Abstract
MicroRNAs (miRNAs) are a set of non-coding small RNA molecules in control of gene expression at posttranscriptional/translational level. They not only play crucial roles in normal developmental progress, but also are commonly dysregulated in human diseases, including cancer. MiR-200 is a family of tumor suppressor miRNAs consisting of five members, which are significantly involved in inhibition of epithelial-to-mesenchymal transition (EMT), repression of cancer stem cells (CSCs) self-renewal and differentiation, modulation of cell division and apoptosis, and reversal of chemoresistance. In this article, we summarize the latest findings with regard to the tumor suppressor signatures of miR-200 and the regulatory mechanisms of miR-200 expression. The collected evidence supports that miR-200 is becoming a new star miRNA in study of human cancer.
Keywords: microRNA, miR-200, EMT, stem cells, ZEB, cell cycle, cancer cell
1. Introduction
MicroRNAs (miRNAs) are a class of short non-coding RNA molecules with a significant regulatory capacity on gene expression [1, 2]. Similar to protein-coding genes, the primary transcripts of miRNA (pri-miRNA) are derived from genomic DNA and synthesized by RNA polymerase II or III into a hairpin structure [3, 4]. After being further processed by the RNase III family enzymes, Drosha and DiGeorge syndrome critical region gene 8 (DGCR8), the excess nucleotides at both the 3′ and 5′ regions of pri-miRNA are cropped off to form precursor miRNA (pre-miRNA) that is ∼70 nt in length [5-8]. Then, pre-miRNA will be exported out of the nucleus by the double-stranded RNA binding protein Exportin-5, and this intermediate product is subsequently cleaved by the RNase III family enzyme, Dicer, into imperfect mature miRNA:miRNA*duplexes in the cytoplasm [9-13]. The miRNA strand of approximately 18-22 nucleotides is incorporated into the RNA-induced silencing complex (RISC), which guides mature miRNA to trigger the target mRNA for subsequent silencing [14, 15]. Although the extent to which miRNAs regulate the human transcriptome is still under investigation, more and more evidence supports the fact that miRNA plays a crucial regulatory role in the control of gene expression.
To date, a total of 2,578 mature miRNA sequences have been identified in humans in accordance to the latest release of the miRBase database (http://www.mirbase.org/). The miRNA-200 family consisting five members (miR-200a, miR-200b, miR-200c, miR-429, and miR-141), is of particular interest for human health and disease. Based on the chromosomal locations, the miR-200 family can be divided into two clusters: the miR-200ba/429 cluster containing miR-200a, miR-200b, and miR-429, which is located on chromosome 1p36, and the miR-200c/141 cluster, which contains miR-200c and miR-141 and is located on chromosome 12p13. As known, in miRNA biology, seed sequences, in terms of the complementary sequences to binding sites of miRNA within the 3′ untranslated regions (UTRs) of the target genes, play a crucial role in regulatory effects of miRNA on gene expression [16]. Theoretically speaking, the members of a miRNA family contain highly conservative seed sequences; and miRNAs with the identical seed sequences may share the same putative target gene profiles. For the miR-200 family, two types of seed sequences are identified, which only have a nucleotide difference. MiR-200b, miR-200c, and miR-429 contain AAUACUG, whereas miR-200a and miR-141 possess AACACUG as their seed sequences. For the convenience of functional analysis, some studies named the miR-200bc/429 and miR-200a/141 clusters based on the seed sequences and potential similar target gene profiles [17], which are distinct from miR-200ba/429 and miR-200c/141 that are defined based on the chromosomal locations.
