Abstract
Voltage-gated potassium (Kv) channels are widely expressed in the central and peripheral nervous system, and are crucial mediators of neuronal excitability. Importantly, these channels also actively participate in cellular and molecular signaling pathways that regulate the life and death of neurons. Injury-mediated increased K+ efflux through Kv2.1 channels promotes neuronal apoptosis, contributing to widespread neuronal loss in neurodegenerative disorders such as Alzheimer’s disease and stroke. In contrast, some forms of neuronal activity can dramatically alter Kv2.1 channel phosphorylation levels and influence their localization. These changes are normally accompanied by modifications in channel voltage-dependence, which may be neuroprotective within the context of ischemic injury. Kv1 and Kv7 channel dysfunction leads to neuronal hyperexcitability that critically contributes to the pathophysiology of human clinical disorders such as episodic ataxia and epilepsy. This review summarizes the neurotoxic, neuroprotective, and neuroregulatory roles of Kv channels, and highlights the consequences of Kv channel dysfunction on neuronal physiology. The studies described in this review thus underscore the importance of normal Kv channel function in neurons, and emphasize the therapeutic potential of targeting Kv channels in the treatment of a wide range of neurological diseases.
Keywords: voltage-gated potassium channels, Kv2.1, apoptosis, ischemia, ischemic preconditioning, neuronal hyperexcitability, epilepsy
I. Introduction
Voltage-gated potassium (Kv) channels are the largest gene family of potassium (K+) channels, and are key regulators of neuronal excitability [1–4]. In humans, they are encoded by forty different genes and categorized into twelve sub-families, Kv1 through Kv12 [5]. Mammalian Kv channels are tetramers, composed of four α-subunits that surround an ion conduction pore. Each α-subunit contains six α-helical transmembrane domains (S1–S6), a membrane-reentering P loop between S5 and S6, and cytosolic N- and C-termini. Four S5-P-S6 segments line the ion conduction pore, while the S1–S4 sequences are critical for channel voltage-sensing and gating.
Kv channels mediate outward K+ currents that contribute to membrane repolarization and hyperpolarization, thus generally serving to limit neuronal excitability. Characterizing the precise molecular correlates of Kv-mediated K+ currents in different cell types has been difficult, owing to the assortment of channels generated from α-subunit heteromerization within Kv families. This diverse channel subunit composition produces a wide spectrum of Kv channels with differing biophysical and pharmacologic profiles. Furthermore, Kv α-subunits can bind to regulatory Kv β-subunits, as well as with other Kv channel-interacting proteins, which can strongly modify channel properties [6–8]. Moreover, post-translational modifications such as phosphorylation, dephosphorylation, and sumoylation all have been shown to alter Kv channel properties significantly [9–11]. Despite these challenges, through electrophysiological studies utilizing pharmacologic agents and Kv channel subunit-specific genetic manipulation, the general functions of Kv channel sub-families in neurons have been relatively well characterized. As such, low-voltage-activated channels such as Kv1, Kv4, and Kv7 regulate the threshold potential for firing, and limit the number of action potentials generated in response to depolarization [12, 13]. In contrast, high-voltage-activated, slowly inactivating Kv2 channels play an important role in influencing action potential duration during periods of high frequency firing [14–17]. In addition to strongly shaping neuronal excitability, Kv channels also critically contribute to cell death and cell survival signaling pathways. In this review, the diverse neurotoxic, neuroprotective, and neuroregulatory roles of Kv channels will be discussed. Additionally, the implications of Kv channel dysfunction, particularly in the context of human neurological diseases, will also be addressed.
II. Neurotoxicity of Kv channels
A. K+ efflux is a requisite component of apoptotic cell death
Apoptotic cell death contributes significantly to the neuronal loss observed in a number of neurological disorders, including Alzheimer’s disease and stroke [18–22]. Therefore, understanding the mechanisms of apoptotic signaling pathways is of paramount importance in order to successfully develop therapeutic strategies for preventing or reducing neuronal damage. Apoptosis was first described as “shrinkage necrosis,” due to the morphological features of shrunken cell size and fragmentation of nuclei, which distinguished apoptotic cells from the swollen appearance of necrotic cells [23]. The key biochemical features of apoptosis have since been characterized, and include DNA fragmentation, mitochondrial damage, and caspase activation. Several critical components of apoptotic cascades occur only in the presence of a reduction in cell volume, termed apoptotic volume decrease (AVD), and decreased intracellular ionic strength, both of which are observed regardless of apoptotic stimulus and cell type [23–32]. Because the net electrochemical gradient of the cell favors the exit of K+, K+ channel-mediated K+ efflux was an early contender for promoting AVD and thus facilitating apoptotic signaling cascades. This idea is supported by several key findings:
Physiological concentrations of K+ inhibit, while lowered K+ levels activate, apoptotic enzymes: In 1997, Cidlowski and colleagues identified a critical relationship between potassium concentrations and apoptotic enzyme activity. They incubated thymocyte nuclei with calcium and magnesium to activate autodigestion, a process that recapitulates apoptotic DNA degradation in vitro. Potassium chloride (KCl) inhibited DNA fragmentation in a dose-dependent fashion, indicating blockade of pro-apoptotic nuclease activity. Importantly, normal physiological levels of intracellular K+ effected near-complete inhibition of nuclease activity [33]. Using cytoplasmic extracts from rats treated with dexamethasone to induce apoptosis, they also showed that caspase-3 activation was reduced with increasing concentrations of KCl. In other in vitro systems of apoptosis, physiologic K+ concentrations have been shown to mitigate DNA fragmentation and chromatin condensation [34], as well as apoptosome formation [35]. In neurons exposed to serum deprivation, low intracellular K+ concentrations enhance the DNA binding activity of pro-apoptotic transcription factors and the mRNA expression of their target genes, while depressing the DNA binding activity of anti-apoptotic factors and mRNA expression of their target genes [36]. This evidence strongly indicates that reduced intracellular K+ concentrations provide a permissive environment for apoptotic signaling cascades.
Apoptotic stimuli cause K+ loss: Reduced K+ concentrations are observed in cortical neurons following serum deprivation [37], and in other cell types following an assortment of apoptotic insults [24, 28, 33, 34, 38]. Important early flow cytometry studies in thymocytes demonstrated that K+ loss after exposure to an apoptotic stimulus is restricted to cells exhibiting apoptotic features such as cell volume reduction, DNA fragmentation, and loss of mitochondrial membrane potential [33, 34].
K+ efflux promotes apoptosis, while blocking K+ efflux supports cell survival: K+ efflux promotes apoptotic signaling and cell death in a range of cell types [37, 39–44]. Ionophores that induce K+ efflux, including nigericin and valinomycin, and the Na+/K+ ATPase inhibitor ouabain, activate LPS-stimulated, caspase-1-mediated maturation of IL-1β in phagocytes [41, 42]. Cortical neurons exposed to valinomycin undergo cell death, displaying the typical morphological and biochemical features of apoptosis [37].
High extracellular K+ concentrations, by decreasing the K+ gradient and thus blocking K+ efflux, oppose apoptotic signaling and promote cell survival. This observation has been well characterized particularly in cerebellar granule neurons (CGNs) [32, 45–51]. Neurons grown in 5 mM KCl exhibit indications of apoptotic cell death, as compared to neurons grown in 25 mM KCl, which are protected from DNA fragmentation and are resistant to TGF-β-induced apoptosis [48, 50, 51]. Accordingly, switching mature CGNs from 25 mM KCl to 5 mM KCl induces vacuole formation, condensing of nuclei, cellular and neurite shrinkage, and apoptotic cell death [46]. Cholesterol enhances apoptosis in CGNs cultured in low K+ medium, but does not influence cell survival in CGNs incubated in high K+ medium [52]. Similar results have been demonstrated in: (i) ciliary and dorsal root ganglion neurons, which display increased survival and differentiation in high extracellular K+ media [53, 54]; (ii) cortical neurons, which are protected by high extracellular K+ from apoptosis induced by oxidants, staurosporine, glutamate, ceramide, neurotoxic amyloid-β (Aβ) peptides, and serum deprivation [37, 55–58]; (iii) septal cholinergic cells, which in high K+ media are resistant to Aβ-induced cell death [59]; and (iv) thymocytes, where high K+ media limits pro-apoptotic caspase activation and DNA fragmentation [33]. Elevated extracellular K+ also inhibits pro-apoptotic enzyme activity. IL-1β processing by caspase-1 is prevented by high K+ growth media in human monocytes and mouse macrophages [41, 42]. In agreement with these findings, K+ channel blockers attenuate apoptotic signaling cascades and cell death in numerous neuronal [37, 56, 57, 60–69] and non-neuronal systems [27, 70–72].
Some studies have suggested that elevated extracellular K+ mitigates apoptotic cell death by increasing calcium (Ca2+) entry through voltage-gated Ca2+ channels, rather than by eliminating pro-apoptotic K+ efflux [38, 45, 48, 55, 73–78]. In rat embryonic sympathetic neurons, withdrawal of Ca2+ from the media or treatment with Ca2+ channel blockers precludes high extracellular K+-induced rescue from NGF deprivation in some cases [73, 74, 77], while thapsigargin-induced Ca2+ influx restricts NGF deprivation-induced apoptosis [73]. Similarly, Ca2+ channel antagonists impede high K+-mediated cell survival in CGNs [45, 48], and prevent rescue by increased extracellular K+ of high oxygen-stimulated apoptotic toxicity in hippocampal neurons, and of staurosporine-mediated cell death in cortical neurons [55, 78]. However, as noted by Yu and colleagues in a landmark paper [37], these studies do not rule out the possibility that reducing K+ efflux inhibits apoptosis and promotes neuronal survival. In fact, increases in intracellular Ca2+ can promote neuronal apoptosis [79, 80], and heightened Ca2+ levels are not always required for high extracellular K+-facilitated survival of NGF-deprived sympathetic neurons [81]. Importantly, in cortical neurons, Ca2+ channel blockers do not eliminate neuroprotection by high extracellular K+ or tetraethylammonium (TEA, a blocker of delayed rectifying Kv channels) in response to serum deprivation, NMDA, Aβ peptide, or ceramide [37, 56, 57, 60]. Additionally, TEA analogs that ablate staurosporine-induced K+ efflux, cell volume loss, caspase cleavage and activation, and neuronal apoptosis, also inhibit high threshold voltage-activated Ca2+ channels, supporting the idea that neuroprotection via K+ channel inhibition does not occur by activation of Ca2+ channels [61]. The specificity for K+ efflux, rather than inhibition of Ca2+ influx, in the promotion of apoptotic signaling cascades has also been demonstrated in monocytes [42], leukocytes [70], Chinese hamster ovary cells [43], and corneal epithelial cells [71, 72].
Chloride ion (Cl−) efflux may accompany pro-apoptotic K+ exit in order to maintain electroneutrality in the cell. In fact, Cl− channel activation and Cl− efflux are observed following an apoptotic stimulus in several cell types [82–86]. Furthermore, Cl− channel blockers attenuate some features of apoptotic signaling and cell death in neurons and other cell types, although these blockers are not invariably as effective as K+ channel inhibitors [83, 87–89]. Cl− exit, while insufficient to facilitate the completion of apoptotic programs, may promote pro-apoptotic K+ efflux and thus contribute to cell death. Although beyond the scope of this review, Cl− efflux in apoptosis merits further investigation for possible therapeutic intervention.
Finally, while K+ efflux is a requisite event for many forms of apoptosis, it is not, in and of itself, completely sufficient to stimulate apoptotic cell death in all injurious contexts. In Chinese hamster ovary cells, which do not express endogenous Kv channels and are resistant to apoptosis induced by hypoxia or serum deprivation, treatment with the K+ ionophore valinomycin stimulates massive cell death characterized by mitochondrial damage and caspase activation [43]. In contrast, lymphocytes cultured under hypotonic conditions undergo a 50% drop in K+ concentrations via a volume regulatory response, but this reduction alone is not sufficient to induce apoptosis [24]. Similarly, serum deprivation along with decreased extracellular K+ is required to stimulate apoptosis in CGNs, while in cortical neurons, caspase activity inhibition blocks oxidant-induced apoptotic cell death, despite the presence of prominent increased outward K+ currents [45–49, 51, 64, 90, 91].
B. Kv currents enable neuronal apoptosis
Delayed rectifier Kv channels are thought to be the principal conduits for the exit of K+ in neuronal apoptosis [37, 51, 56–58, 60, 61, 65, 67, 68, 92–101], although other K+ channels, including A-type K+ channels [27, 42, 64, 69–72, 102], Ca2+-activated K+ channels [28, 62, 103, 104], KATP channels [63], and TASK leak K+ channels [105], may also play an important role in this context. Yu and coworkers have shown that cortical neurons deprived of serum, or exposed to staurosporine, neurotoxic Aβ peptide, or ceramide, manifest a TEA-sensitive increase in delayed rectifying Kv currents, without exhibiting an increase in other major K+ currents, including inwardly rectifying, A-type (with the exception of serum deprivation, which increases these currents slightly), M type, or BK currents [37, 56, 60]. TEA or TEA analogs render neurons resistant to the above-mentioned apoptotic insults, while 4-aminopyridine (4-AP), a Kv1 channel inhibitor that opposes apoptosis in some neuronal and non-neuronal systems [27, 42, 64, 70], does not attenuate the rise in K+ currents or confer neuroprotection against apoptotic stimuli in these studies [37, 56, 60, 68, 87]. A study in septal cholinergic cells has similarly demonstrated Aβ-induced K+ current increase and apoptotic cell death, both of which are blocked by TEA. In a dopaminergic cell line that doesn’t manifest Aβ-induced increased K+ currents, TEA is not protective, while septal cholinergic cells that exhibit minimal basal K+ currents are not susceptible to Aβ-mediated toxicity, consistent with the requirement for increased K+ currents in the completion of apoptotic signaling [59]. In neurons, amplified apoptotic Kv channel currents that can be tempered by TEA, high extracellular K+, Kv siRNA-mediated knockdown, and/or a dominant negative form of the Kv channel, have also been shown in response to peroxynitrite [99], the apoptosis inducer thiol oxidant 2,2′-dithiodipyridine (DTDP) [92, 93, 95, 96, 106–108], the nitric oxide donor S-nitrocysteine (SNOC) [99], low K+/serum-free media [51, 101, 102], 6-hydroxydopamine [94], glutamate [109], and increased intracellular cholesterol [52]. These studies will be discussed in further detail below.
K+ efflux and changes in K+ current behavior have also been observed following ischemic injury in vitro and in vivo [110–119]. For instance, delayed rectifying K+ currents are increased in CA1 pyramidal neurons after transient forebrain ischemia [120, 121]. Moreover, two Kv channel antagonists, tetraethylammonium (TEA) and clofilium, are neuroprotective against cerebral ischemia in mice [98]. In another study, TEA administered to rats post-forebrain ischemia significantly rescues neuronal density, shrunken cells, and nuclei condensation, while treatment with 4-AP does not prevent the apoptotic phenotype [97].
Kv2.1-mediated neuronal apoptosis
Kv2.1, the predominant mediator of delayed rectifying K+ currents in neurons [15, 122, 123], has been identified as the channel responsible for the pro-apoptotic K+ current increase in cortical, hippocampal, and cerebellar granule neurons. Importantly, the increase in K+ current amplitude occurs without changes in the voltage-gated activation or inactivation kinetics of the Kv2.1 channels [37, 52, 93–96, 100, 101, 106, 108].
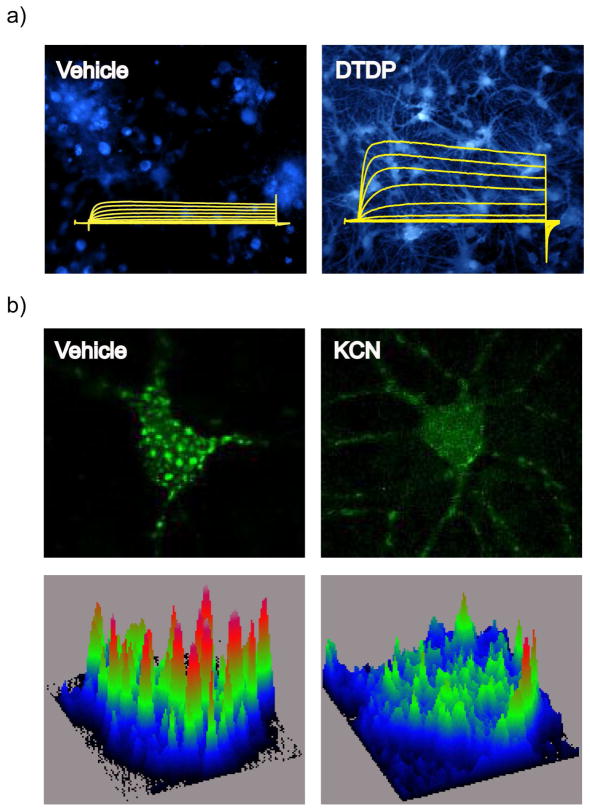
A Kv2.1-mediated neuronal apoptotic pathway stimulated by oxidant treatment has been well characterized (Fig. 1a and Fig. 2, right). Oxidants, such as DTDP, induce an intracellular release of zinc (Zn2+) from metal-binding proteins, which is required to activate two kinase signaling pathways that converge upon increased phosphorylation of Kv2.1 channels, enhanced plasma membrane delivery of Kv2.1 channels, and amplified Kv2.1 K+ currents, producing an intracellular environment that enables DNA fragmentation, caspase activation, and apoptosis [92, 93, 95, 96, 99, 106, 107, 124]. The increased Kv2.1-mediated K+ currents are observed approximately three hours following a brief exposure to the apoptogenic stimulus.
