Abstract
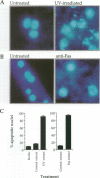
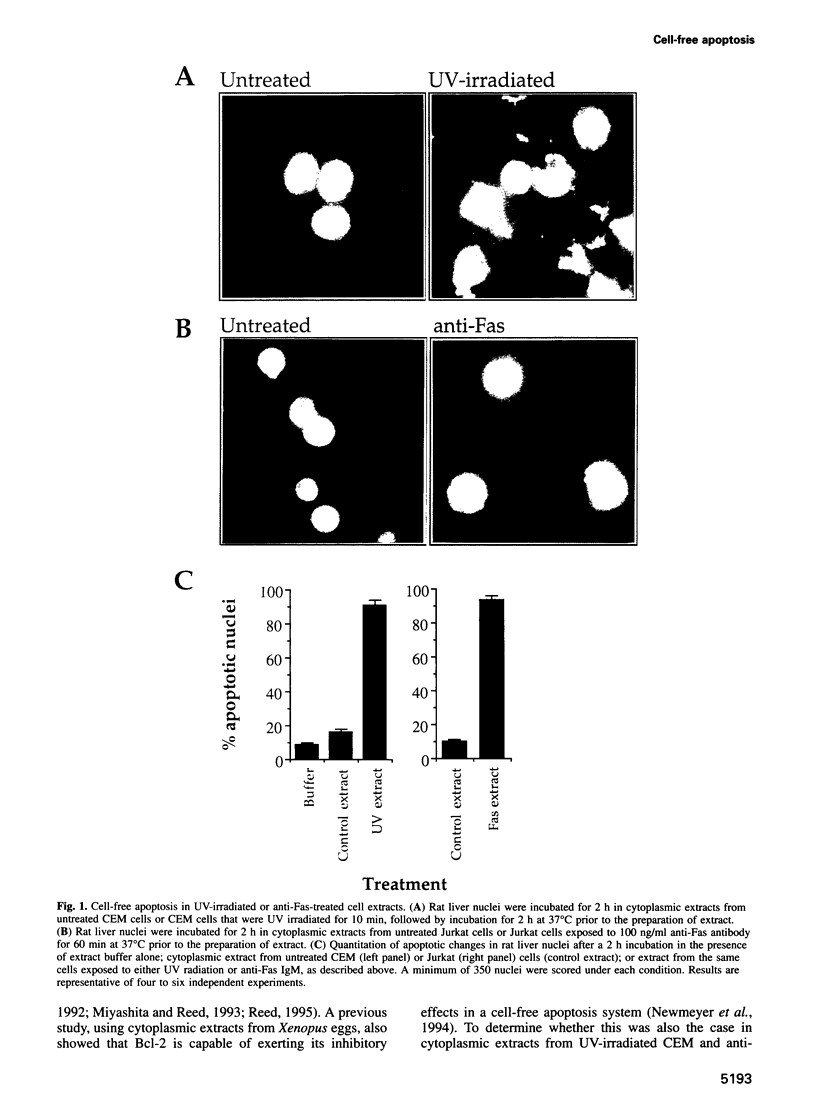
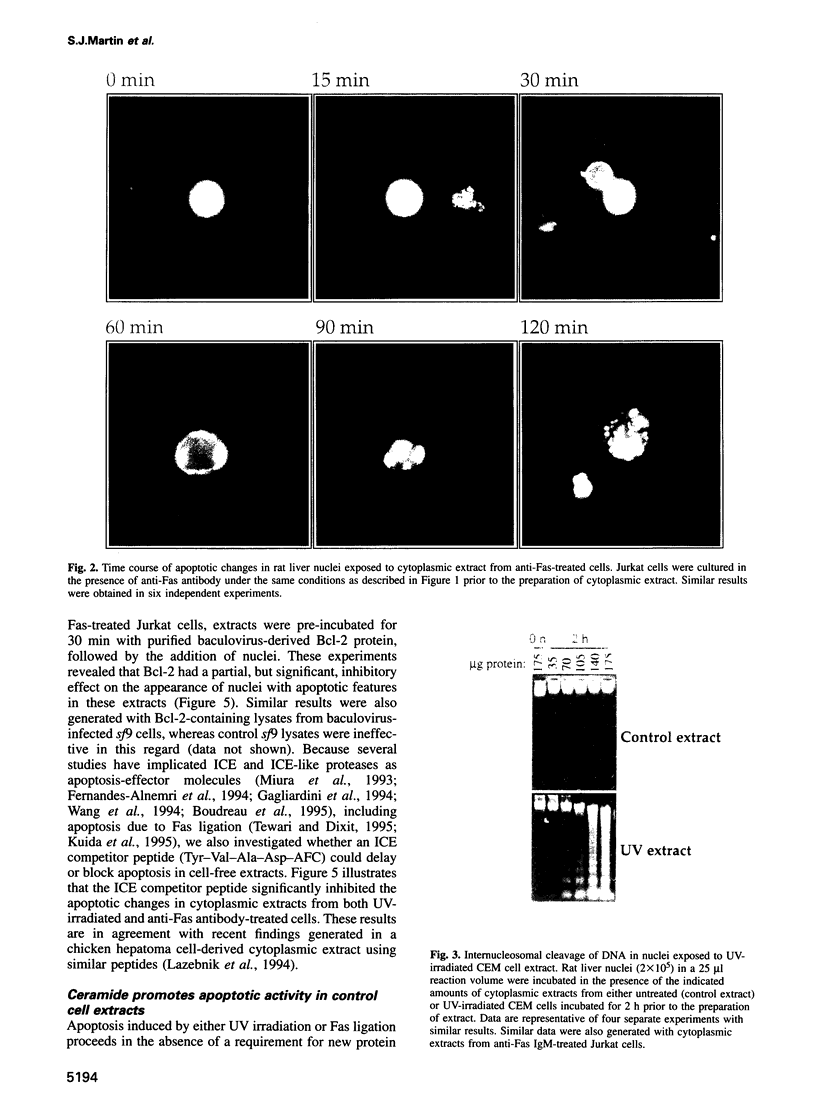
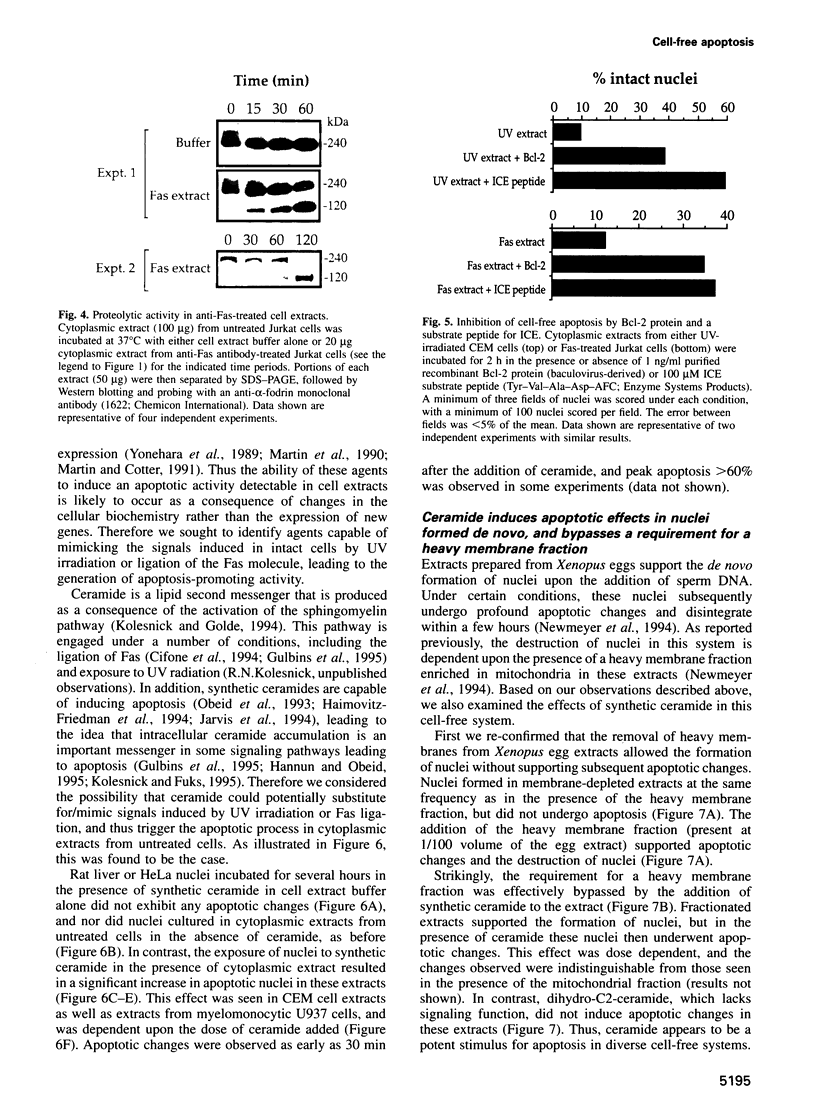
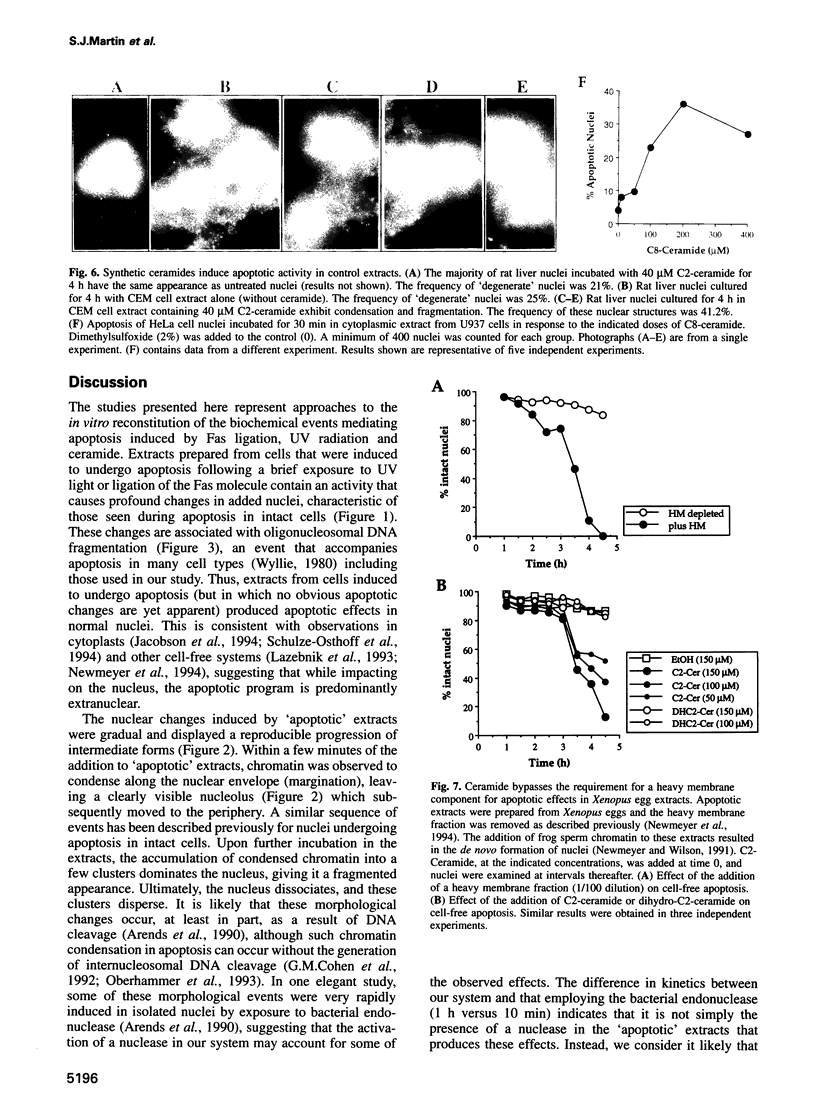
Cell-free systems are valuable tools for the dissection of complex cellular processes. Here we show that cytoplasmic extracts from cells exposed to anti-Fas antibody or UV radiation contain an activity capable of reproducing morphological changes typical of apoptosis in nuclei added to these extracts, as well as internucleosomal cleavage of DNA and proteolysis of a protein known to be cleaved during the apoptosis of intact cells. Extracts from control cell populations were inactive in this respect. These effects were partly blocked by the addition of purified Bcl-2 protein or a competitive inhibitor peptide of interleukin-1 beta-converting enzyme to the extracts. Furthermore, apoptotic activity was induced in cytoplasmic extracts from untreated cells by the addition of ceramide, a lipid second messenger implicated recently in apoptosis signaling. These extracts should prove highly useful in the dissection of molecular events that occur during apoptosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bedi A., Zehnbauer B. A., Barber J. P., Sharkis S. J., Jones R. J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994 Apr 15;83(8):2038–2044. [PubMed] [Google Scholar]
- Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995 Aug 11;82(3):405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Sympson C. J., Werb Z., Bissell M. J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995 Feb 10;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Miller D. K., Anhalt G. J., Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994 Dec 9;269(49):30757–30760. [PubMed] [Google Scholar]
