Abstract
We report the isolation of 10 differentially expressed cDNAs in the process of apoptosis induced by the p53 tamor suppressor. As a global analytical method, we performed a differential display of mRNA between mouse M1 myeloid leukemia cells and derived clone LTR6 cells, which contain a stably transfected temperature-sensitive mutant of p53. At 32 degrees C wild-type p53 function is activated in LTR6 cells, resulting in programmed cell death. Eight genes are activated (TSAP; tumor suppressor activated pathway), and two are inhibited (TSIP, tumor suppressor inhibited pathway) in their expression. None of the 10 sequences has hitherto been recognized as part of the p53 signaling pathway. Three TSAPs are homologous to known genes. TSAP1 corresponds to phospholipase C beta 4. TSAP2 has a conserved domain homologous to a multiple endocrine neoplasia I (ZFM1) candidate gene. TSAP3 is the mouse homologue of the Drosophila seven in absentia gene. These data provide novel molecules involved in the pathway of wild-type p53 activation. They establish a functional link between a homologue of a conserved developmental Drosophila gene and signal transduction in tumor suppression leading to programmed cell death.
Full text
PDF
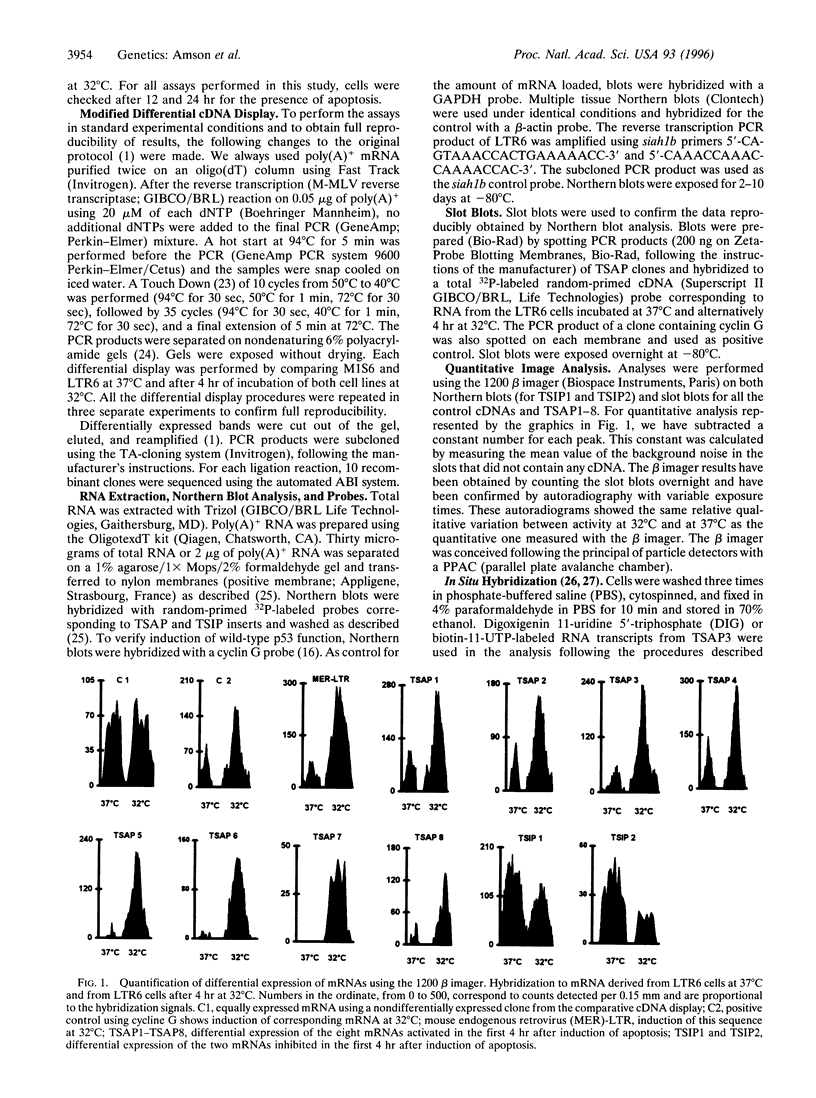



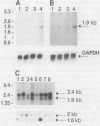
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Localization of mRNAs by in situ hybridization. Methods Cell Biol. 1991;35:37–71. doi: 10.1016/s0091-679x(08)60568-3. [DOI] [PubMed] [Google Scholar]
- Bauer D., Müller H., Reich J., Riedel H., Ahrenkiel V., Warthoe P., Strauss M. Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR). Nucleic Acids Res. 1993 Sep 11;21(18):4272–4280. doi: 10.1093/nar/21.18.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi M. L., Marx S. J., Aurbach G. D., Fitzpatrick L. A. Familial multiple endocrine neoplasia type I: a new look at pathophysiology. Endocr Rev. 1987 Nov;8(4):391–405. doi: 10.1210/edrv-8-4-391. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Neufeld T. P., Rubin G. M. Identification of genes that interact with the sina gene in Drosophila eye development. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11689–11693. doi: 10.1073/pnas.91.24.11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Rubin G. M. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990 Nov 2;63(3):561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- Della N. G., Bowtell D. D., Beck F. Expression of Siah-2, a vertebrate homologue of Drosophila sina, in germ cells of the mouse ovary and testis. Cell Tissue Res. 1995 Feb;279(2):411–419. doi: 10.1007/BF00318499. [DOI] [PubMed] [Google Scholar]