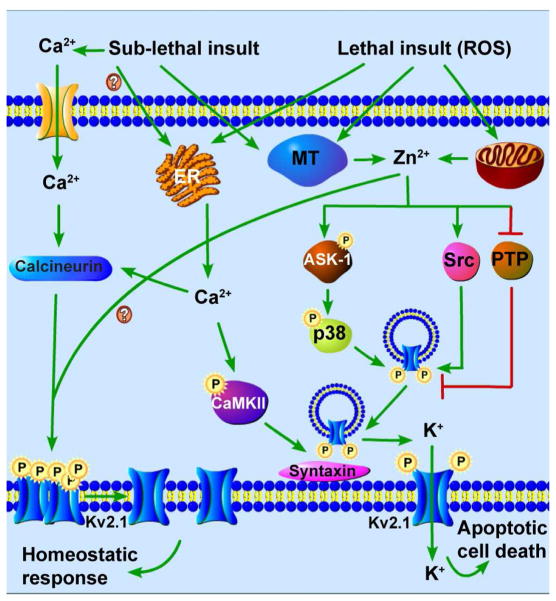
Fig. 1.
Kv2.1 channel-mediated pathways of neuronal apoptosis (right) and neuronal tolerance (left). (Right) An oxidant stimulus induces the release of Zn2+ from mitochondrial stores and metal-binding proteins, such as metallothionein (MT). Zn2+ activates ASK-1, leading to the phosphorylation and activation of p38 kinase. Zn2+ also inhibits PTPε and activates Src kinase. The combined action of both kinase systems results in increased phosphorylation of Kv2.1 channel residues S800 (by p38 kinase activation) and Y124 (by Src kinase activation and PTPε inhibition). Oxidant injury additionally stimulates release of Ca2+ from endoplasmic reticulum (ER) stores, which activates CaMKII. Coordinate phosphorylation of Kv2.1 channels at S800 and Y124, and the interaction of CaMKII with syntaxin, facilitate Kv2.1 channel-syntaxin binding, and subsequent channel delivery to the plasma membrane. Increased K+ currents through these newly inserted Kv2.1 channels permit the completion of the apoptotic signaling pathway by mediating cytoplasmic K+ loss. (Left) Neuronal activity or sub-lethal ischemia induces Ca2+ influx through glutamate receptors or intracellular Ca2+ release from the ER, and release of free Zn2+ from metal-binding proteins. Ca2+ increases calcineurin activity, leading to dephosphorylation and declustering of Kv2.1 channels. These changes are accompanied by a hyperpolarizing shift in the channel’s voltage-gated activation profile. Zn2+ is required for channel de-clustering and the voltage-gated activation shift, but not for Kv2.1 channel dephosphorylation. These changes in Kv2.1 channels reduce neuronal excitability in the context of an ischemic or epileptic insult, and render neurons tolerant to excitotoxic or other forms of injury
Fig. 2.
a) Oxidant exposure in neurons liberates Zn2+ from intracellular metal binding proteins (as detected by an increase in fluorescence using a Zn2+-sensitive indicator such as FluoZin-3), which produces a pro-apoptotic enhancement of Kv2.1 K+ currents. Reprinted with permission and adapted from [124] b) In contrast, neuronal activity or sub-lethal ischemia stimulates Kv2.1 channel dephosphorylation-dependent declustering, which, along with hyperpolarizing voltage-gated activation, induces neuronal tolerance to ischemic or epileptic challenge. Shown are confocal micrographs of rat cortical neurons transfected with plasmid vectors encoding GFP-labeled Kv2.1 channels. Below are fluorescence surface intensity maps, used to quantify the number of clusters present in neurons [163]
Apoptotic enhancement of K+ currents via Kv2.1 channels occurs upstream of caspase activation and requires coordinate channel phosphorylation at two amino acid residues, C-terminal S800 and N-terminal Y124, by p38 kinase and Src kinase, respectively [92, 107]. The oxidant-stimulated Zn2+ release is a necessary early event for p38 kinase activation, via either apoptosis signal-regulating kinase 1 (ASK-1) [96] or mixed-lineage kinase (MLK) [125], and for consequent, p38 kinase-mediated S800 phosphorylation [95, 96, 107]. Inhibiting p38 kinase activity blocks oxidant-induced S800 phosphorylation, increased Kv2.1 currents, caspase activation, and toxicity [92]. Zn2+ also permits the second, Src kinase-mediated phosphorylation step by inhibiting the activity of cytoplasmic protein tyrosine phosphatase ε (Cyt-PTPε), which is normally responsible for dephosphorylating Kv2.1 channels at the Src kinase-phosphorylated site Y124 [107, 126, 127]. In fact, over-expression of Cyt-PTPε blocks the increase in K+ currents and is neuroprotective, while Src kinase activity inhibition blocks the apoptotic K+ current surge [107]. The coordinate, oxidant-induced phosphorylation of Kv2.1 channels at the S800 and Y124 residues permits Kv2.1 channels to interact with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins via a proximal C-terminal region of the channel [106, 126, 128]. This SNARE-Kv2.1 channel interaction, which requires Ca2+-activated Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation, facilitates Kv2.1 channel delivery to the cell surface, enabling pro-apoptotic K+ currents through Kv2.1 channels [129]. Accordingly, oxidant-stimulated Kv2.1 trafficking to the plasma membrane is blocked by co-expression of botulinum toxin fragments, expression of an S800A mutant, or treatment with p38 kinase inhibitor [95, 106]. In summary, interfering with any one of multiple steps of this apoptotic pathway, including ROS production, intracellular Zn2+ release, CAMKII activation, Src- and p38-mediated Kv2.1 phosphorylation, or SNARE-dependent membrane insertion of new Kv2.1 channels, precludes the pro-apoptotic K+ current rise and rescues neurons from oxidant-mediated toxicity. This injurious pathway has also been validated in neurons exposed to activated microglia, which generate peroxynitrite, a well-established Zn2+-liberating agent [100, 130].
Neuronal cell death facilitated by a range of other apoptotic stimuli share several features of DTDP-mediated neurotoxicity, particularly the Kv2.1-mediated current increase, providing a compelling argument for the convergence of apoptotic signaling pathways on a requisite, Kv2.1-facilitated rise in K+ currents in neurons. In CGNs, increased K+ currents and apoptosis follow incubation in low K+, serum-free media, while silencing Kv2.1 gene expression via siRNA knockdown reduces K+ current amplitudes and increases cell viability [51]. Increased intracellular cholesterol potentiates the low K+/serum deprivation-stimulated Kv2.1 current rise, DNA fragmentation, and consequent apoptosis in CGNs, all of which are blocked by TEA or MβCD, a cholesterol-binding agent [52]. The elevated K+ currents are attenuated by inhibition of endoplasmic reticulum/Golgi transport [52], indicating a role for de novo Kv channel plasma membrane insertion in propagating pro-apoptotic K+ efflux, similar to that seen in DTDP-treated neurons [106]. Treatment of cerebrocortical neurons with the nitric oxide donor SNOC facilitates apoptosis characterized by K+ efflux, cell shrinkage, and activation of TEA-sensitive K+ channels. In agreement with the cell death pathway observed in DTDP-treated cortical neurons, this process involves nitric oxide-mediated Zn2+ release, leading to further oxidative injury, mitochondrial function impairment, and p38 kinase activation-mediated enhanced Kv currents, all of which are required for neurotoxicity [99]. p38 kinase activation, and Kv2.1 K+ current-mediated apoptosis is also observed in hippocampal neurons following sustained treatment with the chemokine stromal cell-derived factor-1α (SDF-1α) or exposure to HIV-1 glycoprotein gp120 [131], in dopamine transporter-expressing non-dopaminergic neurons after incubation with 6-hydroxydopamine (6-OHDA), and in 6-OHDA-treated dopaminergic neurons [94]. In another report, serum deprivation in cortical neurons was shown to provoke Kv2.1 K+ current surge-mediated apoptosis that is dependent on SNARE-facilitated channel membrane insertion: the apoptotic stimulus enhances interaction of Kv2.1 and SNARE protein SNAP-25, while blocking this interaction with botulinum toxin completely blocks the serum deprivation-associated enhancement of K+ currents [101].
Additionally, most features of this Kv2.1-facilitated apoptotic pathway have been recapitulated in recombinant cell systems, strongly implicating Kv2.1 channels in an apoptogen-stimulated, requisite K+ current surge that is sufficient for caspase activation and completion of apoptosis [92, 93, 95, 96, 106, 107, 131–133]. Transfection of Kv2.1 in Chinese hamster ovary or HEK293 cells, for example, renders them newly susceptible to apoptosis induced by DTDP or oxygen-glucose deprivation, respectively [93, 132]. Further, these studies have confirmed the involvement of pro-apoptotic p38- and Src-mediated Kv2.1 phosphorylation, as well as de novo Kv2.1 channel membrane insertion [106, 131].
Other signaling components that may participate in Kv2.1-mediated neuronal apoptosis have been identified, but have not yet been thoroughly investigated. For example, the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP response element-binding protein (CREB) pathway has been implicated in K+ channel-mediated apoptosis. In CGNs, cAMP-promoting agents reduce Kv channel-facilitated apoptosis induced by low extracellular K+ or ethanol treatment [46, 48, 51, 66, 91]. In contrast, cAMP/PKA/CREB activation promotes the Kv2.1-mediated rise in K+ currents and subsequent cell death in cholesterol-enhanced, low K+-mediated apoptosis [52]. Kv2.1-facilitated K+ efflux and consequent neuronal apoptosis following exposure to SDF-1α or HIV-1 glycoprotein gp120 depend on calcineurin signaling, and are accompanied by a shift in Kv2.1 voltage-gated kinetics that is not normally observed in oxidant-mediated neurotoxicity [131].
An alternate mechanism of Kv2.1-mediated neuronal apoptosis has been proposed. In this model, oxidant-mediated oligomerization of Kv2.1 channels leads to a rapid decrease, rather than an increase, of Kv2.1 K+ currents that is absent in cells expressing an oxidation-resistant Kv2.1 cysteine mutant. Neurons expressing the mutant are protected from neurotoxic Aβ peptide-stimulated apoptosis, and, interestingly, increased oxidation of Kv2.1 channels is observed in an Alzheimer’s disease mouse model brain [134]. Oxidant-induced toxicity is postulated to proceed via defective Kv2.1 internalization and consequent Kv2.1 oligomer formation, leading to activation of the Src/JNK signaling pathway, although the data does not unequivocally place Kv2.1 oligomerization upstream of Src/JNK activation [135]. Further, while decreased K+ currents are observed acutely following DTDP treatment in this study, the previously described, pro-apoptotic, Kv2.1 K+ current increase is detected approximately three hours after oxidant treatment [58, 92, 93, 95, 96, 107]. The results from these studies, therefore, are not irreconcilable; in fact, there may be oxidation of Kv2.1 channels and reduction of currents immediately following oxidative insult [134, 135], followed by SNARE-dependent trafficking of Kv2.1 channels to the plasma membrane, resulting in K+ current enhancement, caspase activation, and apoptotic cell death [52, 58, 92–95, 99–101, 106, 107, 109].
Evidence collected thus far from numerous studies certainly points to the existence of disparate cell death signaling events in neurons, potentially depending on the nature of apoptotic stimulus and neuronal cell type. However, the fact that several early (e.g. Zn2+ release) and late pro-apoptotic processes are elicited by such a diverse range of toxic stimuli, converging on Kv2.1-mediated K+ current enhancement, strongly suggests that this step represents a key mechanism in neuronal apoptosis that could be therapeutically targeted. In this vein, the hepatitis C virus nonstructural protein 5A (NS5A) was recently discovered to attenuate pro-apoptotic Kv2.1 K+ current enhancement in hepatocytes and cortical neurons [125, 136, 137]. This K+ current blockade has been suggested to occur through NS5A-mediated inhibition of mixed lineage kinase 3 (MLK3), a MAP kinase kinase kinase which promotes the activation of p38 kinase [125]. As described above, p38 kinase is required for Kv2.1 S800 phosphorylation, enabling the pro-apoptotic K+ current increase. However, in another study, NS5A was shown to block Src kinase-facilitated phosphorylation of the Y124 residue, without affecting channel phosphorylation of S800 by p38 kinase. In fact, pseudo-phosphorylation of Kv2.1 channels at S800 does not eliminate NS5A-induced inhibition of K+ currents, whereas Kv2.1 channels expressing a phospho-mimetic substitution at Y124F are no longer susceptible to K+ current attenuation by NS5A, strongly indicating that NS5A exerts its inhibition of Kv2.1 currents and neuroprotective effects through preventing Src kinase-mediated Y124 phosphorylation rather than by blocking p38 kinase-induced S800 phosphorylation [137]. This mechanism warrants further exploration, as NS5A could serve as a model for new neuroprotective agents specifically targeting pro-apoptotic Kv2.1-mediated K+ currents.
Other Kv channels involved in neuronal damage and cell death
In addition to Kv2.1 channels, Kv1.5 channels, which also mediate delayed rectifying K+ currents, have been implicated in playing a role in neuronal cell death, particularly in the context of ischemia. Cell viability following ischemia is increased in rat cortical neurons lacking Kv1.5 and the auxiliary β-subunit Kvβ2 [138]. Ischemic preconditioning in vivo, which limits infarct size following lethal ischemia, produces a decrease in Kv1.5 and Kvβ2 mRNA and protein expression in rat cortex, while preconditioning in rat cortical neurons reduces delayed rectifying K+ currents, suggesting that inhibition of Kv1.5 channel-mediated K+ currents is neuroprotective, and may be a viable therapeutic strategy for reducing neuronal damage and cell death in ischemic stroke [139].
Apoptotic stimuli that enhance delayed rectifier Kv currents have also been shown to increase rapidly inactivating, A-type Kv channel-mediated K+ currents (Ia), implicating these currents in promoting apoptosis, although the molecular mechanisms underlying these processes have not yet been thoroughly characterized [27, 42, 64, 70, 102, 140–145]. Activated macrophages and conditioned media from these inflammatory cells induce an increase in Ia and in apoptotic cell death in hippocampal neurons [143]. Similarly, the HIV-1 glycoprotein gp120 causes a rise in Ia and protein kinase C-mediated apoptotic cell death [69]. In both studies, the Ia increase and toxicity are attenuated by 4-AP. 4-AP also reduces low K+/serum deprivation-mediated Ia current increase and augments viability in CGNs [64, 102, 145], and in UV-treated epithelial cells [71, 72]. However, 4-AP inhibits a relatively broad spectrum of Kv channels that mediate currents which include but are not limited to rapidly inactivating, A-type K+ currents [5], underscoring the need for further exploration of the role of A-type K+ currents in apoptotic cell death pathways.