The first correlative report showing a relationship between the miR-200 family and human health demonstrated its high olfactory enrichment and neural expression patterns, consistent with a role in olfactory neurogenesis [18]. Soon afterwards, its involvement in human disease was confirmed when new studies demonstrated that miR-200 appeared to be downregulated during tumor progression and acted as a key inhibitor for epithelial-to-mesenchymal transition (EMT), tumor cell invasion, and metastasis. These benchmark results have been summarized in several high quality reviews [19-24]. To be complementary to these articles, we focus on presentation of the latest findings on studies of miR-200 tumor suppressive roles in human cancer, which include inhibition of EMT, repression of cancer stem cell (CSC) self-renewal and differentiation, modulation of cell division and apoptosis, and involvement in chemoresistance. Moreover, after sorting out many related publications, we summarize the mechanisms accounting for the regulation of miR-200 expression in various cancer cells. These results have not yet been collated to date but will be of importance to understand the tumor suppressor role of miR-200 in human cancer. We hope that this review article can provide useful insights into translation of the achievements gained from miRNA research into the future clinical applications.
2. Tumor suppressive signatures of miR-200
2.1. Inhibition of epithelial mesenchymal transition (EMT) and tumor metastasis
The involvement of miR-200 in cancer originates from several studies demonstrating a significant role for miR-200 in EMT, which is an important process in tumor progression as well as embryonic development. The expression of miR-200 family members was found to be highly associated with the epithelial phenotype of cancer cells, serving as a class of key markers for E-cadherin-positive and vimentin-negative cancer cell lines [25-27]. ZEB1 and ZEB2 are two members of the zinc-finger E-box binding homeobox family, and they have been defined as the master regulators in EMT [24]. The mechanism by which ZEB1 and ZEB2 facilitate EMT is that ZEBs can efficiently inhibit the cell-cell adhesion molecule E-cadherin, given that the aberrant expression of E-cadherin is a hallmark of EMT [28]. A number of studies have documented that, in epithelial cancer cells, highly expressed miR-200 represses the expression of ZEBs; whereas in mesenchymal cancer cells, impaired expression of miR-200 leads to induction of ZEBs and subsequent repression of E-cadherin [25-27]. Following the studies on the ability of miR-200 to regulate the expression of ZEB1 and ZEB2, dozens of others were carried out in various types of human tumors as summarized in Table 1[29-60]. While expression of the miR-200 family members was determined to be impaired in various human tumor cells leading to EMT and disease progression, increased miR-200 family member expression led to a reversal of EMT in bladder cancer, gastric cancer, nasopharyngeal carcinomas, ovarian cancer, pancreatic cancer, and prostate cancer [29, 39, 40, 44, 46, 52, 54]. In support of these results, studies by using clinical patient samples also indicated strong correlations between miR-200 expression and tumor progression in a variety of tumor types[31-33, 35, 37, 39, 41, 42, 45, 47, 48, 50, 53, 54, 56, 58-60]. Furthermore, it has been noted that low level expression of miR-200 could correlate with poor survival and serve as a prognostic marker for cancer patients [29, 38, 47, 58-60]. As such, the findings from the studies of miR-200 and EMT have become the benchmarks for further research on the tumor suppressive signatures of miR-200.
Table 1. Dysregulation of miR-200 in various human cancers.
| Members of miR-200 family | Cancer types | References |
|---|---|---|
| miR-200a, 200b, 200c, 141 | Bladder cancer | [29] |
| miR-200a, 200b, 200c, 141, 429 | Breast cancer | [30-32] |
| miR-200a, 200b, 200c | Colorectal cancer | [33; 34; 58] |
| miR-200a, 200c | Cutaneous melanoma | [35] |
| miR-200a, 200b, 200c, 141, 429 | Endometrial cancer | [36; 37] |
