Abstract
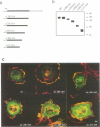
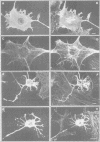
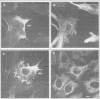
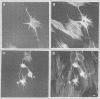
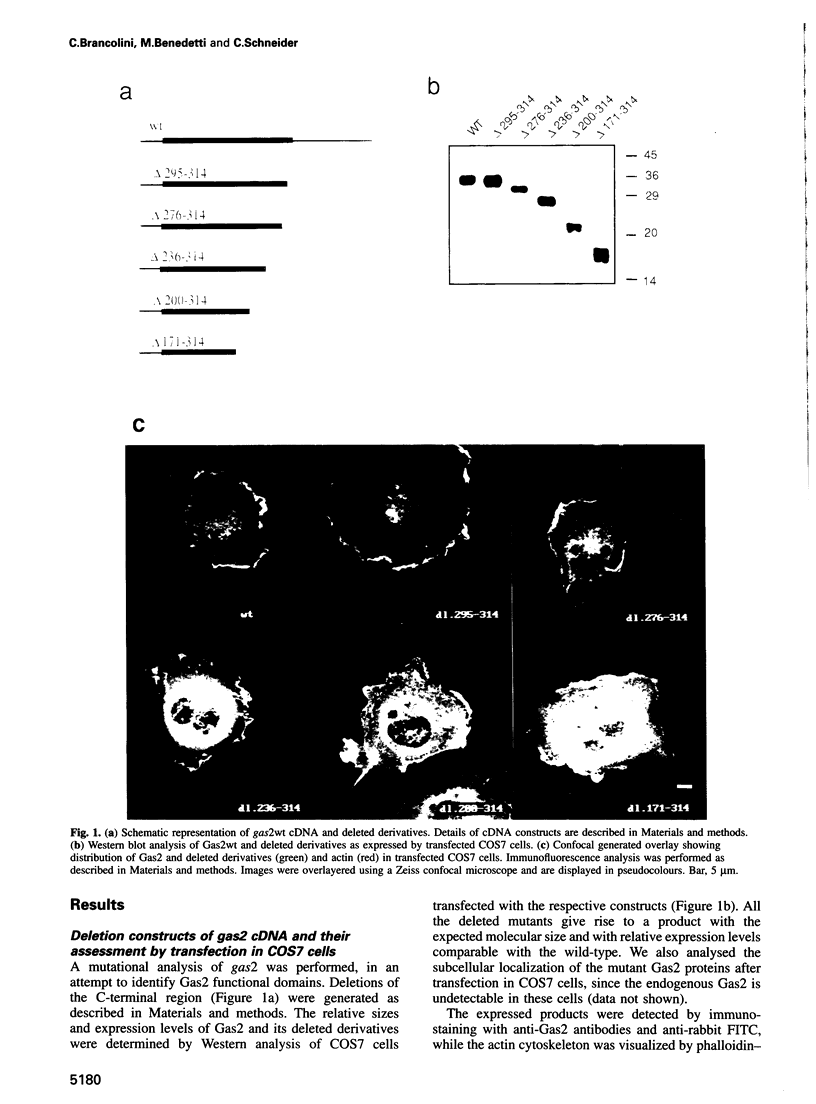
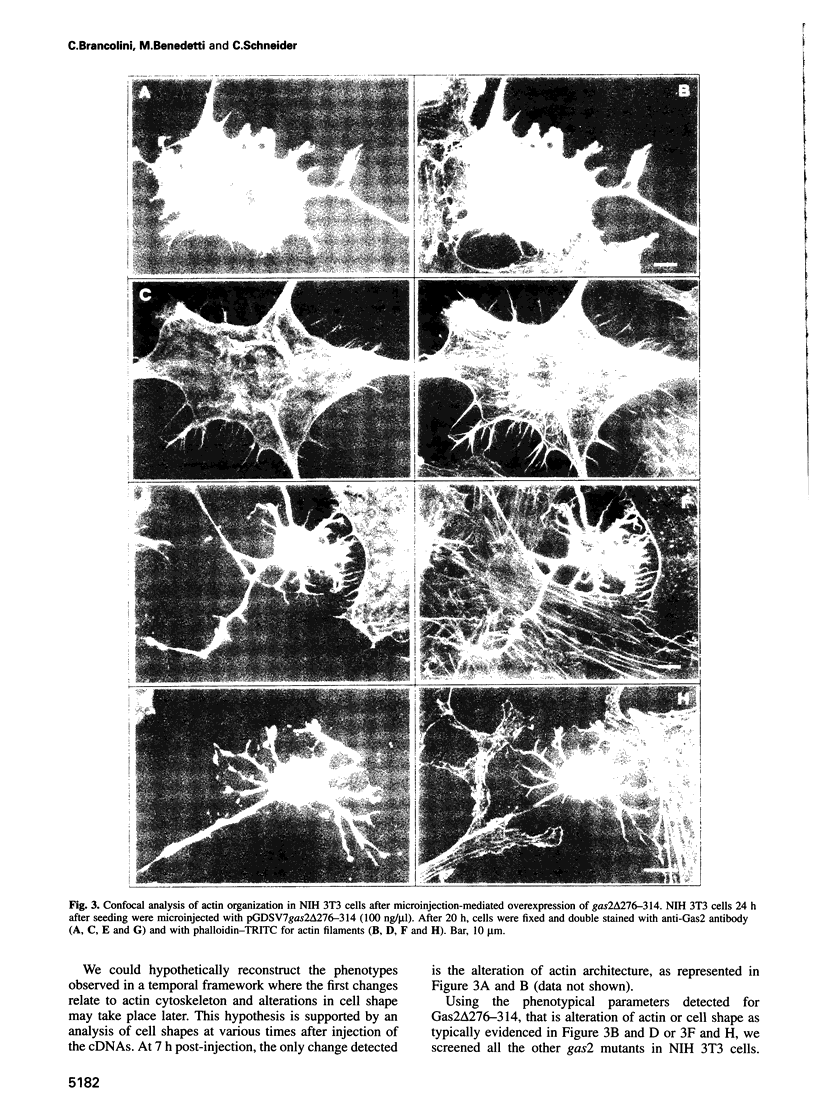
Gas2, a component of the microfilament system, belongs to the class of gas genes whose expression is induced at growth arrest. After serum or growth factor addition to quiescent NIH 3T3 cells, Gas2 is hyperphosphorylated and relocalized at the membrane ruffles. By overexpressing gas2wt and a series of deletion mutants of the C-terminal region, we have analysed its role in the organization of the actin cytoskeleton in different cell lines. Overexpression of Gas2 deleted at its C-terminal region (delta 276-314 and delta 236-314), but not its wild-type form, induces dramatic changes in the actin cytoskeleton and cell morphology. These effects are not due to interference of the deleted forms with the endogenous Gas2wt function but could be ascribed to a gain of function. We demonstrate that during apoptosis the C-terminal domain of Gas2 is removed by proteolytic cleavage, resulting in a protein that is similar in size to the described delta 276-314. Moreover, by using in vitro mutagenesis, we also demonstrate that the proteolytic processing of Gas2 during apoptosis is dependent on an aspartic acid residue at position 279. The evidence accumulated here could thus represent a first example of a mechanism linking apoptosis with the co-ordinated microfilament-dependent cell shape changes, as possibly mediated by an interleukin-1 beta-converting enzyme (ICE)-like dependent proteolytic cleavage of the Gas2 protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin. Trends Biochem Sci. 1992 Oct;17(10):438–443. doi: 10.1016/0968-0004(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Arends M. J., McGregor A. H., Toft N. J., Brown E. J., Wyllie A. H. Susceptibility to apoptosis is differentially regulated by c-myc and mutated Ha-ras oncogenes and is associated with endonuclease availability. Br J Cancer. 1993 Dec;68(6):1127–1133. doi: 10.1038/bjc.1993.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Armstrong D. K., Isaacs J. T., Ottaviano Y. L., Davidson N. E. Programmed cell death in an estrogen-independent human breast cancer cell line, MDA-MB-468. Cancer Res. 1992 Jun 15;52(12):3418–3424. [PubMed] [Google Scholar]
- Berges R. R., Furuya Y., Remington L., English H. F., Jacks T., Isaacs J. T. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8910–8914. doi: 10.1073/pnas.90.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Bottega S., Schneider C. Gas2, a growth arrest-specific protein, is a component of the microfilament network system. J Cell Biol. 1992 Jun;117(6):1251–1261. doi: 10.1083/jcb.117.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Schneider C. Phosphorylation of the growth arrest-specific protein Gas2 is coupled to actin rearrangements during Go-->G1 transition in NIH 3T3 cells. J Cell Biol. 1994 Mar;124(5):743–756. doi: 10.1083/jcb.124.