A-type Kv currents may be particularly relevant in Alzheimer’s disease (AD) as neurotoxic Aβ peptides have been shown to provoke an increase in Ia [140–142]. A specific inhibitor of Kv3.4 channels, which mediate Ia, reduces Aβ peptide-stimulated Ia enhancement and apoptotic nuclear morphology in hippocampal neurons [140]. Kv3.4 co-localizes with Aβ plaques, and its mRNA and protein expression is increased in AD mouse model brain, neurotoxic Aβ-treated PC-12 cells and rat hippocampal neurons, and in post-mortem frontal cortex tissue from patients with early and late AD [140, 141, 146]. mRNA and protein expression of Kv4.2, another channel responsible for A-type K+ currents, is also enhanced in the cortex of rats whose spatial memory is compromised due to an intracerebroventricular injection of Aβ peptide [147]. Of note, increased Kv1.4 and Kv2.1 channel expression is also observed in the hippocampus of these Aβ-injected animals, and in CGNs, the neuroprotective peptide substance P blocks Aβ-induced increases in both delayed rectifier and rapidly inactivating K+ currents, suggesting that both types of K+ currents may be involved in Aβ-mediated neurotoxicity [56, 65, 142]. In contrast to these observations, several groups have suggested a normal physiological role for Aβ in modulating K+ currents in a neuronal cell type-specific manner. One study has shown that aggregated, neurotoxic Aβ peptide has no effect on K+ currents in cortical neurons or cerebellar granule neurons. Non-toxic, unaggregated Aβ peptide, however, increases Kv4.2 protein expression, and A-type and calcium-activated delayed rectifier K+ currents in cerebellar granule neurons, while inhibition of endogenous Aβ production decreases Kv4.2 expression and inhibits K+ currents [148, 149].
Kv1.1 channels have also been implicated in Ia-mediated neuronal apoptosis [150–152]. siRNA knockdown of Kv1.1 blocks Ia in CGNs, and prevents rises in Ia and rescues cell viability in low K+/serum-deprived CGNs [150]. This apoptotic pathway is promoted by protein kinase C signaling, which is sufficient to activate Ia and apoptosis, effects that are mitigated by decreasing Kv1.1 expression. Further, Kv1-specific blockers reduce retinal ganglion cell degeneration after axotomy, while siRNA knockdown of Kv1.1 or Kv1.3 channels augments cell survival [151, 152].
III. Neuroprotective and neuroregulatory roles for Kv channels
A. Kv channels in ischemic neuroprotection
As described above, Kv2.1 channels critically contribute to oxidant injury-induced neuronal apoptosis. As the major mediators of delayed rectifying, outward K+ currents in neurons, Kv2.1 channels also play a key role in maintaining intrinsic neuronal excitability, primarily by promoting slow after-hyperpolarization and by regulating action potential repolarization during high frequency stimulation [3, 14, 15, 93, 122, 123, 153–158]. Excitatory stimuli, such as glutamate treatment, exposure to convulsants, or ischemia, trigger dramatic changes in Kv2.1 voltage-gated activation, in addition to affecting their cellular localization (Fig. 1b). Emerging evidence indicates that these modifications aid in reducing neuronal excitotoxicity in the context of an injurious stimulus (Fig. 2, left).
Trimmer and coworkers first showed that Kv2.1 channels are maintained in highly phosphorylated, somatodendritic clusters in neurons [11, 122, 159, 160]. An excitatory stimulus induces bulk Kv2.1 dephosphorylation in vivo, in rats subjected to kainate-induced seizures or CO2 exposure, for example, and in vitro, in cultured hippocampal or cortical neurons treated with glutamate, NMDA, or chemical ischemia. This dephosphorylation is thought to be critical in promoting two concomitant changes in the channels: dispersal of Kv2.1 channel clusters, and a hyperpolarizing shift in voltage-gated activation of the channel [9, 11, 16, 17, 131, 161–166]. Several lines of evidence support this concept. Phosphorylation of Kv channels promotes depolarizing shifts in voltage dependence, possibly due to an increase in the density of negative surface charges near the voltage sensor, explaining why dephosphorylation may induce a hyperpolarizing shift in the activation voltage [166]. Phospho-mimetic substitutions of seven, normally phosphorylated serine residues on the cytosolic Kv2.1 C-terminus eliminate the hyperpolarizing effects of excitatory stimuli, while serine-to-alanine mutations, which render the residues non-phosphorylatable, result in hyperpolarized voltage-gated activation. Similarly, blocking phosphorylation or inducing dephosphorylation of Kv2.1 channels results in channel declustering as well as hyperpolarizing voltage-gated activation [9, 167].
What signaling mechanisms govern these neuronal activity-induced changes in Kv2.1 channels? Several studies have demonstrated an early requirement for intracellular Zn2+ release and the Ca2+/calmodulin-dependent phosphatase calcineurin. Chelating Zn2+ blocks the channel dephosphorylation and cluster dispersal, but not the hyperpolarizing shift in cortical neurons [163]. Ca2+ influx via a Ca2+ ionophore is sufficient to induce Kv2.1 dephosphorylation, declustering, and the hyperpolarizing activation shift, while inhibiting either Ca2+ influx or calcineurin activity blocks these changes in Kv2.1 in response to an excitatory stimulus in hippocampal and cortical neurons [9, 16, 161, 163–165]. One C-terminal serine residue in particular, Ser603, is highly sensitive to excitatory stimuli-induced, calcineurin-mediated dephosphorylation [165]. Recently, cyclin-dependent kinase 5 (Cdk5) was shown to phosphorylate this residue. Pharmacologic inhibition of Cdk5 kinase activity blocks Kv2.1 Ser603 phosphorylation and stimulates dispersal of channel clusters [167]. Further, neuronal activity blockade promotes precipitous increases in Ser603 phosphorylation, whereas activity-inducing stimuli trigger its dephosphorylation. As the phosphorylation status of Ser603 critically regulates voltage-dependent gating of the channel [9], this residue may serve as a bidirectional sensor of neuronal activity, mediating changes in Kv2.1 channel gating kinetics, and thus regulating neuronal excitability in response to excitatory or inhibitory stimuli.
A few groups have proposed that ischemia-induced changes in Kv2.1 channel properties may be dependent on specific neuronal-glial interactions. In the rat cerebral cortex, Kv2.1 channel clusters are located in the extra-synaptic zone, adjacent to astrocytic processes that contain a high concentration of glutamate transporters [162, 168]. During ischemia, excessive glutamate accumulation in the extracellular space due to compromised glutamate uptake in damaged astrocytes may be responsible for promoting Kv2.1 channel dephosphorylation, cluster dispersal, and hyperpolarizing shifts in voltage-gated activation following NMDA receptor activation [17, 162, 169, 170]. Indeed, NMDA exposure or selective inhibition of astrocytic glutamate uptake in cortical or hippocampal slices is sufficient to promote neuronal Kv2.1 dephosphorylation. Accordingly, NMDA receptor antagonists block the dephosphorylation and hyperpolarizing gating shift activated by exogenous glutamate treatment or inhibition of astrocytic glutamate uptake [17, 162, 169, 170].
The hyperpolarizing shift in Kv2.1 channel voltage-gated activation is thought to reduce excitability and, consequently, excitotoxicity in neurons facing an ischemic or epileptic challenge. Sub-lethal chemical ischemia, which renders rat cortical neurons tolerant to subsequent NMDA receptor-mediated excitotoxicity [171, 172], induces Kv2.1 channel dephosphorylation and declustering, and produces a hyperpolarized shift in voltage-gated activation, implicating these channel modifications in promoting neuroprotection [163]. In hippocampal neurons, ischemia or glutamate treatment reduces spontaneous calcium transients, and spontaneous and current-evoked firing. Combining Kv2.1 channel block with either of these treatments promotes an increase in calcium overload and in firing frequency, demonstrating the requirement for Kv2.1 channel-mediated K+ currents in reducing neuronal hyperexcitability within the context of ischemia [14, 16, 17].
As described above, the changes in Kv2.1 localization, phosphorylation status, and voltage gating have been observed in response to a range of excitatory stimuli in vitro and in vivo. Further, the dephosphorylation and hyperpolarization of voltage-gated activation have been linked to reduction of intrinsic excitability, and neuronal tolerance to otherwise-lethal injury. However, little is known about the mechanism and significance of Kv2.1 channel clustering, and the specific contribution of Kv2.1 declustering towards mediating neuronal hyperactivity. Four C-terminal residues, Ser583, Ser586, Phe587, and Ser589, are critical for Kv2.1 channel clustering. A C-terminal portion of Kv2.1 channels possessing all four of these residues confers Kv2.1-like clustered localization on other Kv channels subtypes, such as Kv2.2 and Kv1.5 [164, 173, 174]. Additionally, a cytoplasmic N-terminal/C-terminal interaction is required for proper channel surface expression and phosphorylation-driven modulation of activation kinetics [175]. As mentioned above, it has been postulated that channels in clusters located at extra-synaptic locations and adjacent to astrocytic processes may be important in sensing ischemia-induced glial dysfunction through glutamate signaling, while the channel declustering following calcineurin activity-dependent dephosphorylation would remove the Kv2.1 channels from the site of calcium release, initiating recovery and precluding a potentially detrimental, prolonged response. This cluster dispersal may occur through excess glutamatergic stimulation of extrasynaptic rather than synaptic NMDA receptors, prompting relocation of Kv2.1 channels to synaptic zones [162, 169, 170]. However, the cellular and molecular mechanisms involved in these processes require further exploration.
Tamkun and colleagues have proposed a somewhat different role for Kv2.1 channel clusters. They have reported that clustered Kv2.1 channels are non-conducting, but retain gating currents that display a hyperpolarized activation profile when compared to that of Kv2.1 ionic currents [176]. Because the channels would detect membrane depolarization at a lower threshold, these studies suggest that Kv2.1 channel clusters may serve as voltage sensors of neuronal activity that convey changes in membrane potential to cytosolic signaling pathways. Supporting this hypothesis is the demonstration that Kv2.1 channel clusters are insertion platforms for trafficking of Kv2.1 and other channels to the plasma membrane, indicating that clustered Kv2.1 channels could be sites of depolarization-driven vesicle trafficking and neurotransmitter release [177–179]. In fact, Lotan and coworkers have shown that in neuroendocrine cells, Kv2.1 channels play an important role in depolarization-induced exocytosis that is independent of their ion conducting properties [180, 181]. However, these investigations have been conducted in recombinant cell expression systems and future studies examining these properties in neurons are necessary. Importantly, it was demonstrated recently that the majority of Kv2.1 channels in hippocampal neurons are non-conducting, lending further credence to the theory that Kv2.1 channel clusters may regulate key neuronal functions unrelated to their ion conducting properties [182].
Other Kv channels may be involved in reducing neuronal excitability and cell death in the context of ischemic injury. Following ischemia, Kv1-mediated delayed rectifying K+ currents increase in large aspiny neurons, which are highly resistant to anoxic cell death [183]. Ischemic injury shortens spike duration in these neurons, which could limit Ca2+ influx and thus mitigate excitotoxicity. Importantly, blocking Kv1 channel function restores action potentials to normal duration in anoxia-treated cells, suggesting a role for Kv1-facilitated K+ currents in regulating neuronal excitability in ischemia. Further, increased Kv1.2 subunit expression is observed in rat brain following transient focal ischemia [184]. An ischemic injury-promoted rise in A-type K+ currents may also be responsible for decreasing excitability and thus limiting excitotoxic cell death in large aspiny neurons [185]. Medium spiny neurons, which are more vulnerable to ischemic neuronal damage, do not manifest an increase in Ia. Importantly, over-expression of Ia-mediating Kv1.4 or Kv4.2 channels in medium spiny neurons reduces oxygen-glucose deprivation-induced toxicity, while neurons lacking Kv1.4 or Kv4.2 channel expression are more sensitive to ischemic cell death [185]. Increased Ia is also observed in CA1 hippocampal neurons after transient forebrain ischemia [121].
B. Loss of Kv1 or Kv7 channel function mediates neuronal hyperexcitability disorders
Kv1 and Kv7 encode K+ channels that are also important contributors to neuronal excitability, with functions including maintenance of resting membrane potential, action potential repolarization and after-hyperpolarization, and regulation of neurotransmitter release [2, 12, 13, 186–204]. Accordingly, loss of proper function of these channels is generally associated with hyperexcitability phenotypes such as episodic ataxia type 1 (EA-1) and epilepsy.
Kv1 channels and EA-1
EA-1 is a rare, autosomal dominant disorder characterized by generalized ataxia attacks and spontaneous muscle quivering [205]. In 1994, Browne and colleagues discovered four mutations in Kv1.1 in each of four families that had multiple members affected by EA-1[206]. Since then, more than a dozen Kv1.1 mutations have been identified in EA-1 patients with variable symptomatic presentations [206–222]. Most of these are point mutations in highly conserved channel residues that generate Kv1.1 loss-of-function phenotypes of varying degrees. For several EA-1 Kv1.1 mutations, the extent of disease in patients correlates to the magnitude of altered channel properties in Xenopus oocyte expression experiments, strongly implicating Kv1.1 channel dysfunction in the pathogenesis of EA-1 [210, 211, 223, 224].
When expressed in oocytes or mammalian cells, the majority of EA-1 Kv1.1 channel mutants exhibit undetectable or reduced K+ currents, compared to expression of wild-type Kv1.1 channels [207–211, 214, 217]. Dysfunctional post-translational modifications and improper plasma membrane trafficking may mediate the reduced currents [217, 223–226]. R417stop Kv1.1 channels, for example, lack a C-terminal targeting determinant, and undergo inefficient phosphorylation and N-glycosylation, forming large intracellular membranous aggregates in COS cells and mammalian neurons [225].
Other modifications that are observed in several EA-1 Kv1.1 mutant channels expressed in oocytes, such as slowed activation kinetics and a depolarizing shift in voltage-gated activation, implicate gating defects as the source of Kv1.1 dysfunction [7, 208–210, 214, 224, 227–230]. Given the importance of Kv1 channels in limiting neuronal excitability, these alterations in Kv1.1 channel kinetics would be expected to increase neuronal activity, providing a possible explanation for the hyperexcitable EA-1 phenotype. Indeed, expressing Kv1.1 R417stop or T226R mutant channels in hippocampal neurons elicits a lower current threshold for action potential firing, and increased neurotransmitter release compared to expression of wild-type Kv1.1 channels [196]. Another EA-1 Kv1.1 mutation, V408A, confers a range of channel gating defects in recombinant cell systems [7, 206, 208, 209, 226, 227, 229, 230]. V408A heterozygous mice show increased frequency and amplitude of cerebellar Purkinje cell inhibitory post-synaptic currents, spontaneous neuromuscular activity, and importantly, stress-induced motor deficits, similar to EA-1 patients [212, 231]. Two other Kv1.1 mutant mouse models that demonstrate variable EA-1 phenotypes have also been reported [232, 233]. However, as most EA-1 Kv1.1 mutational analysis has been conducted in oocyte expression systems, a thorough investigation into the biophysical properties of neurons expressing EA-1 Kv1.1 channel mutations is warranted, given the key role Kv1.1 dysfunction likely plays in this disorder.
Kv1 channels and epilepsy
A subset of patients with familial EA-1 is affected with epileptic seizures, suggesting that Kv1 channel dysfunction may play a role in the pathophysiology of epilepsy [210, 211, 224]. Several reports have also identified patients who are heterozygous for Kv1.1 mutations, and suffer epileptic seizures concomitant with other neurologic abnormalities such as cognitive delay [234, 235]. Injection of dendrotoxin, a Kv1 channel antagonist, into rat hippocampus induces neuronal hyperexcitability, seizures, and cell death [236, 237]. Importantly, Kv1.1-null mice exhibit an epileptic phenotype, undergoing spontaneous behavioral seizures once or twice every hour, which are consistently accompanied by ictal electroencephalographic (EEG) patterns. The threshold for seizure initiation is determined by Kv1.1 gene dosage. Homozygous Kv1.1-null mice are more rapidly susceptible to convulsant-induced seizures than heterozygous Kv1.1-null mice, which are in turn more sensitive than their wild-type littermates [238, 239]. On the cellular level, loss of Kv1.1 channel function in Kv1.1-null mice produces a neuronal hyperexcitability phenotype that is commonly observed in epilepsy models, in the hippocampus, a brain region highly susceptible to epileptogenic activity [13, 196, 238, 240–247]. Neuronal hyperexcitability in Kv1.1-null mice has also been observed in myelinated nerves [242, 243], cerebellar basket neurons [244, 248], and medial nucleus of the trapezoid body neurons in the brainstem [13, 245]. Decreasing network excitability by impairing P/Q-type Ca2+ channel function, or providing inhibitory synaptic input by grafting medial ganglionic GABAergic neuron precursors into the cortex of Kv1.1-null mice, lowers the duration and frequency of spontaneous seizures [246, 249]. In agreement with these findings, in a rodent model of tetanus toxin-induced neocortical epilepsy, lentiviral-mediated delivery of Kv1.1 channels to motor cortex pyramidal neurons along with, or one week after, tetanus toxin injection, attenuates neuronal hyperexcitability and prevents EEG-measured epileptic activity [247].
