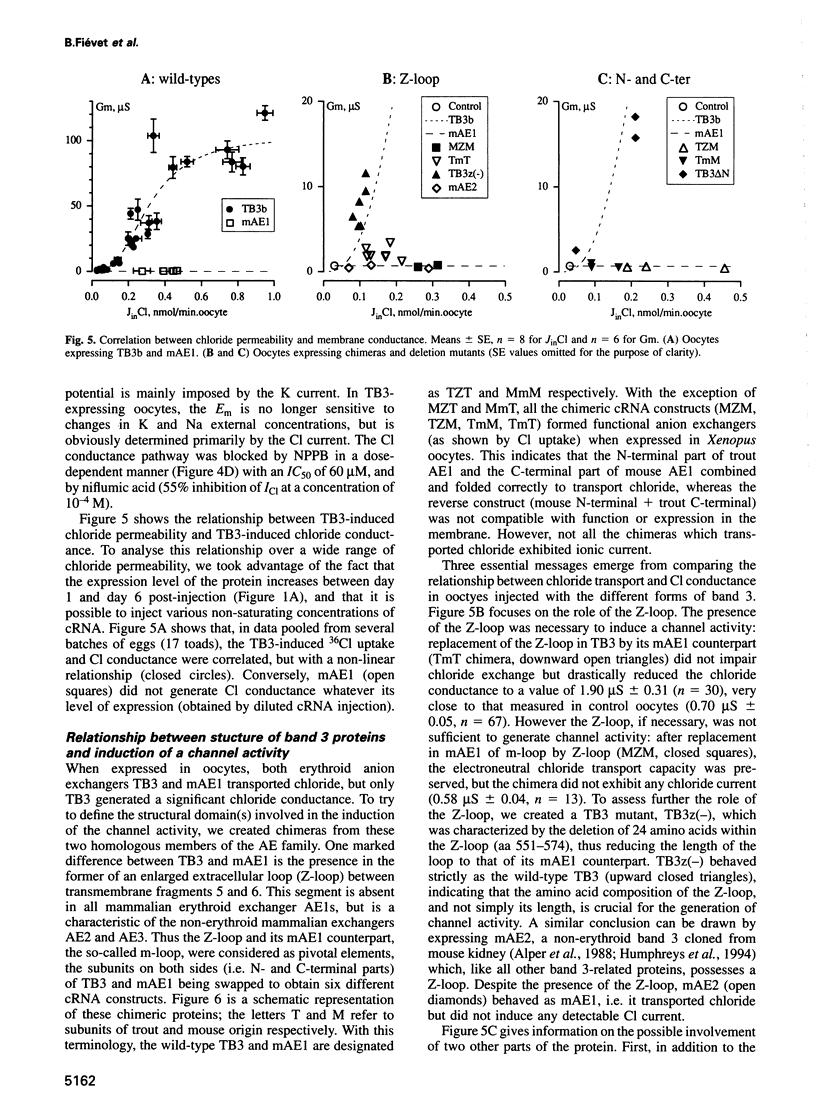
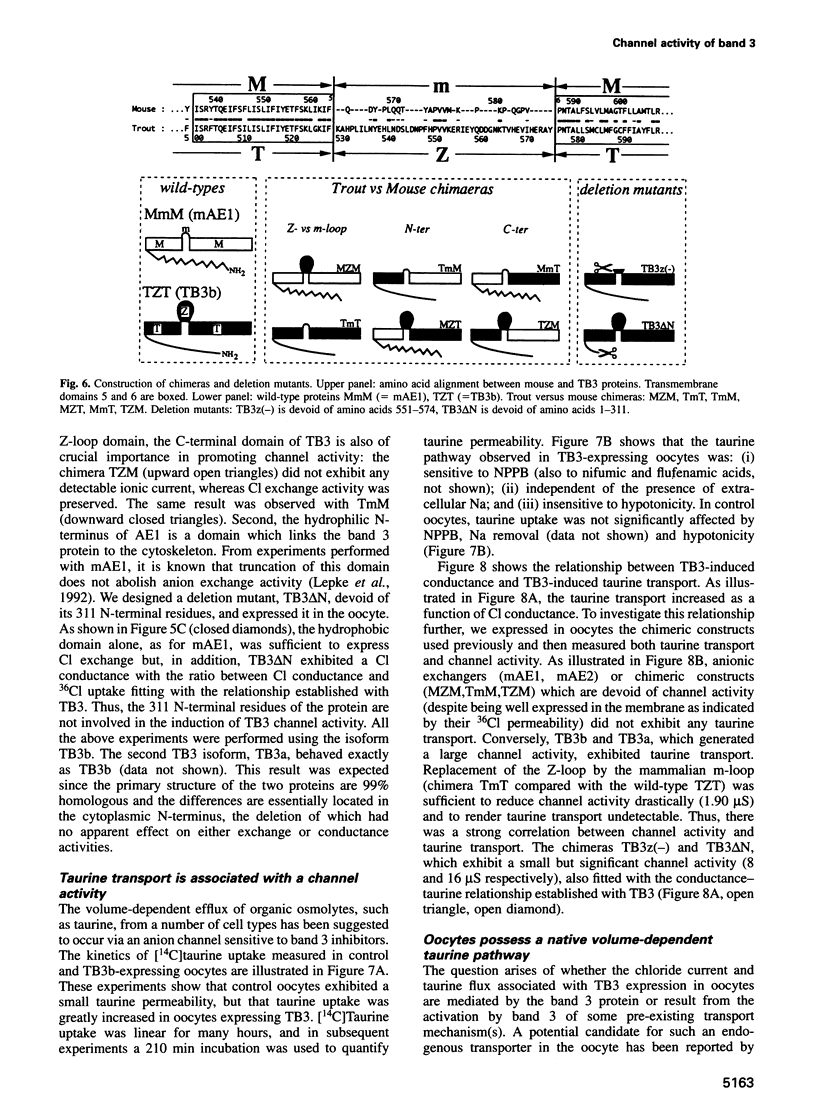
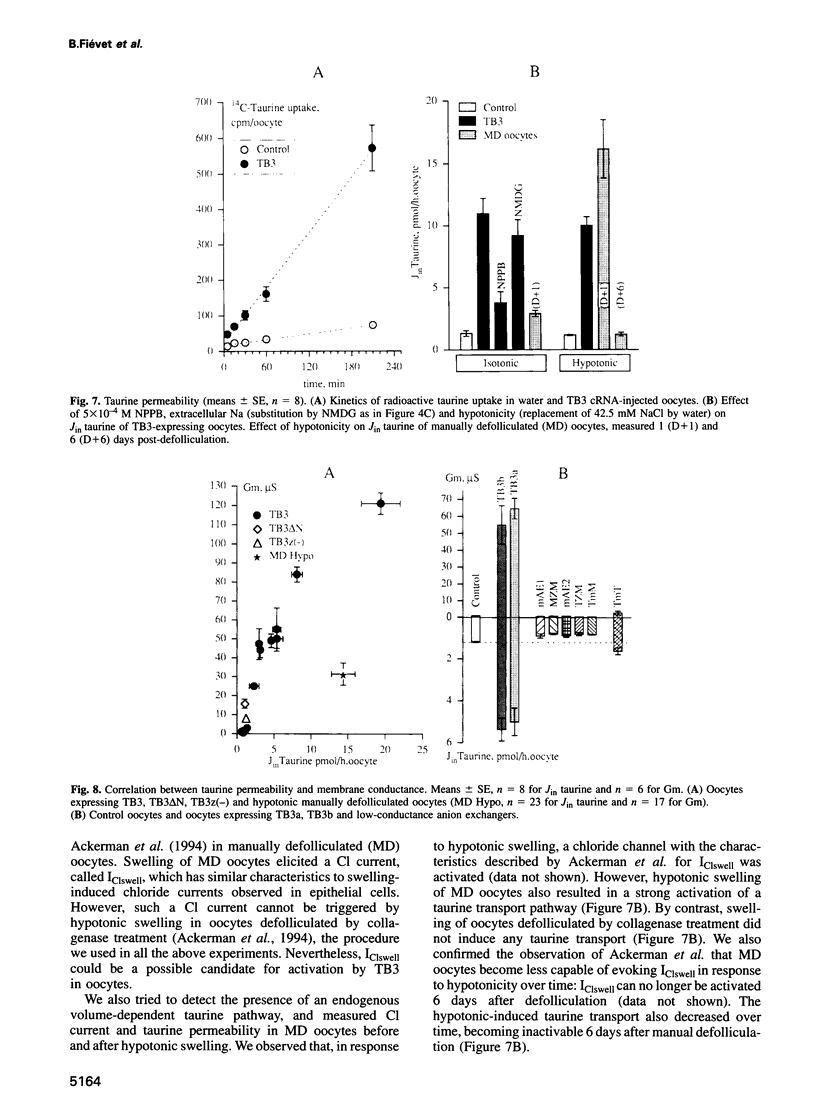
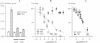Abstract
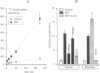
Most, but not all, cell types release intracellular organic solutes (e.g. taurine) in response to cell swelling to achieve cell volume regulation. Although this efflux is blocked by classical inhibitors of the electroneutral anion exchanger band 3 (AE1), it is thought to involve an anion channel. The role of band 3 in volume-dependent taurine transport was determined by expressing, in Xenopus oocytes, band 3 from erythrocytes which do (trout) or do not (mouse) release taurine when swollen. AE1 of both species elicited anion exchange activity, but only trout band 3 showed chloride channel activity and taurine transport. Chimeras constructed from trout and mouse band 3 allowed the identification of some protein domains critically associated with channel activity and taurine transport. The data provide evidence that swelling-induced taurine movements occur via an anion channel which is dependent on, or controlled by, band 3. They suggest the involvement of proteins of the band 3 (AE) family in cell volume regulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S. L., Kopito R. R., Libresco S. M., Lodish H. F. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem. 1988 Nov 15;263(32):17092–17099. [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992 Apr;262(4 Pt 1):C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugelli K., Thoroed S. M. Taurine transport associated with cell volume regulation in flounder erythrocytes under anisosmotic conditions. J Physiol. 