| miR-200a, 200b, 200c, 141 | Gastric cancer | [38-41] |
| miR- 200c | Hepatocellular tumor | [42] |
| miR-200a, 200b, 200c, 141, 429 | Lung cancer | [43; 59] |
| miR-200a | Nasopharyngeal carcinoma | [44] |
| miR-200a, 200b, 200c, 141, 429 | Oral squamous cell carcinoma | [45] |
| miR-200a, 200b, 200c, 141, 429 | Ovarian cancer | [46-51] |
| miR-200a, 200b | Pancreatic cancer | [52; 60] |
| miR-200b, 200c, 141, 429 | Pleural mesothelioma | [53] |
| miR-200a | Prostate cancer | [54] |
| miR-200a, 200b, 200c, 141 | Renal cell carcinoma | [55] |
| miR-200a, 200c, 141, 429 | Spindle cell carcinoma of the head and neck | [56] |
| miR-200a, 200b, 200c, 141 | Thyroid carcinoma | [57] |
EMT is considered the initiating event for cancer metastasis. Two different groups simultaneously reported that miR-200 was significantly downregulated in metastases and metastatic-like primary tumors, thereby relieving the repression conferred by the mesenchymal transcription factor ZEB1 in vivo. Forced expression of miR-200 abrogated the capacity of tumor cells to undergo invasion and metastasis, underscoring the role for miR-200 in the regulation of both EMT and subsequent metastases [61, 62]. A similar conclusion was drawn through studying different human ovarian cancer cell lines with distinct capabilities to metastasize [63], while miR-200 has also been implicated in the reversal of the metastatic phenotype of non-small cell lung cancer, as its re-expression has been shown to downregulate the expression of many prognostic markers for metastasis, such as alpha thalassemia/mental retardation syndrome X-linked gene (ATRX), deleted in liver cancer 1 gene (DLC1), hereditary hemochromatosis gene (HFE), and heterogeneous nuclear ribonucleoprotein A3 gene (HNRNPA3)[43]. Given the mechanistic studies supporting that the Notch signaling pathway plays a crucial role in the regulation of EMT and thus metastasis during cancer progression [64], miR-200 was found to decrease expansion of human metastatic prostate cancer cells by targeting the Notch ligand Jagged1 and the mastermind-like coactivators Maml2 and Maml3, the key components in the Notch pathway [65, 66]. Complementary to these results, Yang et al. found that the Notch ligand Jagged2 was also able to inhibit miR-200 family expression at the transcriptional level by induction of GATA transcription factors, which eventually led to promotion of tumor metastasis in vivo [67]. These findings support a regulatory loop consisting of miR-200 and the Notch signaling pathway; the balance of their interaction can potentially decide the stages of tumor progression (Figure 1A). However, the molecular mechanisms accounting for the tumor suppressor roles of miR-200 are still largely unknown, although the Notch signaling pathway sheds light on understanding of its anti-metastatic activity. A recent study reported the controversial evidence that miR-200 was found to be upregulated in breast cancer 4T1 cells that formed macroscopic metastases in vivo when compared with related cells invading distant tissues but were unable to colonize. The authors proposed that miR-200 might be involved in promotion of the last step of the metastatic cascade when establishing macroscopic metastatic masses at distant sites [68]. Given most current findings supporting miR-200 as a tumor suppressor, additional evidence is needed to confirm such a hypothesis in which miR-200 plays an oncogenic role. Together with the myriad of aforementioned studies from a wide variety of cancers listed in Table 1, these data suggest that the miR-200 family plays a significant role in combating not only EMT, but also tumor cell invasion and metastases. Thereby, miR-200 has great potential to become a novel class of biomarkers for tumor prognosis and targets for new drug development against tumor progression.
Figure 1.
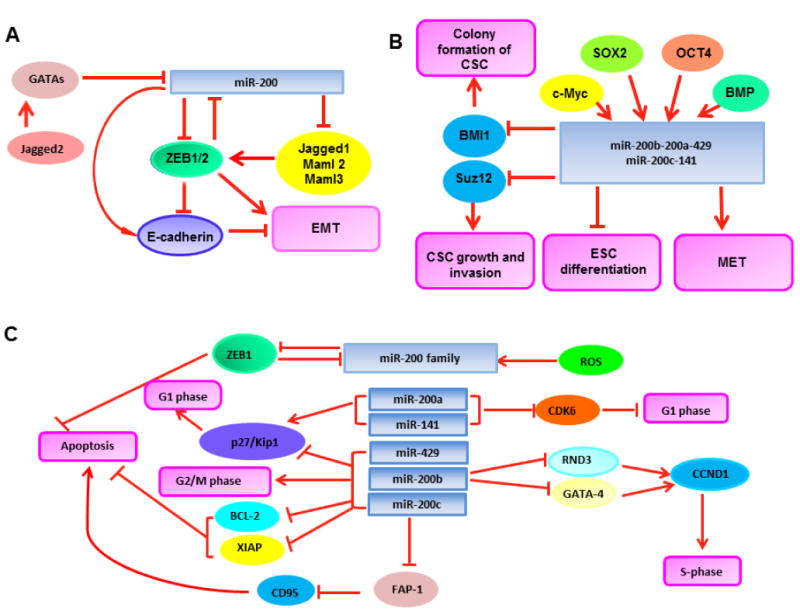
Tumor suppressive signatures of miR-200. (A) MiR-200 inhibits EMT by interacting with ZEB1/2 and the Notch pathway. (B) MiR-200 represses self-renewal and differentiation in CSCs. (C) MiR-200 is involved in the regulation of cell division and apoptosis.