Kv1.2 channel dysfunction in neuronal hyperexcitability has also been reported. Early studies revealed that Kv1.1 α-subunits co-localize and likely form heteromers with Kv1.2 channel subunits in most parts of the brain where both channels are expressed [186, 250–254]. When co-expressed in fibroblast cells, trafficking of EA-1 R417stop Kv1.1 mutant channels and wild-type Kv1.2 channels is impaired, implying heteromerization and suggesting that loss of Kv1.2 channel function, as a result of Kv1.1 mutations, may play a role in familial EA-1 [225]. Further, most pharmacologic agents that block Kv1.1 channels and induce neuronal hyperexcitability, inhibit Kv1.2 channels as well [189, 197, 236]. Several studies indicate that loss of Kv1.2 channel function alone is sufficient to promote neuronal hyperexcitability, and may mediate epileptic pathology. For example, Kv1.2-specific inhibitors instigate hyperexcitability in cerebellar and brainstem neurons [186, 192]. Additionally, decreased Kv1.2 protein expression, which can be rescued by anti-convulsant agents, is detected in the hippocampus of seizure-prone or convulsant-treated mice [232, 255]. Although no Kv1.2 mutations have been detected in patients with epilepsy, Kv1.2-null mice display increased susceptibility to seizures and decreased life span [256]. In contrast to studies demonstrating impairment of Kv1.2 channel function due to Kv1.1 dysfunction in EA-1 [225], some investigators have suggested that Kv1.2 subunits may play a compensatory role in neurons when Kv1.1 function is compromised [13].
As described above, neuronal hyperexcitability due to Kv1 channel loss-of-function is associated with the pathogenesis of some forms of epilepsy. However, epilepsy is a complex disorder that encompasses network excitability abnormalities arising from dysfunction of a wide range of molecular components in various cell types and in different brain regions. The effects of reduced Kv1 K+ currents on epileptic pathology, therefore, may be varied depending on the location of the epileptogenic focus, and the affected neuronal cell type. Kv1.1 channel loss-of-function is associated with promotion of epileptic activity in the hippocampus, whereas in an animal model of absence epilepsy associated with defects in thalamocortical circuitry, eliminating Kv1.1 channel function rescues the seizure phenotype [249]. Moreover, in cortical, fast-spiking inhibitory neurons, decreased intrinsic excitability via up-regulation of Kv1.1 channel activity may promote seizure susceptibility [257].
Spinal cord injury and multiple sclerosis are additional examples of clinical disorders in which increased neuronal signaling via blockade of Kv1 channel activity may be beneficial. In these diseases, outward K+ currents through exposed Kv1 channels along damaged, demyelinated axons may impair action potential propagation. In fact, fampridine, a slow-release formulation of the Kv channel blocker 4-AP, was recently approved by the Food and Drug Administration (FDA) to improve walking in patients with multiple sclerosis [258].
A success story: Kv7 channel activators in the treatment of epilepsy
Heteromeric Kv7.2/Kv7.3 channels mediate the low-voltage-activated, slowly activating, non-inactivating M currents in central and peripheral neurons [12, 259, 260]. These channels critically contribute to the after-hyperpolarizing potential, aid in maintaining resting membrane potential and firing thresholds, and importantly, reduce intrinsic burst firing and repetitive action potential firing in response to excitatory stimuli [12, 198, 200, 201, 203, 259, 261–265]. Increasing Kv7 channel function decreases excitability, while suppressing Kv7 channel K+ currents enhances excitability in hippocampal pyramidal, and superior cervical and dorsal root ganglionic neurons, and promotes epileptiform activity in hippocampal neurons [202, 264–271]. Mice expressing dominant negative mutant Kv7.2 channels display spontaneous seizures, behavioral hyperactivity, and increased hippocampal neuronal excitability and cell death [202].
Mutations in Kv7.2 and Kv7.3 channels are associated with sporadic neonatal seizures, and benign familial neonatal convulsions (BFNC), an autosomal dominant disease of frequent generalized epileptic seizures beginning in the first week of life and generally disappearing within a few months [272–279]. However, several neonatal seizure-associated Kv7.2 mutations are linked to more severe abnormalities in patients, such as increased risk of seizures and therapy-refractory epilepsy later in life, epileptic encephalopathy, myokymia, and slowed psychomotor development [280–287]. These studies further confirm the involvement of Kv7 channel dysfunction in some forms of epilepsy, and implicate central and peripheral neuronal Kv7 channel dysfunction in diverse clinical phenotypes generally correlating with neuronal hyperexcitability.
Most Kv7.2 and Kv7.3 mutations associated with BFNC and more severe disorders occur in the cytosolic C-terminus, voltage-sensing domain, or pore-forming region. Expression of mutant channels in oocytes or hippocampal neurons reveals a range of channel defects. Several mutations, particularly those in the voltage-sensing domain of the channel, confer slower activation kinetics and depolarizing shifts in voltage-gated activation [274, 285, 286, 288–290], while C-terminal frameshift, insertion, or truncation mutant Kv7 channels exhibit reduced current amplitudes due to intracellular trafficking defects, inefficient membrane targeting, or increased degradation [274, 290–294]. Two transgenic BFNC mouse models, expressing Kv7.2 A306T or Kv7.3 G311V channels, present with generalized seizures likely of hippocampal origin, but display minimal synaptic reorganization or permanent neuronal damage in the hippocampus, recapitulating the major features of human BFNC. Additionally, Kv7 current density in homozygous mutant hippocampal slices is decreased, while deactivation kinetics are accelerated [271, 295]. Heterozygous adult mice show reduced threshold to electroconvulsant-induced seizures and similar, albeit less severe, Kv7 current alterations to homozygous mice.
Retigabine, also known as ezogabine, is a Kv7 channel activator that was approved by the FDA in 2011 for adjuvant treatment of partial-onset seizures in adults [277, 296–299], following demonstration of seizure reduction in animal models of epilepsy [300, 301] and in human clinical trials [302–304]. Retigabine enhances Kv7 channel activation by inducing a hyperpolarizing effect on voltage-gated channel activation. This mechanism of action limits neuronal excitability, as evidenced by the reduction of depolarization-induced action potential firing in neurons treated with retigabine [305, 306]. Since the discovery of retigabine’s anticonvulsant properties, numerous novel Kv7 activators are being explored for their therapeutic potential in treating epilepsy [277, 307–311]. Notably, in addition to epilepsy, Kv7 channel activators may also be effective in treating other diseases in which neuronal hyperexcitability represents a primary pathological component, including inflammatory or neuropathic pain [312–314], tinnitus [315], as well as neuropsychiatric disorders [316, 317].
D. A role for Kv channels in neuro-cardiac regulation
Recently, Kv channels have been associated with sudden unexplained death in epilepsy (SUDEP), an event which occurs in two to eighteen percent of chronic, idiopathic epileptic patients, and is thought to arise from neurologically-driven cardiac dysfunction [318–321]. Kv1.1-null mice display a range of cardiac abnormalities, some of which are ameliorated by inhibiting parasympathetic innervation from the vagus nerve (where Kv1.1 is normally expressed) to the heart [322]. Additionally, about half of Kv1.1-null mice die suddenly between the third and fourth week of life, with several of these mice exhibiting severe generalized seizures prior to death [238, 239], suggesting that they may be experiencing SUDEP. In another study, mice carrying a human long QT syndrome mutation in Kv7.1 channels exhibit cardiac arrhythmias and epileptiform activity, with a mouse in this report experiencing seizures that developed into status epilepticus accompanied by severe cardiac abnormalities, culminating in cardiac arrest [319]. These studies implicate Kv channels in the pathophysiology of a disastrous complication of epilepsy, highlighting the importance of Kv channels in neurological regulation of cardiac function.
IV. Conclusion
The Kv channel family is a diverse group of channels mediating outward K+ currents that play important roles in normal and pathological processes in neurons. Increased efflux of currents through Kv2.1 channels promotes apoptotic signaling (Fig. 1a and Fig. 2, right), while neuronal activity-regulated alterations in channel localization, phosphorylation, and voltage-gated activation reduce neuronal excitability, suggesting a role for these modifications in neuroprotection against ischemic or epileptic injury (Fig. 1b and Fig. 2, left). Loss of Kv1 or Kv7 promotes neuronal hyperexcitability, which manifests pathological consequences in disorders such as epilepsy or EA-1. Further, Kv channelopathy is likely to contribute to the pathophysiology of several other neurological diseases, including spinal cord injury, multiple sclerosis, inflammatory and neuropathic pain, and neuropsychiatric disorders. Significant challenges, however, exist for developing Kv channel-directed therapeutic agents. Kv channels are widely expressed in most organs, including the brain, heart, liver, lungs, pancreas, and kidney [5, 310]. As such, drugs targeting these channels in neuronal diseases may cause potentially harmful, off-target effects. Additionally, the precise molecular composition of Kv channels mediating specific K+ currents in different neuronal cell types is often difficult to pinpoint, given the diversity of α-subunit heteromerization patterns and the presence of modulatory binding partners. However, as evidenced by the successful clinical use of retigabine to activate Kv7 channels in the treatment of epilepsy, targeting Kv channels is likely to be a viable therapeutic strategy for a wide range of neurological diseases in the near future.
Table 1.
Kv channels implicated in neuronal pathology and human neurological disease
| Subtype | K+ current type | Associated pathology | Sources |
|---|---|---|---|
| Kv1 | Delayed rectifying (Kv1.1–1.3, Kv1.5–1.8), A-type (Kv1.4) | Episodic ataxia, epilepsy (Kv1.1, Kv1.2, Kv1.4) Neuronal apoptosis (Kv1.1, Kv1.3) Ischemic cell death (Kv1.5) |
7,13,192,196,197,206–257,322,323 150–152 138,139 |
| Kv2 | Delayed rectifying | Neuronal apoptosis (Kv2.1) | 37,46,48,51,52,58, 66,91–96,99–101,106–109,125–137 |
| Kv3 | Delayed rectifying (Kv3.1, Kv3.2), A-type (Kv3.3, Kv3.4) | Alzheimer’s disease (Kv3.4) Epilepsy (Kv3.2) |
140,141,146 324 |
| Kv4 | A-type | Alzheimer’s disease (Kv4.2) Epilepsy (Kv4.2, Kv4.3) |
147–149 323, 325–330 |
| Kv7 | Delayed rectifying, M-type | Epilepsy, tinnitus, pain, neuropsychiatric disorders (Kv7.1–7.5) | 202,264–317,319 |
Acknowledgments
We wish to thank our colleague Edwin Levitan (University of Pittsburgh School of Medicine), whose input has been essential to our K+ channel work over the years. We would also like to acknowledge the work of current and prior members of the Aizenman laboratory who have critically contributed to the development of our research program in this area, including Sumon Pal, BethAnn McLaughlin, Megan Knoch, Hirokazu Hara, Mandar Aras, Patrick Redman, Callie Norris, Mia Jefferson, Karen Hartnett, Kai He, and Meghan McCord. We thank Shan Ping Yu (Emory University), Dennis Choi (SUNY, Stony Brook), and John Cidlowski (NIEHS), for illuminating discussions during our early work in this field. Finally, we thank Dandan Sun and Kristopher T. Kahle for inviting us to contribute to this special issue of Translational Stroke Research. Our work has been supported by the National Institutes of Health (grant NS043277). Ms. Hegde Shah is supported by a predoctoral award from the American Heart Association (12PRE11070001).
Footnotes
Compliance with Ethics Requirements. Niyathi Hegde Shah and Elias Aizenman declare that they have no conflict of interest. This is a review article and thus there are no new experiments described that utilize human or animal subjects.
References
- 1.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419(6902):35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 2.Guan D, Lee J, Higgs M, Spain WJ, Foehring RC. Functional roles of Kv1 channels in neocortical pyramidal neurons. Journal of neurophysiology. 2007;97(3):1931–40. doi: 10.1152/jn.00933.2006. [DOI] [PubMed] [Google Scholar]
- 3.Guan D, Armstrong WE, Foehring RC. Kv2 channels regulate firing rate in pyramidal neurons from rat sensorimotor cortex. The Journal of physiology. 2013 doi: 10.1113/jphysiol.2013.257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston J, Forsythe ID, Kopp-Scheinpflug C. SYMPOSIUM REVIEW: Going native: voltage-gated potassium channels controlling neuronal excitability. The Journal of physiology. 2010;588(17):3187–200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, Mckinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacological reviews. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 6.Schulte U, Thumfart J-O, Klöcker N, Sailer CA, Bildl W, Biniossek M, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvβ1. Neuron. 2006;49(5):697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Imbrici P, D’Adamo MC, Kullmann DM, Pessia M. Episodic ataxia type 1 mutations in the KCNA1 gene impair the fast inactivation properties of the human potassium channels Kv1. 4-1.1/Kvβ1. 1 and Kv1. 4-1.1/Kvβ1. 2. European Journal of Neuroscience. 2006;24(11):3073–83. doi: 10.1111/j.1460-9568.2006.05186.x. [DOI] [PubMed] [Google Scholar]
- 8.McKeown L, Swanton L, Robinson P, Jones OT. Surface expression and distribution of voltage-gated potassium channels in neurons (Review) Molecular membrane biology. 2008;25(4):332–43. doi: 10.1080/09687680801992470. [DOI] [PubMed] [Google Scholar]
- 9.Park K-S, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2. 1 potassium channel by variable phosphorylation. Science. 2006;313(5789):976–9. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 10.Benson MD, Li Q-J, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, et al. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1. 5. Proceedings of the National Academy of Sciences. 2007;104(6):1805–10. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2. 1 K+ channel alters voltage-dependent activation. Molecular pharmacology. 1997;52(5):821–8. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. British journal of pharmacology. 2009;156(8):1185–95. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1. 1. The Journal of physiology. 2003;548(1):1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2. 1. The Journal of physiology. 2000;522(1):19–31. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 α-subunits and regulate tonic firing in mammalian sympathetic neurons. The Journal of Neuroscience. 2002;22(23):10094–105. doi: 10.1523/JNEUROSCI.22-23-10094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium-and metabolic state-dependent modulation of the voltage-dependent Kv2. 1 channel regulates neuronal excitability in response to ischemia. The Journal of Neuroscience. 2005;25(48):11184–93. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohapatra DP, Misonou H, Sheng-Jun P, Held JE, Surmeier DJ, Trimmer JS. Regulation of intrinsic excitability in hippocampal neurons by activity-dependent modulation of the KV2. 1 potassium channel. Channels. 2009;3(1):46–56. doi: 10.4161/chan.3.1.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi DW. Ischemia-induced neuronal apoptosis. Current opinion in neurobiology. 1996;6(5):667–72. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 19.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer I, Friguls B, Dalfo E, Justicia C, Planas A. Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathology and applied neurobiology. 2003;29(5):472–81. doi: 10.1046/j.1365-2990.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 21.Lobysheva NV, Tonshin AA, Selin AA, Yaguzhinsky LS, Nartsissov YR. Diversity of neurodegenerative processes in the model of brain cortex tissue ischemia. Neurochemistry international. 2009;54(5):322–9. doi: 10.1016/j.neuint.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993;24(12):2002–8. doi: 10.1161/01.str.24.12.2002. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. The Journal of pathology. 1971;105(1):13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 24.Bortner CD, Hughes FM, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. Journal of Biological Chemistry. 1997;272(51):32436–42. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 25.Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. American Journal of Physiology-Cell Physiology. 1996;271(3):C950–C61. doi: 10.1152/ajpcell.1996.271.3.C950. [DOI] [PubMed] [Google Scholar]