- Cifone M. G., De Maria R., Roncaioli P., Rippo M. R., Azuma M., Lanier L. L., Santoni A., Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994 Oct 1;180(4):1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Clem R. J., Miller L. K. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J Virol. 1993 Jul;67(7):3730–3738. doi: 10.1128/jvi.67.7.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Hannun Y. A. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992 Mar 15;267(8):5048–5051. [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Evans C. A., Owen-Lynch P. J., Whetton A. D., Dive C. Activation of the Abelson tyrosine kinase activity is associated with suppression of apoptosis in hemopoietic cells. Cancer Res. 1993 Apr 15;53(8):1735–1738. [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994 Dec 9;269(49):30761–30764. [PubMed] [Google Scholar]
- Gagliardini V., Fernandez P. A., Lee R. K., Drexler H. C., Rotello R. J., Fishman M. C., Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994 Feb 11;263(5148):826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Bissonnette R., Mahboubi A., Martin S., Nishioka W., Brunner T., Baier G., Baier-Bitterlich G., Byrd C., Lang F. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995 Apr;2(4):341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Baier G., Telford D., Langlet C., Baier-Bitterlich G., Bonnefoy-Berard N., Burn P., Wittinghofer A., Altman A. Direct stimulation of Vav guanine nucleotide exchange activity for Ras by phorbol esters and diglycerides. Mol Cell Biol. 1994 Jul;14(7):4749–4758. doi: 10.1128/mcb.14.7.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., Raff M. C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994 Apr 15;13(8):1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph C. K., Byun H. S., Bittman R., Kolesnick R. N. Substrate recognition by ceramide-activated protein kinase. Evidence that kinase activity is proline-directed. J Biol Chem. 1993 Sep 25;268(27):20002–20006. [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R., Fuks Z. Ceramide: a signal for apoptosis or mitogenesis? J Exp Med. 1995 Jun 1;181(6):1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2: a repressor of lymphocyte death. Immunol Today. 1992 Aug;13(8):285–288. doi: 10.1016/0167-5699(92)90037-8. [DOI] [PubMed] [Google Scholar]
- Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995 Mar 31;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Lazebnik Y. A., Cole S., Cooke C. A., Nelson W. G., Earnshaw W. C. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J Cell Biol. 1993 Oct;123(1):7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y. A., Kaufmann S. H., Desnoyers S., Poirier G. G., Earnshaw W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994 Sep 22;371(6495):346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb 10;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Cotter T. G. Ultraviolet B irradiation of human leukaemia HL-60 cells in vitro induces apoptosis. Int J Radiat Biol. 1991 Apr;59(4):1001–1016. doi: 10.1080/09553009114550891. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Apoptosis and cancer: the failure of controls on cell death and cell survival. Crit Rev Oncol Hematol. 1995 Feb;18(2):137–153. doi: 10.1016/1040-8428(94)00124-c. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R., Cotter T. G. Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci. 1994 Jan;19(1):26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995 Aug 11;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- Martin S. J., Matear P. M., Vyakarnam A. HIV-1 infection of human CD4+ T cells in vitro. Differential induction of apoptosis in these cells. J Immunol. 1994 Jan 1;152(1):330–342. [PubMed] [Google Scholar]
- Martin S. J., O'Brien G. A., Nishioka W. K., McGahon A. J., Mahboubi A., Saido T. C., Green D. R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995 Mar 24;270(12):6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- McGahon A., Bissonnette R., Schmitt M., Cotter K. M., Green D. R., Cotter T. G. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood. 1994 Mar 1;83(5):1179–1187. [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Newmeyer D. D., Farschon D. M., Reed J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994 Oct 21;79(2):353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Wilson K. L. Egg extracts for nuclear import and nuclear assembly reactions. Methods Cell Biol. 1991;36:607–634. doi: 10.1016/s0091-679x(08)60299-x. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C., Mannherz H. G., Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol. 1994 Feb;4(2):37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin A., Adams D. H., Henkart P. A. Protease inhibitors selectively block T cell receptor-triggered programmed cell death in a murine T cell hybridoma and activated peripheral T cells. J Exp Med. 1993 Nov 1;178(5):1693–1700. doi: 10.1084/jem.178.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Walczak H., Dröge W., Krammer P. H. Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol. 1994 Oct;127(1):15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Shi L., Kam C. M., Powers J. C., Aebersold R., Greenberg A. H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992 Dec 1;176(6):1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Squìer M. K., Miller A. C., Malkinson A. M., Cohen J. J. Calpain activation in apoptosis. J Cell Physiol. 1994 May;159(2):229–237. doi: 10.1002/jcp.1041590206. [DOI] [PubMed] [Google Scholar]
- Tewari M., Dixit V. M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995 Feb 17;270(7):3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Voelkel-Johnson C., Entingh A. J., Wold W. S., Gooding L. R., Laster S. M. Activation of intracellular proteases is an early event in TNF-induced apoptosis. J Immunol. 1995 Feb 15;154(4):1707–1716. [PubMed] [Google Scholar]
- Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994 Sep 9;78(5):739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Rose K. A., Morris R. G., Steel C. M., Foster E., Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: growth, metastasis and endogenous oncogene expression. Br J Cancer. 1987 Sep;56(3):251–259. doi: 10.1038/bjc.1987.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]