2.2. Repression of cancer stem cell self-renewal and differentiation
The most essential feature of stem cells is the capability of self-renewal to produce dozens of differentiated cell types. The increased evidence shows that miRNAs play a crucial role in regulating stem cell self-renewal and differentiation. Tumor initiating cells, also called cancer stem cells (CSCs), have been identified in various types of cancer. The first report linking miR-200 and stem cell physiology came in 2009 from a study in which all five members of the miR-200 family were shown to be downregulated in human breast CSCs as well as in normal human and murine mammary stem/progenitor cells [69]. By targeting B lymphoma Mo-MLV insertion region 1 homolog (BMI1), a known regulator for stem cell self-renewal, miR-200c was shown to strongly suppress the ability of normal mammary stem cells from forming mammary ducts and tumor formation driven by human breast CSC in vivo [69]. Not only can miR-200c suppress tumor formation driven by breast CSCs, but miR-200b can downregulate CSC growth through targeting the Polycomb family embryonic stem (ES) cell pluripotency maintenance factor Suz12 [70]. Moreover, Lim et al. reported that the immortalized human mammary epithelial cells convert from a non-stem to a stem cell-like phenotype accompanied with a loss of miR-200 expression. Restoration of miR-200 expression decreased stem-like properties while promoting a transition to an epithelial phenotype [71]. Thus, it is apparent that miR-200 family members play important roles in multiple events related to CSCs.
Many of the molecular factors needed to maintain pluripotency of stem cells have been found to regulate the expression of the miR-200 family of miRNAs, such as c-Myc, octamer-binding transcription factor 4 (Oct4), and sex determining region Y-box 2 (Sox2). Lin et al. reported that the expression of the miR-200 family was regulated by c-Myc in ES cells. The transcriptional induction of these miRNAs by c-Myc significantly attenuated the downregulation of pluripotency markers, which indicates that in ES cells, c-Myc acts, at least in part, through the miR-200 family to attenuate differentiation [72]. In addition, Wang et al. found that Oct4 and Sox2 induced the transcriptional activation of the miR-200 family, which promoted the mesenchymal-to-epithelial transition (MET) and the generation of induced pluripotent stem cells (iPSCs) by targeting ZEB2 [73], thus leading to somatic cell reprogramming. However, in proliferating vMH (ventral midbrain/hindbrain) neural progenitors, miR-200 was required to promote cell-cycle exit and neuronal differentiation through targeting the expression of Sox2 and the cell cycle regulator E2F3 [74]. Samavarchi-Tehrani et al. also found that during the initiation phase of reprogramming, the bone morphogenetic protein (BMP) signaling could induce the expression of miR-200 and miR-205, forming a multistep mechanism that incorporates a BMP-miRNA-MET axis to promote somatic cell reprogramming [75]. Thus, as illustrated in Figure 1B, the miR-200 family has been demonstrated to bear integral importance in the process of self-renewal and differentiation of CSCs.