- 26.Bortner CD, Cidlowski JA. A necessary role for cell shrinkage in apoptosis. Biochemical pharmacology. 1998;56(12):1549–59. doi: 10.1016/s0006-2952(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 27.Beauvais F, Michel L, Dubertret L. Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. Journal of leukocyte biology. 1995;57(6):851–5. doi: 10.1002/jlb.57.6.851. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy JV, Cotter TG. Cell shrinkage and apoptosis: a role for potassium and sodium ion efflux. Cell death and differentiation. 1997;4(8):756–70. doi: 10.1038/sj.cdd.4400296. [DOI] [PubMed] [Google Scholar]
- 29.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proceedings of the National Academy of Sciences. 2000;97(17):9487–92. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proceedings of the National Academy of Sciences. 2000;97(17):9360–2. doi: 10.1073/pnas.97.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson R, Heer S, Dive C, Watson A. Characterization of cell volume loss in CEM-C7A cells during dexamethasone-induced apoptosis. American Journal of Physiology-Cell Physiology. 1996;270(4):C1190–C203. doi: 10.1152/ajpcell.1996.270.4.C1190. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Enríquez B, Guemez-Gamboa A, Morán J. Reactive oxygen species are related to ionic fluxes and volume decrease in apoptotic cerebellar granule neurons: role of NOX enzymes. Journal of neurochemistry. 2011;117(4):654–64. doi: 10.1111/j.1471-4159.2011.07231.x. [DOI] [PubMed] [Google Scholar]
- 33.Hughes FM, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. Journal of Biological Chemistry. 1997;272(48):30567–76. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 34.Dallaporta B, Hirsch T, Susin SA, Zamzami N, Larochette N, Brenner C, et al. Potassium leakage during the apoptotic degradation phase. The Journal of Immunology. 1998;160(11):5605–15. [PubMed] [Google Scholar]
- 35.Cain K, Langlais C, Sun X-M, Brown DG, Cohen GM. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. Journal of Biological Chemistry. 2001;276(45):41985–90. doi: 10.1074/jbc.M107419200. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Yan D, Wang Y. K+ regulates DNA binding of transcription factors to control gene expression related to neuronal apoptosis. Neuroreport. 2006;17(11):1199–204. doi: 10.1097/01.wnr.0000224. [DOI] [PubMed] [Google Scholar]
- 37.Yu SP, Yeh C-H, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278(5335):114–7. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 38.Barbiero G, Duranti F, Bonelli G, Amenta JS, Baccino FM. Intracellular ionic variations in the apoptotic death of L cells by inhibitors of cell cycle progression. Experimental cell research. 1995;217(2):410–8. doi: 10.1006/excr.1995.1104. [DOI] [PubMed] [Google Scholar]
- 39.Ojcius DM, Zychlinsky A, Zheng LM, Young JD-E. Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Experimental cell research. 1991;197(1):43–9. doi: 10.1016/0014-4827(91)90477-c. [DOI] [PubMed] [Google Scholar]
- 40.Deckers C, Lyons A, Samuel K, Sanderson A, Maddy A. Alternative pathways of apoptosis induced by methylprednisolone and valinomycin analyzed by flow cytometry. Experimental cell research. 1993;208(2):362–70. doi: 10.1006/excr.1993.1257. [DOI] [PubMed] [Google Scholar]
- 41.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. Journal of Biological Chemistry. 1994;269(21):15195–203. [PubMed] [Google Scholar]
- 42.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. The EMBO journal. 1995;14(8):1607. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdalah R, Wei L, Francis K, Yu SP. Valinomycin-induced apoptosis in Chinese hamster ovary cells. Neuroscience letters. 2006;405(1):68–73. doi: 10.1016/j.neulet.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 44.Nadeau H, McKinney S, Anderson D, Lester H. ROMK1 (Kir1. 1) causes apoptosis and chronic silencing of hippocampal neurons. Journal of neurophysiology. 2000;84(2):1062–75. doi: 10.1152/jn.2000.84.2.1062. [DOI] [PubMed] [Google Scholar]
- 45.Gallo V, Kingsbury A, Balazs R, Jorgensen O. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. The Journal of Neuroscience. 1987;7(7):2203–13. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proceedings of the National Academy of Sciences. 1993;90(23):10989–93. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan G-M, Ni B, Weller M, Wood KA, Paul SM. Depolarization or glutamate receptor activation blocks apoptotic cell death of cultured cerebellar granule neurons. Brain research. 1994;656(1):43–51. doi: 10.1016/0006-8993(94)91364-1. [DOI] [PubMed] [Google Scholar]
- 48.Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. The Journal of Neuroscience. 1995;15(2):1172–9. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. The Journal of Neuroscience. 1996;16(15):4696–706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Luca A, Weller M, Fontana A. TGF-β-induced apoptosis of cerebellar granule neurons is prevented by depolarization. The Journal of Neuroscience. 1996;16(13):4174–85. doi: 10.1523/JNEUROSCI.16-13-04174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiao S, Liu Z, Ren WH, Ding Y, Zhang YQ, Zhang ZH, et al. cAMP/protein kinase A signalling pathway protects against neuronal apoptosis and is associated with modulation of Kv2. 1 in cerebellar granule cells. Journal of neurochemistry. 2007;100(4):979–91. doi: 10.1111/j.1471-4159.2006.04261.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhou MH, Yang G, Jiao S, Hu CL, Mei YA. Cholesterol enhances neuron susceptibility to apoptotic stimuli via cAMP/PKA/CREB-dependent up-regulation of Kv2. 1. Journal of neurochemistry. 2012;120(4):502–14. doi: 10.1111/j.1471-4159.2011.07593.x. [DOI] [PubMed] [Google Scholar]
- 53.Collins F, Schmidt MF, Guthrie PB, Kater S. Sustained increase in intracellular calcium promotes neuronal survival. The Journal of Neuroscience. 1991;11(8):2582–7. doi: 10.1523/JNEUROSCI.11-08-02582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chalazonitis A, Fischbach GD. Elevated potassium induces morphological differentiation of dorsal root ganglionic neurons in dissociated cell culture. Developmental biology. 1980;78(1):173–83. doi: 10.1016/0012-1606(80)90327-9. [DOI] [PubMed] [Google Scholar]
- 55.Koh J-Y, Wie MB, Gwag BJ, Sensi SL, Canzoniero LM, Demaro J, et al. Staurosporine-induced neuronal apoptosis. Experimental neurology. 1995;135(2):153–9. doi: 10.1006/exnr.1995.1074. [DOI] [PubMed] [Google Scholar]
- 56.Yu SP, Farhangrazi ZS, Ying HS, Yeh C-H, Choi DW. Enhancement of outward potassium current may participate in β-amyloid peptide-induced cortical neuronal death. Neurobiology of disease. 1998;5(2):81–8. doi: 10.1006/nbdi.1998.0186. [DOI] [PubMed] [Google Scholar]
- 57.Yu S, Yeh C-H, Strasser U, Tian M, Choi D. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284(5412):336–9. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- 58.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of Neuronal Apoptosis by Thiol Oxidation. Journal of neurochemistry. 2000;75(5):1878–88. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 59.Colom LV, Diaz ME, Beers DR, Neely A, Xie Wj, Appel SH. Role of potassium channels in amyloid-induced cell death. Journal of neurochemistry. 1998;70(5):1925–34. doi: 10.1046/j.1471-4159.1998.70051925.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu SP, Yeh CH, Gottron F, Wang X, Grabb MC, Choi DW. Role of the Outward Delayed Rectifier K+ Current in Ceramide-Induced Caspase Activation and Apoptosis in Cultured Cortical Neurons. Journal of neurochemistry. 1999;73(3):933–41. doi: 10.1046/j.1471-4159.1999.0730933.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Xiao AY, Ichinose T, Yu SP. Effects of tetraethylammonium analogs on apoptosis and membrane currents in cultured cortical neurons. Journal of Pharmacology and Experimental Therapeutics. 2000;295(2):524–30. [PubMed] [Google Scholar]
- 62.Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted β-amyloid-precursor protein. 1996. [DOI] [PubMed] [Google Scholar]
- 63.Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmér E, et al. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. Journal of neurochemistry. 2003;86(4):966–79. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- 64.Hu C-L, Liu Z, Zeng X-M, Liu Z-Q, Chen X-H, Zhang Z-H, et al. 4-aminopyridine, a Kv channel antagonist, prevents apoptosis of rat cerebellar granule neurons. Neuropharmacology. 2006;51(4):737–46. doi: 10.1016/j.neuropharm.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Yu Hb, Li Zb, Zhang Hx, Wang Xl. Role of potassium channels in Aβ1–40-activated apoptotic pathway in cultured cortical neurons. Journal of neuroscience research. 2006;84(7):1475–84. doi: 10.1002/jnr.21054. [DOI] [PubMed] [Google Scholar]
- 66.Mei Y, Vaudry D, Basille M, Castel H, Fournier A, Vaudry H, et al. PACAP inhibits delayed rectifier potassium current via a cAMP/PKA transduction pathway: evidence for the involvement of IK in the anti-apoptotic action of PACAP. European Journal of Neuroscience. 2004;19(6):1446–58. doi: 10.1111/j.1460-9568.2004.03227.x. [DOI] [PubMed] [Google Scholar]
- 67.Shen QJ, Zhao YM, Cao DX, Wang XL. Contribution of Kv channel subunits to glutamate-induced apoptosis in cultured rat hippocampal neurons. Journal of neuroscience research. 2009;87(14):3153–60. doi: 10.1002/jnr.22136. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Chi S, Liu M, Yang W, Wei T, Qi Z, et al. Inhibitory effect of ganglioside GD1b on K+ current in hippocampal neurons and its involvement in apoptosis suppression. Journal of lipid research. 2005;46(12):2580–5. doi: 10.1194/jlr.M500252-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, Liu J, Xu C, Keblesh J, Zang W, Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents. PLoS One. 2011;6(10):e25994. doi: 10.1371/journal.pone.0025994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Xu D, Dai W, Lu L. An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. Journal of Biological Chemistry. 1999;274(6):3678–85. doi: 10.1074/jbc.274.6.3678. [DOI] [PubMed] [Google Scholar]
- 71.Singleton KR, Will DS, Schotanus MP, Haarsma LD, Koetje LR, Bardolph SL, et al. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Experimental eye research. 2009;89(2):140–51. doi: 10.1016/j.exer.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Investigative ophthalmology & visual science. 2003;44(12):5102–9. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- 73.Lampe PA, Cornbrooks EB, Juhasz A, Johnson EM, Franklin JL. Suppression of programmed neuronal death by a thapsigargin-induced Ca2+ influx. Journal of neurobiology. 1995;26(2):205–12. doi: 10.1002/neu.480260205. [DOI] [PubMed] [Google Scholar]
- 74.Franklin J, Sanz-Rodriguez C, Juhasz A, Deckwerth T, Johnson E. Chronic depolarization prevents programmed death of sympathetic neurons in vitro but does not support growth: requirement for Ca2+ influx but not Trk activation. The Journal of Neuroscience. 1995;15(1):643–64. doi: 10.1523/JNEUROSCI.15-01-00643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franklin JL, Johnson EM., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends in neurosciences. 1992;15(12):501–8. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- 76.Johnson EM, Jr, Koike T, Franklin J. A “calcium set-point hypothesis” of neuronal dependence on neurotrophic factor. Experimental neurology. 1992;115(1):163–6. doi: 10.1016/0014-4886(92)90242-i. [DOI] [PubMed] [Google Scholar]
- 77.KoIKE T, Martin DP, Johnson EM. Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proceedings of the National Academy of Sciences. 1989;86(16):6421–5. doi: 10.1073/pnas.86.16.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Enokido Y, Hatanaka H. Apoptotic cell death occurs in hippocampal neurons cultured in a high oxygen atmosphere. Neuroscience. 1993;57(4):965–72. doi: 10.1016/0306-4522(93)90041-d. [DOI] [PubMed] [Google Scholar]
- 79.Gwag B, Canzoniero L, Sensi S, Demaro J, Koh J, Goldberg M, et al. Calcium ionophores can induce either apoptosis or necrosis in cultured cortical neurons. Neuroscience. 1999;90(4):1339–48. doi: 10.1016/s0306-4522(98)00508-9. [DOI] [PubMed] [Google Scholar]
- 80.Song J, Lee JH, Lee SH, Park KA, Lee WT, Lee JE. TRPV1 Activation in Primary Cortical Neurons Induces Calcium-Dependent Programmed Cell Death. Experimental neurobiology. 2013;22(1):51–7. doi: 10.5607/en.2013.22.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murrell RD, Tolkovsky AM. Role of Voltage-gated Ca2+ Channels and Intracellular Ca2+ in Rat Sympathetic Neuron Survival and Function Promoted by High K+ and Cyclic AMP in the Presence or Absence of NGF. European Journal of Neuroscience. 1993;5(10):1261–72. doi: 10.1111/j.1460-9568.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 82.Nilius B, Sehrer J, De Smet P, Van Driessche W, Droogmans G. Volume regulation in a toad epithelial cell line: role of coactivation of K+ and Cl-channels. The Journal of physiology. 1995;487(Pt 2):367–78. doi: 10.1113/jphysiol.1995.sp020886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szabò I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proceedings of the National Academy of Sciences. 1998;95(11):6169–74. doi: 10.1073/pnas.95.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl-channel. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6770–3. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. The Journal of membrane biology. 2006;209(1):21–9. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- 86.Dupere-Minier G, Hamelin C, Desharnais P, Bernier J. Apoptotic volume decrease, pH acidification and chloride channel activation during apoptosis requires CD45 expression in HPB-ALL T cells. Apoptosis. 2004;9(5):543–51. doi: 10.1023/B:APPT.0000038031.84705.84. [DOI] [PubMed] [Google Scholar]
- 87.Wei L, Xiao AY, Jin C, Yang A, Lu ZY, Yu SP. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflügers Archiv. 2004;448(3):325–34. doi: 10.1007/s00424-004-1277-2. [DOI] [PubMed] [Google Scholar]
- 88.RASOLA A, FAR DF, HOFMAN P, ROSSI B. Lack of internucleosomal DNA fragmentation is related to Cl− efflux impairment in hematopoietic cell apoptosis. The FASEB Journal. 1999;13(13):1711–23. doi: 10.1096/fasebj.13.13.1711. [DOI] [PubMed] [Google Scholar]
- 89.Inoue H, Ohtaki H, Nakamachi T, Shioda S, Okada Y. Anion channel blockers attenuate delayed neuronal cell death induced by transient forebrain ischemia. Journal of neuroscience research. 2007;85(7):1427–35. doi: 10.1002/jnr.21279. [DOI] [PubMed] [Google Scholar]
- 90.Gerhardt E, Kügler S, Leist M, Beier C, Berliocchi L, Volbracht C, et al. Cascade of caspase activation in potassium-deprived cerebellar granule neurons: targets for treatment with peptide and protein inhibitors of apoptosis. Molecular and Cellular Neuroscience. 2001;17(4):717–31. doi: 10.1006/mcne.2001.0962. [DOI] [PubMed] [Google Scholar]
- 91.Castel H, Vaudry D, MEI YA, Lefebvre T, Basille M, Desrues L, et al. The delayed rectifier channel current IK plays a key role in the control of programmed cell death by PACAP and ethanol in cerebellar granule neurons. Annals of the New York Academy of Sciences. 2006;1070(1):173–9. doi: 10.1196/annals.1317.008. [DOI] [PubMed] [Google Scholar]
- 92.McLaughlin B, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, et al. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. The Journal of Neuroscience. 2001;21(10):3303–11. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2. 1-encoded potassium channels. The Journal of Neuroscience. 2003;23(12):4798–802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Redman PT, Jefferson BS, Ziegler CB, Mortensen OV, Torres GE, Levitan ES, et al. A vital role for voltage-dependent potassium channels in dopamine transporter-mediated 6-hydroxydopamine neurotoxicity. Neuroscience. 2006;143(1):1–6. doi: 10.1016/j.neuroscience.2006.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, et al. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2. 1. Proceedings of the National Academy of Sciences. 2007;104(9):3568–73. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aras MA, Aizenman E. Obligatory role of ASK1 in the apoptotic surge of K+ currents. Neuroscience letters. 2005;387(3):136–40. doi: 10.1016/j.neulet.2005.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H, Gao TM, Gong L-W, Zhuang Z-Y, Li X. Potassium channel blocker TEA prevents CA1 hippocampal injury following transient forebrain ischemia in adult rats. Neuroscience letters. 2001;305(2):83–6. doi: 10.1016/s0304-3940(01)01821-3. [DOI] [PubMed] [Google Scholar]
- 98.Wei L, Yu SP, Gottron F, Snider BJ, Zipfel GJ, Choi DW. Potassium channel blockers attenuate hypoxia-and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34(5):1281–6. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 99.Bossy-Wetzel E, Talantova MV, Lee WD, Schölzke MN, Harrop A, Mathews E, et al. Crosstalk between Nitric Oxide and Zinc Pathways to Neuronal Cell Death Involving Mitochondrial Dysfunction and p38-Activated K+ Channels. Neuron. 2004;41(3):351–65. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 100.Knoch ME, Hartnett KA, Hara H, Kandler K, Aizenman E. Microglia induce neurotoxicity via intraneuronal Zn2+ release and a K+ current surge. Glia. 2008;56(1):89–96. doi: 10.1002/glia.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao H, Zhou K, Yan D, Li M, Wang Y. The Kv2.1 channels mediate neuronal apoptosis induced by excitotoxicity. Journal of neurochemistry. 2009;108(4):909–19. doi: 10.1111/j.1471-4159.2008.05834.x. [DOI] [PubMed] [Google Scholar]