1986 May;374:245–261. doi: 10.1113/jphysiol.1986.sp016077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu F., Cossins A. R., Motais R. Cell volume regulation by trout erythrocytes: characteristics of the transport systems activated by hypotonic swelling. J Physiol. 1991;440:547–567. doi: 10.1113/jphysiol.1991.sp018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. M., Lodish H. F. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J Biol Chem. 1989 Nov 25;264(33):19607–19613. [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Brill S. R. Volume-activated taurine efflux from skate erythrocytes: possible band 3 involvement. Am J Physiol. 1991 May;260(5 Pt 2):R1014–R1020. doi: 10.1152/ajpregu.1991.260.5.R1014. [DOI] [PubMed] [Google Scholar]
- Hardy S. P., Goodfellow H. R., Valverde M. A., Gill D. R., Sepúlveda V., Higgins C. F. Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels. EMBO J. 1995 Jan 3;14(1):68–75. doi: 10.1002/j.1460-2075.1995.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. Volume-activated chloride currents associated with the multidrug resistance P-glycoprotein. J Physiol. 1995 Jan;482:31S–36S. doi: 10.1113/jphysiol.1995.sp020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys B. D., Jiang L., Chernova M. N., Alper S. L. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol. 1994 Nov;267(5 Pt 1):C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- Huxtable R. J. Physiological actions of taurine. Physiol Rev. 1992 Jan;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Hübner S., Michel F., Rudloff V., Appelhans H. Amino acid sequence of band-3 protein from rainbow trout erythrocytes derived from cDNA. Biochem J. 1992 Jul 1;285(Pt 1):17–23. doi: 10.1042/bj2850017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. S., Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993 Dec;265(6 Pt 