2.3. Modulatory role of miR-200 in cell division and apoptosis
Cell division is a vital process used by a single fertilized egg to develop into a mature organism, as well as to renew cells, tissues, and thus organs. The control of the cell cycle by miRNA is well established, and ectopic expression of certain miRNAs may contribute to tumor development by perturbing important cell cycle regulators. Uhlmann et al. were the first to describe a role for miR-200 in regulating cell cycle progression in breast cancer [17]. They found that overexpression of miR-200a/141 resulted in G1 arrest, which may be due to increased expression of cyclin dependent kinase inhibitor 1B (p27/Kip1) and decreased expression of cyclin dependent kinase 6 (CDK6). In contrast, elevating the expression miR-200bc/429 cluster caused a reduction of p27/Kip1 expression and upregulation of inhibitory phosphorylation of cell division cycle 25C gene (CDC25C), thereby decreasing the G1 population and increasing the G2/M population [17]. It is noticed that, in this study, the miR-200bc/429 and miR-200a/141 clusters are defined by using the different seed sequences and target gene pools, which account for, at least in part, their distinct functions in regulation of cell cycle. Xia et al. found that, in HeLa cells, miR-200b directly downregulated Rho family GTPase 3 (RND3), thereby promoting expression of the downstream cell cycle regulatory factor cyclin D1 (CCND1) that controls S-phase entry [76]. More recently, Yao et al. demonstrated that miR-200b was a critical regulator of the zinc finger transcription factor GATA-4, which regulates the expression of CCND1 at the transcriptional level. Their results supported that miR-200b could regulate tumor cell growth and differentiation by targeting GATA-4 to downregulate the expression of CCND1 [77]. Thus, these findings support that miR-200 can exert control over the cell cycle at many levels through the modulation of several different factors.
In addition to playing a role in the regulation of cell cycle, the miR-200 family has been shown to modulate apoptosis. Schickel et al. found that altering levels of miR-200c changed the sensitivity of cells to death receptor CD95-mediated apoptosis [78]. In addition, they identified the apoptosis inhibitor, Fas-associated phosphatase-1 (FAP-1), as a target of miR-200c, which was demonstrated to be responsible for the reduced sensitivity of CD95-mediated apoptosis in cells with inhibited miR-200. It was also reported that reactive oxygen species (ROS) could induce miR-200 family expression with subsequent downmodulation of ZEB1, which was likely to play a key role in ROS-induced apoptosis and senescence [79]. Figure 1C illustrates the involvement of miR-200 in regulation of cell division and apoptosis.
2.4 Reversal of chemoresistance
Although the mechanisms have not yet been completely understood, the chemoresistance to a few drugs, such as gemcitabine, paclitaxel, cisplatin, doxorubicin, docetaxel, EGFR inhibitors, and vincristine, has been reported to be associated with the downregulation of miR-200 [80-88]. Interestingly, some of these studies also reported that the restoration of miR-200 could effectively reverse the chemoresistance to certain drugs [82, 87, 88]. Meng et al. first reported that inhibition of miR-200b expression could increase the sensitivity of cholangiocarcinoma cells to the nucleoside analog gemcitabine [80]. Ali et al. reported that the curcumin analogue CDF (a novel turmeric spice analogue) was able to cause reactivation of miR-200b/c, which in turn resulted in the reversal of the EMT phenotype and thus sensitized pancreatic cancer cells to gemcitabine [81]. Cochrane et al. found that restoration of miR-200c could enhance the sensitivity of the antimicrotubule agent paclitaxel in resistant cancer cells through targeting TUBB3, which encodes class III β tubulin [82]. In breast cancer MCF7 cells, the miR-200 family was specifically down-regulated in those cells resistant to two therapeutic agents commonly used in the treatment of breast cancer, doxorubicin or cisplatin [83, 84]. By miRNA microarray, Wang et al. found that miR-200b was identified as the most downregulated miRNA in docetaxel-resistant human lung adenocarcinoma (SPC-A1/DTX) cells compared with the parental (SPC-A1) cells [85]. When examining the miRNA expression from lung adenocarcinoma patients treated with docetaxel-based chemotherapy, aberrant expression of miR-200b was correlated with decreased sensitivity to the antimitotic agent docetaxel [86]. Additionally, miR-200 expression correlated with sensitivity to epidermal growth factor receptor (EGFR) blocking agents in bladder cancer, and restoration of miR-200 increased the sensitivity in mesenchymal-like cell lines [87]. Recently, Zhu et al. reported that the miR-200bc/429 cluster was impaired in vincristine-resistant gastric cancer cells and cisplatin-resistant lung cancer cells. However, the restoration of the miR-200bc/429 cluster could sensitize the tumors cells to chemotherapy. The mechanistic studies suggested that this effect resulted from, at least partially, recurred apoptosis led by the suppressive effect of miR-200 on two anti-apoptotic factors, B-cell lymphoma 2 gene (BCL-2) and X-linked inhibitor of apoptosis protein gene (XIAP)[88].