- 102.Hu CL, Liu Z, Gao ZY, Zhang ZH, Mei YA. 2-Iodomelatonin prevents apoptosis of cerebellar granule neurons via inhibition of A-type transient outward K+ currents. Journal of pineal research. 2005;38(1):53–61. doi: 10.1111/j.1600-079X.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 103.Chen M, Sun H-Y, Hu P, Wang C-F, Li B-X, Li S-J, et al. Activation of BKCa Channels Mediates Hippocampal Neuronal Death After Reoxygenation and Reperfusion. Molecular neurobiology. 2013:1–14. doi: 10.1007/s12035-013-8467-x. [DOI] [PubMed] [Google Scholar]
- 104.Jalonen TO, Charniga CJ, Wielt DB. β-Amyloid peptide-induced morphological changes coincide with increased K+ and Cl− channel activity in rat cortical astrocytes. Brain research. 1997;746(1):85–97. doi: 10.1016/s0006-8993(96)01189-4. [DOI] [PubMed] [Google Scholar]
- 105.Lauritzen I, Zanzouri M, Honoré E, Duprat F, Ehrengruber MU, Lazdunski M, et al. K+-dependent Cerebellar Granule Neuron Apoptosis ROLE OF TASK LEAK K+ CHANNELS. Journal of Biological Chemistry. 2003;278(34):32068–76. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- 106.Pal S, Takimoto K, Aizenman E, Levitan E. Apoptotic surface delivery of K+ channels. Cell Death & Differentiation. 2005;13(4):661–7. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. The Journal of physiology. 2009;587(18):4393–404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dallas ML, Boyle JP, Milligan CJ, Sayer R, Kerrigan TL, McKinstry C, et al. Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2. 1. The FASEB Journal. 2011;25(5):1519–30. doi: 10.1096/fj.10-173450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Y-M, Sun L-N, Zhou H-Y, Wang X-L. Voltage-dependent potassium channels are involved in glutamate-induced apoptosis of rat hippocampal neurons. Neuroscience letters. 2006;398(1):22–7. doi: 10.1016/j.neulet.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 110.Jiang C, Sigworth F, Haddad G. Oxygen deprivation activates an ATP-inhibitable K+ channel in substantia nigra neurons. The Journal of Neuroscience. 1994;14(9):5590–602. doi: 10.1523/JNEUROSCI.14-09-05590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang C, Haddad GG. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. Journal of neurophysiology. 1991;66(1):103–11. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- 112.Jiang C, Haddad GG. A direct mechanism for sensing low oxygen levels by central neurons. Proceedings of the National Academy of Sciences. 1994;91(15):7198–201. doi: 10.1073/pnas.91.15.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang C, Haddad G. Oxygen deprivation inhibits a K+ channel independently of cytosolic factors in rat central neurons. The Journal of physiology. 1994;481(Pt 1):15–26. doi: 10.1113/jphysiol.1994.sp020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yushmanov VE, Kharlamov A, Yanovski B, LaVerde G, Boada FE, Jones SC. Correlated sodium and potassium imbalances within the ischemic core in experimental stroke: A 23Na MRI and histochemical imaging study. Brain research. 2013 doi: 10.1016/j.brainres.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leblond J, Krnjevic K. Hypoxic changes in hippocampal neurons. Journal of neurophysiology. 1989;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- 116.Jiang C, Haddad GG. Short periods of hypoxia activate a K+ current in central neurons. Brain research. 1993;614(1):352–6. doi: 10.1016/0006-8993(93)91055-w. [DOI] [PubMed] [Google Scholar]
- 117.Chi X, Xu Z. Alterations of single potassium channel activity in CA1 pyramidal neurons after transient forebrain ischemia. Neuroscience. 2001;108(4):535–40. doi: 10.1016/s0306-4522(01)00549-8. [DOI] [PubMed] [Google Scholar]
- 118.HANSEN AJ, ZEUTHEN T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta physiologica Scandinavica. 1981;113(4):437–45. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 119.Gido G, Kristian T, Siesjo BK. Extracellular potassium in a neocortical core area after transient focal ischemia. Stroke. 1997;28(1):206–10. doi: 10.1161/01.str.28.1.206. [DOI] [PubMed] [Google Scholar]
- 120.Xuan Chi X, Xu ZC. Potassium currents in CA1 neurons of rat hippocampus increase shortly after transient cerebral ischemia. Neuroscience letters. 2000;281(1):5–8. doi: 10.1016/s0304-3940(00)00812-0. [DOI] [PubMed] [Google Scholar]
- 121.Chi XX, Xu ZC. Differential changes of potassium currents in CA1 pyramidal neurons after transient forebrain ischemia. Journal of neurophysiology. 2000;84(6):2834–43. doi: 10.1152/jn.2000.84.6.2834. [DOI] [PubMed] [Google Scholar]
- 122.Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proceedings of the National Academy of Sciences. 1991;88(23):10764–8. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murakoshi H, Trimmer JS. Identification of the Kv2. 1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. The Journal of Neuroscience. 1999;19(5):1728–35. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sensi SL, Paoletti P, Koh J-Y, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. The Journal of Neuroscience. 2011;31(45):16076–85. doi: 10.1523/JNEUROSCI.3454-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amako Y, Igloi Z, Mankouri J, Kazlauskas A, Saksela K, Dallas M, et al. Hepatitis C virus NS5A inhibits mixed lineage kinase 3 to block apoptosis. Journal of Biological Chemistry. 2013 doi: 10.1074/jbc.M113.491985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2. 1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase ε. Journal of Biological Chemistry. 2003;278(19):17509–14. doi: 10.1074/jbc.M212766200. [DOI] [PubMed] [Google Scholar]
- 127.Sobko A, Peretz A, Attali B. Constitutive activation of delayed-rectifier potassium channels by a src family tyrosine kinase in Schwann cells. The EMBO journal. 1998;17(16):4723–34. doi: 10.1093/emboj/17.16.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leung YM, Kang Y, Gao X, Xia F, Xie H, Sheu L, et al. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2. 1 to regulate channel gating and trafficking. Journal of Biological Chemistry. 2003;278(19):17532–8. doi: 10.1074/jbc.M213088200. [DOI] [PubMed] [Google Scholar]
- 129.McCord MC, Aizenman E. Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2.1 K+ currents. Proc Natl Acad Sci U S A. 2013 Aug 5; doi: 10.1073/pnas.1306238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, et al. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. The Journal of Neuroscience. 2004;24(47):10616–27. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shepherd AJ, Loo L, Gupte RP, Mickle AD, Mohapatra DP. Distinct Modifications in Kv2. 1 Channel via Chemokine Receptor CXCR4 Regulate Neuronal Survival-Death Dynamics. The Journal of Neuroscience. 2012;32(49):17725–39. doi: 10.1523/JNEUROSCI.3029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan H, Wang W-P, Feng N, Wang L, Wang X-L. Donepezil attenuated oxygen–glucose deprivation insult by blocking Kv2. 1 potassium channels. European journal of pharmacology. 2011;657(1):76–83. doi: 10.1016/j.ejphar.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 133.Al-Owais MM, Scragg JL, Dallas ML, Boycott HE, Warburton P, Chakrabarty A, et al. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. Journal of Biological Chemistry. 2012;287(29):24754–64. doi: 10.1074/jbc.M112.357012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cotella D, Hernandez-Enriquez B, Wu X, Li R, Pan Z, Leveille J, et al. Toxic role of K+ channel oxidation in mammalian brain. The Journal of Neuroscience. 2012;32(12):4133–44. doi: 10.1523/JNEUROSCI.6153-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu X, Hernandez-Enriquez B, Banas M, Xu R, Sesti F. Molecular Mechanisms Underlying the Apoptotic Effect of KCNB1 K+ Channel Oxidation. Journal of Biological Chemistry. 2013;288(6):4128–34. doi: 10.1074/jbc.M112.440933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mankouri J, Dallas ML, Hughes ME, Griffin SD, Macdonald A, Peers C, et al. Suppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistence. Science Signaling. 2009;106(37):15903. doi: 10.1073/pnas.0906798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norris CA, He K, Springer MG, Hartnett KA, Horn JP, Aizenman E. Regulation of neuronal proapoptotic potassium currents by the hepatitis C virus nonstructural protein 5A. The Journal of Neuroscience. 2012;32(26):8865–70. doi: 10.1523/JNEUROSCI.0937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong Z-g, et al. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Science Signaling. 2010;3(111):ra15. doi: 10.1126/scisignal.2000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. The Lancet. 2003;362(9389):1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 140.Pannaccione A, Boscia F, Scorziello A, Adornetto A, Castaldo P, Sirabella R, et al. Up-regulation and increased activity of KV3. 4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Molecular pharmacology. 2007;72(3):665–73. doi: 10.1124/mol.107.034868. [DOI] [PubMed] [Google Scholar]
- 141.Pannaccione A, Secondo A, Scorziello A, Calì G, Taglialatela M, Annunziato L. Nuclear factor-κB activation by reactive oxygen species mediates voltage-gated K+ current enhancement by neurotoxic β-amyloid peptides in nerve growth factor-differentiated PC-12 cells and hippocampal neurones. Journal of neurochemistry. 2005;94(3):572–86. doi: 10.1111/j.1471-4159.2005.03075.x. [DOI] [PubMed] [Google Scholar]
- 142.Pieri M, Amadoro G, Carunchio I, Ciotti M, Quaresima S, Florenzano F, et al. SP protects cerebellar granule cells against β-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology. 2010;58(1):268–76. doi: 10.1016/j.neuropharm.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 143.Hu D, Liu J, Keblesh J, Xiong H. Involvement of the 4-aminopyridine-sensitive transient A-type K+ current in macrophage-induced neuronal injury. European Journal of Neuroscience. 2010;31(2):214–22. doi: 10.1111/j.1460-9568.2009.07063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogita K, Okuda H, Watanabe M, Nagashima R, Sugiyama C, Yoneda Y. In vivo treatment with the K+ channel blocker 4-aminopyridine protects against kainate-induced neuronal cell death through activation of NMDA receptors in murine hippocampus. Neuropharmacology. 2005;48(6):810–21. doi: 10.1016/j.neuropharm.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 145.Jiao S, Wu MM, Hu CL, Zhang ZH, Mei YA. Melatonin receptor agonist 2-iodomelatonin prevents apoptosis of cerebellar granule neurons via K+ current inhibition. Journal of pineal research. 2004;36(2):109–16. doi: 10.1046/j.1600-079x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 146.Angulo E, Noé V, Casadó V, Mallol J, Gomez-Isla T, Lluis C, et al. Up-regulation of the Kv3. 4 potassium channel subunit in early stages of Alzheimer’s disease. Journal of neurochemistry. 2004;91(3):547–57. doi: 10.1111/j.1471-4159.2004.02771.x. [DOI] [PubMed] [Google Scholar]
- 147.Pan Y, Xu X, Tong X, Wang X. Messenger RNA and protein expression analysis of voltage-gated potassium channels in the brain of Aβ25–35-treated rats. Journal of neuroscience research. 2004;77(1):94–9. doi: 10.1002/jnr.20134. [DOI] [PubMed] [Google Scholar]
- 148.Plant LD, Webster NJ, Boyle JP, Ramsden M, Freir DB, Peers C, et al. Amyloid β peptide as a physiological modulator of neuronal ‘A’-type K+ current. Neurobiology of aging. 2006;27(11):1673–83. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 149.Ramsden M, Plant LD, Webster NJ, Vaughan PF, Henderson Z, Pearson HA. Differential effects of unaggregated and aggregated amyloid β protein (1–40) on K+ channel currents in primary cultures of rat cerebellar granule and cortical neurones. Journal of neurochemistry. 2001;79(3):699–712. doi: 10.1046/j.1471-4159.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 150.Hu CL, Zeng XM, Zhou MH, Shi YT, Cao H, Mei YA. Kv 1.1 is associated with neuronal apoptosis and modulated by protein kinase C in the rat cerebellar granule cell. Journal of neurochemistry. 2008;106(3):1125–37. doi: 10.1111/j.1471-4159.2008.05449.x. [DOI] [PubMed] [Google Scholar]
- 151.Koeberle P, Wang Y, Schlichter L. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death & Differentiation. 2009;17(1):134–44. doi: 10.1038/cdd.2009.113. [DOI] [PubMed] [Google Scholar]
- 152.Koeberle P, Schlichter LC. Targeting KV channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels. 2010;4(5):337–46. doi: 10.4161/chan.4.5.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guan D, Tkatch T, Surmeier D, Armstrong W, Foehring R. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. The Journal of physiology. 2007;581(3):941–60. doi: 10.1113/jphysiol.2007.128454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2. 1 and Kv3. 1/3.2 K+ channels. The Journal of Neuroscience. 1999;19(15):6394–404. doi: 10.1523/JNEUROSCI.19-15-06394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baranauskas G. Ionic channel function in action potential generation: current perspective. Molecular neurobiology. 2007;35(2):129–50. doi: 10.1007/s12035-007-8001-0. [DOI] [PubMed] [Google Scholar]
- 156.Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. Journal of neurophysiology. 2000;83(1):70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- 157.Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. The Journal of physiology. 2000;525(3):611–20. doi: 10.1111/j.1469-7793.2000.t01-2-00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. The Journal of physiology. 2000;525(3):621–39. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trimmer JS. Expression of Kv2.1 delayed rectifier K+ channel isoforms in the developing rat brain. FEBS letters. 1993;324(2):205–10. doi: 10.1016/0014-5793(93)81394-f. [DOI] [PubMed] [Google Scholar]
- 160.Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2. 1 K+ channels expressed in transfected mammalian cells. Journal of Biological Chemistry. 1994;269(37):23204–11. [PubMed] [Google Scholar]
- 161.Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nature neuroscience. 2004;7(7):711–8. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 162.Misonou H, Thompson SM, Cai X. Dynamic regulation of the Kv2. 1 voltage-gated potassium channel during brain ischemia through neuroglial interaction. The Journal of Neuroscience. 2008;28(34):8529–38. doi: 10.1523/JNEUROSCI.1417-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aras MA, Saadi RA, Aizenman E. Zn2+ regulates Kv2.1 voltage-dependent gating and localization following ischemia. European Journal of Neuroscience. 2009;30(12):2250–7. doi: 10.1111/j.1460-9568.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mohapatra DP, Trimmer JS. The Kv2. 1 C terminus can autonomously transfer Kv2. 1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. The Journal of Neuroscience. 2006;26(2):685–95. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Misonou H, Menegola M, Mohapatra DP, Guy LK, Park K-S, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. The Journal of Neuroscience. 2006;26(52):13505–14. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Perozo E, Bezanilla F. Phosphorylation affects voltage gating of the delayed rectifier K+ channel by electrostatic interactions. Neuron. 1990;5(5):685–90. doi: 10.1016/0896-6273(90)90222-2. [DOI] [PubMed] [Google Scholar]
- 167.Cerda O, Trimmer JS. Activity-dependent phosphorylation of neuronal Kv2. 1 potassium channels by CDK5. Journal of Biological Chemistry. 2011;286(33):28738–48. doi: 10.1074/jbc.M111.251942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Du J, Tao-Cheng J-H, Zerfas P, McBain C. The K+ channel, Kv2. 1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84(1):37–48. doi: 10.1016/s0306-4522(97)00519-8. [DOI] [PubMed] [Google Scholar]
- 169.Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2. 1 potassium channels. The Journal of Neuroscience. 2008;28(35):8801–9. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mulholland PJ, Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Ethanol disrupts NMDA receptor and astroglial EAAT2 modulation of Kv2. 1 potassium channels in hippocampus. Alcohol. 2009;43(1):45–50. doi: 10.1016/j.alcohol.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, et al. Caspase 3 activation is essential for neuroprotection in preconditioning. Proceedings of the National Academy of Sciences. 2003;100(2):715–20. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aras MA, Hara H, Hartnett KA, Kandler K, Aizenman E. Protein kinase C regulation of neuronal zinc signaling mediates survival during preconditioning. Journal of neurochemistry. 2009;110(1):106–17. doi: 10.1111/j.1471-4159.2009.06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim ST, Antonucci DE, Scannevin RH, Trimmer JS. A Novel Targeting Signal for Proximal Clustering of the Kv2. 1 K+ Channel in Hippocampal Neurons. Neuron. 2000;25(2):385–97. doi: 10.1016/s0896-6273(00)80902-2. [DOI] [PubMed] [Google Scholar]