1):C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990 Dec 6;348(6301):510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Kirk K., Ellory J. C., Young J. D. Transport of organic substrates via a volume-activated channel. J Biol Chem. 1992 Nov 25;267(33):23475–23478. [PubMed] [Google Scholar]
- Kirk K., Kirk J. Volume-regulatory taurine release from a human lung cancer cell line. Evidence for amino acid transport via a volume-activated chloride channel. FEBS Lett. 1993 Dec 20;336(1):153–158. doi: 10.1016/0014-5793(93)81630-i. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Fuhrmann G. F., Rothstein S., Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977 Mar;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G. B., Ackerman M. J., Gordon E. A., Krapivinsky L. D., Clapham D. E. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994 Feb 11;76(3):439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K. Regulation of taurine transport in Ehrlich ascites tumor cells. J Membr Biol. 1993 Jan;131(1):67–79. doi: 10.1007/BF02258535. [DOI] [PubMed] [Google Scholar]
- Lepke S., Becker A., Passow H. Mediation of inorganic anion transport by the hydrophobic domain of mouse erythroid band 3 protein expressed in oocytes of Xenopus laevis. Biochim Biophys Acta. 1992 Apr 29;1106(1):13–16. doi: 10.1016/0005-2736(92)90215-8. [DOI] [PubMed] [Google Scholar]
- Motais R., Guizouarn H., Garcia-Romeu F. Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochim Biophys Acta. 1991 Oct 10;1075(2):169–180. doi: 10.1016/0304-4165(91)90248-f. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Davis E. M., Goldstein L. Oligomeric forms of skate erythrocyte band 3. Effect of volume expansion. J Biol Chem. 1994 Aug 5;269(31):19683–19686. [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]