Youn J, Gabrilovich D. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191:17–23. doi: 10.4049/jimmunol.1300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava MK, Andersson A, Zhu L, Harris-White M, Lee JM, Dubinett S, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy. 2012;4:291–304. doi: 10.2217/imt.11.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–44. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 16.Talmadge JE, Gabrilovich DI. History of myeloid derived suppressor cells (MDSCs) in the macro- and micro-environment of tumour-bearing hosts. Nat Rev Cancer. 2013 doi: 10.1038/nrc3581. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–24. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 18.Bankoti J, Burnett A, Navarro S, Miller AK, Rase B, Shepherd DM. Effects of TCDD on the fate of naive dendritic cells. Toxicol Sci. 2010;115:422–34. doi: 10.1093/toxsci/kfq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.