- 174.Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2. 1 K+ channel. The Journal of cell biology. 1996;135(6):1619–32. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohapatra DP, Siino DF, Trimmer JS. Interdomain cytoplasmic interactions govern the intracellular trafficking, gating, and modulation of the Kv2. 1 channel. The Journal of Neuroscience. 2008;28(19):4982–94. doi: 10.1523/JNEUROSCI.0186-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O’Connell KM, Loftus R, Tamkun MM. Localization-dependent activity of the Kv2. 1 delayed-rectifier K+ channel. Proceedings of the National Academy of Sciences. 2010;107(27):12351–6. doi: 10.1073/pnas.1003028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. Journal of cell science. 2005;118(10):2155–66. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- 178.O’Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2. 1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. The Journal of Neuroscience. 2006;26(38):9609–18. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deutsch E, Weigel AV, Akin EJ, Fox P, Hansen G, Haberkorn CJ, et al. Kv2. 1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Molecular biology of the cell. 2012;23(15):2917–29. doi: 10.1091/mbc.E12-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Singer-Lahat D, Chikvashvili D, Lotan I. Direct interaction of endogenous Kv channels with syntaxin enhances exocytosis by neuroendocrine cells. PLoS One. 2008;3(1):e1381. doi: 10.1371/journal.pone.0001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Feinshreiber L, Singer-Lahat D, Ashery U, Lotan I. Voltage-gated Potassium Channel as a Facilitator of Exocytosis. Annals of the New York Academy of Sciences. 2009;1152(1):87–92. doi: 10.1111/j.1749-6632.2008.03997.x. [DOI] [PubMed] [Google Scholar]
- 182.Fox PD, Loftus RJ, Tamkun MM. Regulation of Kv2.1 K(+) conductance by cell surface channel density. The Journal of Neuroscience. 2013;33(3):1259–70. doi: 10.1523/JNEUROSCI.3008-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Deng P, Pang Z-P, Zhang Y, Xu Z. Increase of delayed rectifier potassium currents in large aspiny neurons in the neostriatum following transient forebrain ischemia. Neuroscience. 2005;131(1):135–46. doi: 10.1016/j.neuroscience.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 184.Chung YH, Kim HS, Shin CM, Kim MJ, Cha CI. Immunohistochemical study on the distribution of voltage-gated K+ channels in rat brain following transient focal ischemia. Neuroscience letters. 2001;308(3):157–60. doi: 10.1016/s0304-3940(01)01996-6. [DOI] [PubMed] [Google Scholar]
- 185.Deng P, Pang Z-P, Lei Z, Shikano S, Xiong Q, Harvey BK, et al. Up-regulation of A-type potassium currents protects neurons against cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2011;31(9):1823–35. doi: 10.1038/jcbfm.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. The Journal of Neuroscience. 1998;18(3):948–55. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ Channels at the Axon Initial Segment Dampen Near-Threshold Excitability of Neocortical Fast-Spiking GABAergic Interneurons. Neuron. 2008;58(3):387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Geiger JR, Jonas P. Dynamic Control of Presynaptic Ca2+ Inflow by Fast-Inactivating K+ Channels in Hippocampal Mossy Fiber Boutons. Neuron. 2000;28(3):927–39. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 189.Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proceedings of the National Academy of Sciences. 2007;104(27):11453–8. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55(4):633–47. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 191.Hsiao C-F, Kaur G, Vong A, Bawa H, Chandler SH. Participation of Kv1 channels in control of membrane excitability and burst generation in mesencephalic V neurons. Journal of neurophysiology. 2009;101(3):1407–18. doi: 10.1152/jn.91053.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dodson PD, Billups B, Rusznák Z, Szûcs G, Barker MC, Forsythe ID. Presynaptic rat Kv1. 2 channels suppress synaptic terminal hyperexcitability following action potential invasion. The Journal of physiology. 2003;550(1):27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends in neurosciences. 2004;27(4):210–7. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 194.Lambe EK, Aghajanian GK. The role of Kv1. 2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. The Journal of Neuroscience. 2001;21(24):9955–63. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Higgs MH, Spain WJ. Kv1 channels control spike threshold dynamics and spike timing in cortical pyramidal neurones. The Journal of physiology. 2011;589(21):5125–42. doi: 10.1113/jphysiol.2011.216721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heeroma JH, Henneberger C, Rajakulendran S, Hanna MG, Schorge S, Kullmann DM. Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release. Disease models & mechanisms. 2009;2(11–12):612–9. doi: 10.1242/dmm.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bekkers JM, Delaney AJ. Modulation of excitability by α-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. The Journal of Neuroscience. 2001;21(17):6553–60. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. The Journal of physiology. 2005;566(3):689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vervaeke K, Gu N, Agdestein C, Hu H, Storm J. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. The Journal of physiology. 2006;576(1):235–56. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tzingounis AV, Nicoll RA. Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proceedings of the National Academy of Sciences. 2008;105(50):19974–9. doi: 10.1073/pnas.0810535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tzingounis AV, Heidenreich M, Kharkovets T, Spitzmaul G, Jensen HS, Nicoll RA, et al. The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proceedings of the National Academy of Sciences. 2010;107(22):10232–7. doi: 10.1073/pnas.1004644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nature neuroscience. 2004;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 203.Jentsch TJ. Neuronal KCNQ potassium channels: physislogy and role in disease. Nature Reviews Neuroscience. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 204.Foust AJ, Yu Y, Popovic M, Zecevic D, McCormick DA. Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons. The Journal of Neuroscience. 2011;31(43):15490–8. doi: 10.1523/JNEUROSCI.2752-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gancher ST, Nutt JG. Autosomal dominant episodic ataxia: a heterogeneous syndrome. Movement Disorders. 1986;1(4):239–53. doi: 10.1002/mds.870010404. [DOI] [PubMed] [Google Scholar]
- 206.Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nature genetics. 1994;8(2):136–40. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 207.Scheffer H, Brunt E, Mol G, Van der Vlies P, Verlind E, Mantel G, et al. Three novel KCNA1 mutations in episodic ataxia type I families. Human genetics. 1998;102(4):464–6. doi: 10.1007/s004390050722. [DOI] [PubMed] [Google Scholar]
- 208.Zerr P, Adelman JP, Maylie J. Episodic ataxia mutations in Kv1. 1 alter potassium channel function by dominant negative effects or haploinsufficiency. The Journal of Neuroscience. 1998;18(8):2842–8. doi: 10.1523/JNEUROSCI.18-08-02842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.D’Adamo MC, Liu Z, Adelman JP, Maylie J, Pessia M. Episodic ataxia type-1 mutations in the hKv1. 1 cytoplasmic pore region alter the gating properties of the channel. The EMBO journal. 1998;17(5):1200–7. doi: 10.1093/emboj/17.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Spauschus A, Eunson L, Hanna MG, Kullmann DM. Functional characterization of a novel mutation in KCNA1 in episodic ataxia type 1 associated with epilepsy. Annals of the New York Academy of Sciences. 1999;868(1):442–6. doi: 10.1111/j.1749-6632.1999.tb11310.x. [DOI] [PubMed] [Google Scholar]
- 211.Zuberi S, Eunson L, Spauschus A, De Silva R, Tolmie J, Wood N, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1. 1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122(5):817–25. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]
- 212.Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, et al. A mouse model of episodic ataxia type-1. Nature neuroscience. 2003;6(4):378–83. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- 213.Rajakulendran S, Schorge S, Kullmann DM, Hanna MG. Episodic ataxia type 1: a neuronal potassium channelopathy. Neurotherapeutics. 2007;4(2):258–66. doi: 10.1016/j.nurt.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 214.Zerr P, Adelman JP, Maylie J. Characterization of three episodic ataxia mutations in the human Kv1. 1 potassium channel. FEBS letters. 1998;431(3):461–4. doi: 10.1016/s0014-5793(98)00814-x. [DOI] [PubMed] [Google Scholar]
- 215.Browne D, Brunt E, Griggs R, Nutt J, Gancher S, Smith E, et al. Identification of two new KCNA1 mutations in episodic ataxia/myokymia families. Human molecular genetics. 1995;4(9):1671–2. doi: 10.1093/hmg/4.9.1671. [DOI] [PubMed] [Google Scholar]
- 216.Poujois A, Antoine J-C, Combes A, Touraine RL. Chronic neuromyotonia as a phenotypic variation associated with a new mutation in the KCNA1 gene. Journal of neurology. 2006;253(7):957–9. doi: 10.1007/s00415-006-0134-y. [DOI] [PubMed] [Google Scholar]
- 217.Zhu J, Alsaber R, Zhao J, Ribeiro-Hurley E, Thornhill WB. Characterization of the Kv1. 1 I262T and S342I mutations associated with episodic ataxia 1 with distinct phenotypes. Archives of Biochemistry and Biophysics. 2012;524(2):99–105. doi: 10.1016/j.abb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 218.Klein A, Boltshauser E, Jen J, Baloh R. Episodic ataxia type 1 with distal weakness: a novel manifestation of a potassium channelopathy. Neuropediatrics. 2004;35(02):147–9. doi: 10.1055/s-2004-817921. [DOI] [PubMed] [Google Scholar]
- 219.Tomlinson SE, Tan SV, Kullmann DM, Griggs RC, Burke D, Hanna MG, et al. Nerve excitability studies characterize Kv1. 1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain. 2010;133(12):3530–40. doi: 10.1093/brain/awq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Çomu S, Narayanan V, Giuliani M. Episodic ataxia and myokymia syndrome: a new mutation of potassium channel gene Kv1. 1. Annals of neurology. 1996;40(4):684–7. doi: 10.1002/ana.410400422. [DOI] [PubMed] [Google Scholar]
- 221.Shook SJ, Mamsa H, Jen JC, Baloh RW, Zhou L. Novel mutation in KCNA1 causes episodic ataxia with paroxysmal dyspnea. Muscle & nerve. 2008;37(3):399–402. doi: 10.1002/mus.20904. [DOI] [PubMed] [Google Scholar]
- 222.Lee H, Wang H, Jen JC, Sabatti C, Baloh RW, Nelson SF. A novel mutation in KCNA1 causes episodic ataxia without myokymia. Human mutation. 2004;24(6):536. doi: 10.1002/humu.9295. [DOI] [PubMed] [Google Scholar]
- 223.Rea R, Spauschus A, Eunson LH, Hanna MG, Kullmann DM. Variable K+ channel subunit dysfunction in inherited mutations of KCNA1. The Journal of physiology. 2002;538(1):5–23. doi: 10.1113/jphysiol.2001.013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Eunson L, Rea R, Zuberi S, Youroukos S, Panayiotopoulos C, Liguori R, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA 1 reveal new phenotypic variability. Annals of neurology. 2000;48(4):647–56. [PubMed] [Google Scholar]
- 225.Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. Episodic ataxia type-1 mutations in the Kv1. 1 potassium channel display distinct folding and intracellular trafficking properties. Journal of Biological Chemistry. 2001;276(52):49427–34. doi: 10.1074/jbc.M109325200. [DOI] [PubMed] [Google Scholar]
- 226.Bretschneider F, Wrisch A, Lehmann-Horn F, Grissmer S. Expression in mammalian cells and electrophysiological characterization of two mutant Kv1. 1 channels causing episodic ataxia type 1 (EA-1) European Journal of Neuroscience. 1999;11(7):2403–12. doi: 10.1046/j.1460-9568.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 227.Adelman JP, Bond CT, Pessia M, Mayliet J. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 1995;15(6):1449–54. doi: 10.1016/0896-6273(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 228.Imbrici P, Cusimano A, D’Adamo M, De Curtis A, Pessia M. Functional characterization of an episodic ataxia type-1 mutation occurring in the S1 segment of hKv1. 1 channels. Pflügers Archiv. 2003;446(3):373–9. doi: 10.1007/s00424-002-0962-2. [DOI] [PubMed] [Google Scholar]
- 229.Maylie B, Bissonnette E, Virk M, Adelman JP, Maylie JG. Episodic ataxia type 1 mutations in the human Kv1. 1 potassium channel alter hKvβ1-induced N-type inactivation. The Journal of Neuroscience. 2002;22(12):4786–93. doi: 10.1523/JNEUROSCI.22-12-04786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Peters CJ, Werry D, Gill HS, Accili EA, Fedida D. Mechanism of Accelerated Current Decay Caused by an Episodic Ataxia Type-1-Associated Mutant in a Potassium Channel Pore. The Journal of Neuroscience. 2011;31(48):17449–59. doi: 10.1523/JNEUROSCI.2940-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Brunetti O, Imbrici P, Botti FM, Pettorossi VE, D’Adamo MC, Valentino M, et al. Kv1.1 knock-in ataxic mice exhibit spontaneous myokymic activity exacerbated by fatigue, ischemia and low temperature. Neurobiology of disease. 2012;47(3):310–21. doi: 10.1016/j.nbd.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Petersson S, Persson AS, Johansen JE, Ingvar M, Nilsson J, Klement G, et al. Truncation of the Shaker-like voltage-gated potassium channel, Kv1. 1, causes megencephaly. European Journal of Neuroscience. 2003;18(12):3231–40. doi: 10.1111/j.1460-9568.2003.03044.x. [DOI] [PubMed] [Google Scholar]
- 233.Ishida S, Sakamoto Y, Nishio T, Baulac S, Kuwamura M, Ohno Y, et al. Kcna1-mutant rats dominantly display myokymia, neuromyotonia and spontaneous epileptic seizures. Brain research. 2012;1435:154–66. doi: 10.1016/j.brainres.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 234.Liguori R, Avoni P, Baruzzi A, Di Stasi V, Montagna P. Familial continuous motor unit activity and epilepsy. Muscle & nerve. 2001;24(5):630–3. doi: 10.1002/mus.1048. [DOI] [PubMed] [Google Scholar]
- 235.Demos MK, Macri V, Farrell K, Nelson TN, Chapman K, Accili E, et al. A novel KCNA1 mutation associated with global delay and persistent cerebellar dysfunction. Movement Disorders. 2009;24(5):778–82. doi: 10.1002/mds.22467. [DOI] [PubMed] [Google Scholar]
- 236.Bagetta G, Nisticó G, Dolly JO. Production of seizures and brain damage in rats by α-dendrotoxin, a selective K+ channel blocker. Neuroscience letters. 1992;139(1):34–40. doi: 10.1016/0304-3940(92)90851-w. [DOI] [PubMed] [Google Scholar]
- 237.Lalic T, Pettingill P, Vincent A, Capogna M. Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia. 2011;52(1):121–31. doi: 10.1111/j.1528-1167.2010.02756.x. [DOI] [PubMed] [Google Scholar]
- 238.Smart SL, Lopantsev V, Zhang C, Robbins CA, Wang H, Chiu S, et al. Deletion of the KV1.1 Potassium Channel Causes Epilepsy in Mice. Neuron. 1998;20(4):809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 239.Rho JM, Szot P, Tempel BL, Schwartzkroin PA. Developmental seizure susceptibility of kv1. 1 potassium channel knockout mice. Developmental neuroscience. 2011;21(3–5):320–7. doi: 10.1159/000017381. [DOI] [PubMed] [Google Scholar]
- 240.Simeone TA, Simeone KA, Samson KK, Kim DY, Rho JM. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiology of disease. 2013 doi: 10.1016/j.nbd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lopantsev V, Tempel BL, Schwartzkroin PA. Hyperexcitability of CA3 pyramidal cells in mice lacking the potassium channel subunit Kv1. 1. Epilepsia. 2003;44(12):1506–12. doi: 10.1111/j.0013-9580.2003.44602.x. [DOI] [PubMed] [Google Scholar]
- 242.Zhou L, Zhang C-L, Messing A, Chiu SY. Temperature-sensitive neuromuscular transmission in Kv1. 1 null mice: role of potassium channels under the myelin sheath in young nerves. The Journal of Neuroscience. 1998;18(18):7200–15. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhou L, Messing A, Chiu SY. Determinants of excitability at transition zones in Kv1. 1-deficient myelinated nerves. The Journal of Neuroscience. 1999;19(14):5768–81. doi: 10.1523/JNEUROSCI.19-14-05768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang C-L, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar Purkinje cells in mice lacking the potassium channel Kv1. 1. The Journal of Neuroscience. 1999;19(8):2852–64. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. The Journal of Neuroscience. 2003;23(27):9199–207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1. 1 mutant mice. Proceedings of the National Academy of Sciences. 2009;106(36):15472–7. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Science translational medicine. 2012;4(161):161ra52. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen G, Gao W, Reinert KC, Popa LS, Hendrix CM, Ross ME, et al. Involvement of Kv1 potassium channels in spreading acidification and depression in the cerebellar cortex. Journal of neurophysiology. 2005;94(2):1287–98. doi: 10.1152/jn.00224.2005. [DOI] [PubMed] [Google Scholar]
- 249.Glasscock E, Qian J, Yoo JW, Noebels JL. Masking epilepsy by combining two epilepsy genes. Nature neuroscience. 2007;10(12):1554–8. doi: 10.1038/nn1999. [DOI] [PubMed] [Google Scholar]
- 250.Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. The Journal of Neuroscience. 2000;20(1):114–22. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 β-subunits with Kv1 α-subunits in mammalian brain K+ channel complexes. The Journal of Neuroscience. 1997;17(21):8246–58. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang H, Kunkel D, Schwartzkroin P, Tempel B. Localization of Kv1. 1 and Kv1. 2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. The Journal of Neuroscience. 1994;14(8):4588–99. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. 1993. [DOI] [PubMed] [Google Scholar]