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. F., Herget T., Broad S., Rozengurt E. The expression of 80K/MARCKS, a major substrate of protein kinase C (PKC), is down-regulated through both PKC-dependent and -independent pathways. Effects of bombesin, platelet-derived growth factor, and cAMP. J Biol Chem. 1992 Jul 15;267(20):14212–14218. [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Miller D. K., Anhalt G. J., Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994 Dec 9;269(49):30757–30760. [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. C., Stossel T. P., Kwiatkowski D. J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991 Mar 8;251(4998):1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Gustincich S., Ruaro E., Schneider C. New lambda and plasmid vectors for expression cloning in mammalian cells. Biotechniques. 1994 Jan;16(1):134–138. [PubMed] [Google Scholar]
- Del Sal G., Ruaro M. E., Philipson L., Schneider C. The growth arrest-specific gene, gas1, is involved in growth suppression. Cell. 1992 Aug 21;70(4):595–607. doi: 10.1016/0092-8674(92)90429-g. [DOI] [PubMed] [Google Scholar]
- Fabbretti E., Edomi P., Brancolini C., Schneider C. Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A. Genes Dev. 1995 Aug 1;9(15):1846–1856. doi: 10.1101/gad.9.15.1846. [DOI] [PubMed] [Google Scholar]
- Faucheu C., Diu A., Chan A. W., Blanchet A. M., Miossec C., Hervé F., Collard-Dutilleul V., Gu Y., Aldape R. A., Lippke J. A. A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 1995 May 1;14(9):1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Buckley K. M. The synaptic vesicle protein synaptotagmin promotes formation of filopodia in fibroblasts. Nature. 1993 Aug 5;364(6437):537–540. doi: 10.1038/364537a0. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994 Dec 9;269(49):30761–30764. [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Friederich E., Huet C., Arpin M., Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989 Nov 3;59(3):461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Füchtbauer A., Jockusch B. M., Maruta H., Kilimann M. W., Isenberg G. Disruption of microfilament organization after injection of F-actin capping proteins into living tissue culture cells. 1983 Jul 28-Aug 3Nature. 304(5924):361–364. doi: 10.1038/304361a0. [DOI] [PubMed] [Google Scholar]
- Gagliardini V., Fernandez P. A., Lee R. K., Drexler H. C., Rotello R. J., Fishman M. C., Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994 Feb 11;263(5148):826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- Harrington E. A., Bennett M. R., Fanidi A., Evan G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994 Jul 15;13(14):3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Kwiatkowski D. J. Actin-binding proteins. Curr Opin Cell Biol. 1991 Feb;3(1):87–97. doi: 10.1016/0955-0674(91)90170-4. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Herget T., Brooks S. F., Broad S., Rozengurt E. Expression of the major protein kinase C substrate, the acidic 80-kilodalton myristoylated alanine-rich C kinase substrate, increases sharply when Swiss 3T3 cells move out of cycle and enter G0. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2945–2949. doi: 10.1073/pnas.90.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. D., Kostura M. J., Thornberry N., Ding G. J., Limjuco G., Weidner J., Salley J. P., Hogquist K. A., Chaplin D. D., Mumford R. A. IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J Immunol. 1991 Nov 1;147(9):2964–2969. [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1995 May;20(5):198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kinoshita M., Noda M., Copeland N. G., Jenkins N. A. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994 Jul 15;8(14):1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y. A., Kaufmann S. H., Desnoyers S., Poirier G. G., Earnshaw W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994 Sep 22;371(6495):346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb 10;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R., Cotter T. G. Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci. 1994 Jan;19(1):26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Clarke M. F. The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol. 1994 Nov;4(11):399–403. doi: 10.1016/0962-8924(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Schneider C., Philipson L., Ansorge W. Single cell assay with an automated capillary microinjection system. Exp Cell Res. 1988 Oct;178(2):369–376. doi: 10.1016/0014-4827(88)90406-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Membrane ruffling and signal transduction. Bioessays. 1994 May;16(5):321–327. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Sleath P. R., Hendrickson R. C., Kronheim S. R., March C. J., Black R. A. Substrate specificity of the protease that processes human interleukin-1 beta. J Biol Chem. 1990 Aug 25;265(24):14526–14528. [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T. Activation of signal transduction pathways protects quiescent Balb/c-3T3 fibroblasts against death due to serum deprivation. J Cell Physiol. 1991 Jul;148(1):85–95. doi: 10.1002/jcp.1041480111. [DOI] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Thulasi R., Harbour D. V., Thompson E. B. Suppression of c-myc is a critical step in glucocorticoid-induced human leukemic cell lysis. J Biol Chem. 1993 Aug 25;268(24):18306–18312. [PubMed] [Google Scholar]
- Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994 Sep 9;78(5):739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Widmer F., Caroni P. Phosphorylation-site mutagenesis of the growth-associated protein GAP-43 modulates its effects on cell spreading and morphology. J Cell Biol. 1993 Jan;120(2):503–512. doi: 10.1083/jcb.120.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. The genetic regulation of apoptosis. Curr Opin Genet Dev. 1995 Feb;5(1):97–104. doi: 10.1016/s0959-437x(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]