- Della N. G., Senior P. V., Bowtell D. D. Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina). Development. 1993 Apr;117(4):1333–1343. doi: 10.1242/dev.117.4.1333. [DOI] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Simon M. A., Rubin G. M. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992 Feb 6;355(6360):559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- Gobl A. E., Chowdhary B. P., Shu W., Eriksson L., Larsson C., Weber G., Oberg K., Skogseid B. Assignment of the mouse homologue of a human MEN1 candidate gene, phospholipase C-beta 3 (Plcb3), to chromosome region 19B by FISH. Cytogenet Cell Genet. 1995;71(3):257–259. doi: 10.1159/000134122. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Weinberg R. A. Tumor suppressor genes. Curr Opin Genet Dev. 1994 Feb;4(1):135–141. doi: 10.1016/0959-437x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Juven T., Barak Y., Zauberman A., George D. L., Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993 Dec;8(12):3411–3416. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kim M. J., Bahk Y. Y., Min D. S., Lee S. J., Ryu S. H., Suh P. G. Cloning of cDNA encoding rat phospholipase C-beta 4, a new member of the phospholipase C. Biochem Biophys Res Commun. 1993 Jul 30;194(2):706–712. doi: 10.1006/bbrc.1993.1879. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Park D. J., Lee K. H., Kim C. G., Rhee S. G. Purification, molecular cloning, and sequencing of phospholipase C-beta 4. J Biol Chem. 1993 Oct 5;268(28):21318–21327. [PubMed] [Google Scholar]
- Lee S. B., Rhee S. G. Significance of PIP2 hydrolysis and regulation of phospholipase C isozymes. Curr Opin Cell Biol. 1995 Apr;7(2):183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Linares-Cruz G., Rigaut J. P., Vassy J., De Oliveira T. C., De Cremoux P., Olofsson B., Calvo F. Reflectance in situ hybridization (RISH): detection, by confocal reflectance laser microscopy, of gold-labelled riboprobes in breast cancer cell lines and histological specimens. J Microsc. 1994 Jan;173(Pt 1):27–38. doi: 10.1111/j.1365-2818.1994.tb03425.x. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Beach D. Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 1994 Oct 17;13(20):4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Multiple forms of phospholipase C isozymes and their activation mechanisms. Adv Second Messenger Phosphoprotein Res. 1992;26:35–61. [PubMed] [Google Scholar]
- Toda T., Iida A., Miwa T., Nakamura Y., Imai T. Isolation and characterization of a novel gene encoding nuclear protein at a locus (D11S636) tightly linked to multiple endocrine neoplasia type 1 (MEN1). Hum Mol Genet. 1994 Mar;3(3):465–470. doi: 10.1093/hmg/3.3.465. [DOI] [PubMed] [Google Scholar]
- Watson K. L., Justice R. W., Bryant P. J. Drosophila in cancer research: the first fifty tumor suppressor genes. J Cell Sci Suppl. 1994;18:19–33. doi: 10.1242/jcs.1994.supplement_18.4. [DOI] [PubMed] [Google Scholar]
- Wu X., Bayle J. H., Olson D., Levine A. J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993 Jul;7(7A):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Bargonetti J., Walker K., Prives C., Levine A. J. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992 Jul;6(7):1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]
- Zauberman A., Barak Y., Ragimov N., Levy N., Oren M. Sequence-specific DNA binding by p53: identification of target sites and lack of binding to p53 - MDM2 complexes. EMBO J. 1993 Jul;12(7):2799–2808. doi: 10.1002/j.1460-2075.1993.tb05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]