- 254.Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel α and β subunits in rat hippocampal formation. The Journal of Neuroscience. 2001;21(16):5973–83. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tsaur M-L, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8(6):1055–67. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- 256.Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1. 2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. Journal of neurophysiology. 2007;98(3):1501–25. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- 257.Li K-X, Lu Y-M, Xu Z-H, Zhang J, Zhu J-M, Zhang J-M, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1. 1 and acts in epilepsy. Nature neuroscience. 2011;15(2):267–73. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- 258.Preiningerova JL, Baumhackl U, Csepany T, Czaplinski A, Deisenhammer F, Derfuss T, et al. Recommendations for the Use of Prolonged-Release Fampridine in Patients with Multiple Sclerosis (MS) CNS neuroscience & therapeutics. 2013;19(5):302–6. doi: 10.1111/cns.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang H-S, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282(5395):1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 260.Shah M, Mistry M, Marsh S, Brown D, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. The Journal of physiology. 2002;544(1):29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, et al. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proceedings of the National Academy of Sciences. 2000;97(9):4914–9. doi: 10.1073/pnas.090092797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. The Journal of Neuroscience. 2001;21(24):9529–40. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences. 2008;105(22):7869–74. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Miranda P, Cadaveira-Mosquera A, González-Montelongo R, Villarroel A, González-Hernández T, Lamas JA, et al. The Neuronal Serum-and Glucocorticoid-Regulated Kinase 1.1 Reduces Neuronal Excitability and Protects against Seizures through Upregulation of the M-Current. The Journal of Neuroscience. 2013;33(6):2684–96. doi: 10.1523/JNEUROSCI.3442-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sun J, Kapur J. M-type potassium channels modulate Schaffer collateral–CA1 glutamatergic synaptic transmission. The Journal of physiology. 2012;590(16):3953–64. doi: 10.1113/jphysiol.2012.235820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Maslarova A, Salar S, Lapilover E, Friedman A, Veh RW, Heinemann U. Increased susceptibility to acetylcholine in the entorhinal cortex of pilocarpine-treated rats involves alterations in KCNQ channels. Neurobiology of disease. 2013 doi: 10.1016/j.nbd.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 267.Andreasen M, Nedergaard S. Heterogeneous firing behavior during ictal-like epileptiform activity in vitro. Journal of neurophysiology. 2012;107(5):1379–92. doi: 10.1152/jn.00309.2011. [DOI] [PubMed] [Google Scholar]
- 268.Peña F, Alavez-Pérez N. Epileptiform Activity Induced by Pharmacologic Reduction of M-Current in the Developing Hippocampus in Vitro. Epilepsia. 2006;47(1):47–54. doi: 10.1111/j.1528-1167.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 269.Otto JF, Yang Y, Frankel WN, Wilcox KS, White HS. Mice Carrying the Szt1 Mutation Exhibit Increased Seizure Susceptibility and Altered Sensitivity to Compounds Acting at the M-Channel. Epilepsia. 2004;45(9):1009–16. doi: 10.1111/j.0013-9580.2004.65703.x. [DOI] [PubMed] [Google Scholar]
- 270.Otto JF, Yang Y, Frankel WN, White HS, Wilcox KS. A spontaneous mutation involving Kcnq2 (Kv7. 2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. The Journal of Neuroscience. 2006;26(7):2053–9. doi: 10.1523/JNEUROSCI.1575-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Otto JF, Singh NA, Dahle EJ, Leppert MF, Pappas CM, Pruess TH, et al. Electroconvulsive seizure thresholds and kindling acquisition rates are altered in mouse models of human Kcnq2 and Kcnq3 mutations for benign familial neonatal convulsions. Epilepsia. 2009;50(7):1752–9. doi: 10.1111/j.1528-1167.2009.02100.x. [DOI] [PubMed] [Google Scholar]
- 272.Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279(5349):403–6. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 273.Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nature genetics. 1998;18(1):25–9. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 274.Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, et al. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain. 2003;126(12):2726–37. doi: 10.1093/brain/awg286. [DOI] [PubMed] [Google Scholar]
- 275.Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nature genetics. 1998;18(1):53–5. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- 276.Hirose S, Zenri F, Akiyoshi H, Fukuma G, Iwata H, Inoue T, et al. A novel mutation of KCNQ3 (c. 925T→ C) in a Japanese family with benign familial neonatal convulsions. Annals of neurology. 2000;47(6):822–6. [PubMed] [Google Scholar]
- 277.Miceli F, Soldovieri MV, Iannotti FA, Barrese V, Ambrosino P, Martire M, et al. The voltage-sensing domain of kv7. 2 channels as a molecular target for epilepsy-causing mutations and anticonvulsants. Frontiers in pharmacology. 2011:2. doi: 10.3389/fphar.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sadewa AH, Sasongko TH, Lee MJ, Daikoku K, Yamamoto A, Yamasaki T, et al. Germ-line mutation of KCNQ2, p. R213W, in a Japanese family with benign familial neonatal convulsion. Pediatrics International. 2008;50(2):167–71. doi: 10.1111/j.1442-200X.2008.02539.x. [DOI] [PubMed] [Google Scholar]
- 279.Ishii A, Fukuma G, Uehara A, Miyajima T, Makita Y, Hamachi A, et al. A de novo KCNQ2 mutation detected in non-familial benign neonatal convulsions. Brain and Development. 2009;31(1):27–33. doi: 10.1016/j.braindev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 280.Dedek K, Fusco L, Teloy N, Steinlein OK. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy research. 2003;54(1):21–7. doi: 10.1016/s0920-1211(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 281.Borgatti R, Zucca C, Cavallini A, Ferrario M, Panzeri C, Castaldo P, et al. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology. 2004;63(1):57–65. doi: 10.1212/01.wnl.0000132979.08394.6d. [DOI] [PubMed] [Google Scholar]
- 282.Schmitt B, Wohlrab G, Sander T, Steinlein OK, Hajnal BL. Neonatal seizures with tonic clonic sequences and poor developmental outcome. Epilepsy research. 2005;65(3):161–8. doi: 10.1016/j.eplepsyres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 283.Steinlein O, Conrad C, Weidner B. Benign familial neonatal convulsions: Always benign? Epilepsy research. 2007;73(3):245–9. doi: 10.1016/j.eplepsyres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 284.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Annals of neurology. 2012;71(1):15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 285.Dedek K, Kunath B, Kananura C, Reuner U, Jentsch TJ, Steinlein OK. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proceedings of the National Academy of Sciences. 2001;98(21):12272–7. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wuttke T, Jurkat-Rott K, Paulus W, Garncarek M, Lehmann-Horn F, Lerche H. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 2007;69(22):2045–53. doi: 10.1212/01.wnl.0000275523.95103.36. [DOI] [PubMed] [Google Scholar]
- 287.Zhou X, Ma A, Liu X, Huang C, Zhang Y, Shi R, et al. Infantile seizures and other epileptic phenotypes in a Chinese family with a missense mutation of KCNQ2. European journal of pediatrics. 2006;165(10):691–5. doi: 10.1007/s00431-006-0157-5. [DOI] [PubMed] [Google Scholar]
- 288.Castaldo P, del Giudice EM, Coppola G, Pascotto A, Annunziato L, Taglialatela M. Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels. J Neurosci. 2002;22(2):C199. doi: 10.1523/JNEUROSCI.22-02-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Uehara A, Nakamura Y, Shioya T, Hirose S, Yasukochi M, Uehara K. Altered KCNQ3 potassium channel function caused by the W309R pore-helix mutation found in human epilepsy. Journal of Membrane Biology. 2008;222(2):55–63. doi: 10.1007/s00232-008-9097-5. [DOI] [PubMed] [Google Scholar]
- 290.Volkers L, Rook MB, Das JH, Verbeek NE, Groenewegen WA, van Kempen MJ, et al. Functional analysis of novel KCNQ2 mutations found in patients with Benign Familial Neonatal Convulsions. Neuroscience letters. 2009;462(1):24–9. doi: 10.1016/j.neulet.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 291.Lerche H, Biervert C, Alekov A, Schleithoff L, Lindner M, Klingler W, et al. A reduced K+ current due to a novel mutation in KCNQ 2 causes neonatal convulsions. Annals of neurology. 1999;46(3):305–12. doi: 10.1002/1531-8249(199909)46:3<305::aid-ana5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 292.Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. Journal of Biological Chemistry. 2000;275(18):13343–8. doi: 10.1074/jbc.275.18.13343. [DOI] [PubMed] [Google Scholar]
- 293.Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proceedings of the National Academy of Sciences. 2006;103(23):8870–5. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Su J, Cao X, Wang K. A novel degradation signal derived from distal C-terminal frameshift mutations of KCNQ2 protein which cause neonatal epilepsy. Journal of Biological Chemistry. 2011;286(50):42949–58. doi: 10.1074/jbc.M111.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Singh NA, Otto JF, Jill Dahle E, Pappas C, Leslie JD, Vilaythong A, et al. Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization. The Journal of physiology. 2008;586(14):3405–23. doi: 10.1113/jphysiol.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Blackburn-Munro G, Dalby-Brown W, Mirza N, Mikkelsen J, Blackburn-Munro R. Retigabine: chemical synthesis to clinical application. CNS Drug Reviews. 2005;11(1):1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Orhan G, Wuttke TV, Nies AT, Schwab M, Lerche H. Retigabine/Ezogabine, a KCNQ/KV7 channel opener: pharmacological and clinical data. Expert opinion on pharmacotherapy. 2012;13(12):1807–16. doi: 10.1517/14656566.2012.706278. [DOI] [PubMed] [Google Scholar]
- 298.Weisenberg JL, Wong M. Profile of ezogabine (retigabine) and its potential as an adjunctive treatment for patients with partial-onset seizures. Neuropsychiatric disease and treatment. 2011;7:409. doi: 10.2147/NDT.S14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Amabile CM, Vasudevan A. Ezogabine: A Novel Antiepileptic for Adjunctive Treatment of Partial-Onset Seizures. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2013;33(2):187–94. doi: 10.1002/phar.1185. [DOI] [PubMed] [Google Scholar]
- 300.Tober C, Rostock A, Rundfeldt C, Bartsch R. D-23129: a potent anticonvulsant in the amygdala kindling model of complex partial seizures. European journal of pharmacology. 1996;303(3):163–9. doi: 10.1016/0014-2999(96)00073-8. [DOI] [PubMed] [Google Scholar]
- 301.Rostock A, Tober C, Rundfeldt C, Bartsch R, Engel J, Polymeropoulos EE, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy research. 1996;23(3):211–23. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 302.Brodie M, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75(20):1817–24. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- 303.French J, Abou-Khalil B, Leroy R, Yacubian E, Shin P, Hall S, et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76(18):1555–63. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- 304.Porter R, Partiot A, Sachdeo R, Nohria V, Alves W. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68(15):1197–204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 305.Tatulian L, Delmas P, Abogadie F, Brown D. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. The Journal of Neuroscience. 2001;21(15):5535–45. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7. 2 (KCNQ2) channel by binding to its activation gate. Molecular pharmacology. 2005;67(4):1009–17. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- 307.Dalby-Brown W, Jessen C, Hougaard C, Jensen ML, Jacobsen TA, Nielsen KS, et al. Characterization of a novel high-potency positive modulator of Kv7 channels. European journal of pharmacology. 2013 doi: 10.1016/j.ejphar.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 308.Qi J, Zhang F, Mi Y, Fu Y, Xu W, Zhang D, et al. Design, synthesis and biological activity of pyrazolo [1, 5-a] pyrimidin-7 (4H)-ones as novel Kv7/KCNQ potassium channel activators. European journal of medicinal chemistry. 2011;46(3):934–43. doi: 10.1016/j.ejmech.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 309.Kasteleijn-Nolst Trenité DG, Biton V, French JA, Abou-Khalil B, Rosenfeld WE, Diventura B, et al. Kv7 potassium channel activation with ICA-105665 reduces photoparoxysmal EEG responses in patients with epilepsy. Epilepsia. 2013 doi: 10.1111/epi.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nature Reviews Drug Discovery. 2009;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Roeloffs R, Wickenden AD, Crean C, Werness S, McNaughton-Smith G, Stables J, et al. In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3, 4-difluoro-benzamide], a potent and selective KCNQ2/Q3 (Kv7. 2/Kv7. 3) activator in rodent anticonvulsant models. Journal of Pharmacology and Experimental Therapeutics. 2008;326(3):818–28. doi: 10.1124/jpet.108.137794. [DOI] [PubMed] [Google Scholar]
- 312.Hirano K, Kuratani K, Fujiyoshi M, Tashiro N, Hayashi E, Kinoshita M. Kv7.2–7.5 voltage-gated potassium channel (KCNQ2–5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neuroscience letters. 2007;413(2):159–62. doi: 10.1016/j.neulet.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 313.Munro G, Dalby-Brown W. Kv7 (KCNQ) channel modulators and neuropathic pain. Journal of medicinal chemistry. 2007;50(11):2576–82. doi: 10.1021/jm060989l. [DOI] [PubMed] [Google Scholar]
- 314.Bi Y, Chen H, Su J, Cao X, Bian X, Wang K. Visceral hyperalgesia induced by forebrain-specific suppression of native Kv7/KCNQ/M-current in mice. Molecular pain. 2011;7(1):84. doi: 10.1186/1744-8069-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7. 2/3 channel activity is essential for the induction of tinnitus. Proceedings of the National Academy of Sciences. 2013;110(24):9980–5. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Redrobe JP, Nielsen AN. Effects of neuronal Kv7 potassium channel activators on hyperactivity in a rodent model of mania. Behavioural brain research. 2009;198(2):481–5. doi: 10.1016/j.bbr.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 317.Sotty F, Damgaard T, Montezinho LP, Mørk A, Olsen CK, Bundgaard C, et al. Antipsychotic-like effect of retigabine [N-(2-Amino-4-(fluorobenzylamino)-phenyl) carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. Journal of Pharmacology and Experimental Therapeutics. 2009;328(3):951–62. doi: 10.1124/jpet.108.146944. [DOI] [PubMed] [Google Scholar]
- 318.Stöllberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP) Epilepsy research. 2004;59(1):51–60. doi: 10.1016/j.eplepsyres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 319.Goldman A, Glasscock E, Yoo J, Chen T, Klassen T, Noebels J. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Science translational medicine. 2009;1(2):2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia. 2007;48(5):859–71. doi: 10.1111/j.1528-1167.2007.01082.x. [DOI] [PubMed] [Google Scholar]
- 321.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. The Lancet Neurology. 2008;7(11):1021–31. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- 322.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1. 1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. The Journal of Neuroscience. 2010;30(15):5167–75. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Monaghan MM, Menegola M, Vacher H, Rhodes KJ, Trimmer JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156(3):550–62. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lau D, de Miera EV-S, Contreras D, Ozaita A, Harvey M, Chow A, et al. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3. 2 K+ channel proteins. The Journal of Neuroscience. 2000;20(24):9071–85. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Barnwell LFS, Lugo JN, Lee WL, Willis SE, Gertz SJ, Hrachovy RA, et al. Kv4. 2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia. 2009;50(7):1741–51. doi: 10.1111/j.1528-1167.2009.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, et al. Altered phosphorylation and localization of the A-type channel, Kv4. 2 in status epilepticus. Journal of neurochemistry. 2008;106(4):1929–40. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Aronica E, Boer K, Doorn K, Zurolo E, Spliet W, van Rijen P, et al. Expression and localization of voltage dependent potassium channel Kv4. 2 in epilepsy associated focal lesions. Neurobiology of disease. 2009;36(1):81–95. doi: 10.1016/j.nbd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 328.Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, et al. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiology of disease. 2006;24(2):245–53. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 329.Lei Z, Deng P, Li J, Xu ZC. Alterations of A-type potassium channels in hippocampal neurons after traumatic brain injury. Journal of Neurotrauma. 2012;29(2):235–45. doi: 10.1089/neu.2010.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305(5683):532–5. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]