In summary, aside from the well-documented inhibitory effect on EMT, many novel tumor suppressive signatures of miR-200 have been identified in various human cancer cells, such as inhibition of CSC self-renewal and differentiation, modulation of cell division and apoptosis, and reversal of chemoresistance. Figure 1 illustrates the tumor suppressor roles of miR-200 that have been discussed in this article. These findings will support that miR-200 not only is a valuable biomarker for tumorigenesis and progression, but can also become a potential target for new drug development.
3. Regulation of miR-200 expression
Given that miRNA is a class of non-coding small RNA molecules, the transcription factor involved regulation and epigenetic modulation are two major mechanisms that contr o l miRNA expression. Due to the increased interest in miR-200 as well as its tumor suppressor role in a variety of human tumors, better understanding of miR-200 regulatory mechanisms can provide insights into the future translation of miR-200's tumor suppressive signatures into development of novel strategies for treating human cancer. Here, we will summarize the latest research results on studies of miR-200 expression regulation.
3.1 Transcription factor involved regulation
Regulatory mechanisms for miR-200's expression have been studied in dozens of cell types, during which several essential transcription factors have been determined to be involved. In addition to the master inducers of EMT, ZEB1 and ZEB2 are known as the transcription factors containing zinc-finger domains [24]. Within the putative promoter region of miR-200c/141 and in spacers between the miR-200c and miR-141 stem loops, there are two highly conserved Z-box and four E-box transcription factor binding motifs to which ZEB1 can bind for suppression of this family's polycistronic transcription [89]. Similarly, ZEB1 and ZEB2 can also repress miR-200ba/429 polycistronic transcription by binding to their regulatory E-boxes [90]. Interestingly, ZEB1 and ZEB2 were also demonstrated as targets of miR-200 so that they form a mutually inhibitory feedforward loop as shown in Figure 1A [24-27]. Additionally, two other related transcription factors known to be associated with EMT, Snail and Slug, were shown to be able to negatively regulate transcription of miR-200, providing additional evidence in support of the involvement of miR-200 in EMT [91, 92].
Members of various other transcription factor families have also been shown to be involved in the regulated expression of miR-200. Mizuguchi et al. reported that regulation of miR-200 transcription by ZEB1 could be modulated via the involvement of the transcription factors P300 and PCAF [93]. They found that the activation of miR-200c/141 transcription occurred when P300 and PCAF physically interacted to form a transcriptional complex involving ZEB1, while disruption of the P300-PCAF interaction significantly suppressed the transcriptional activity [93]. A recent study report that the proto-oncogene c-Myb was able to upregulate all miR-200 miRNA family members through the transcriptional regulation, whereas this inductive effect could be completely attenuated by ZEB1 at the onset of EMT [94]. A previous study also reported that miR-200b, miR-200c, and miR-429 target c-Myb and repress its expression [95]. Therefore, a reciprocal feedback loop may involve the mutual regulation between miR-200 and c-Myb. Another factor recently demonstrated to directly modulate the expression of miR-200a and miR-141 is the gene named proline, glutamic acid and leucine rich protein 1 (PELP1), a nuclear receptor that can be self-upregulated during the metastatic progression of breast cancer cells [96]. PELP1 is found to form a complex with histone deacetylating ezymes (HDAC2) that binds to the miR-200's promoters, thereby downregulating their expression [96]. Kim et al. reported that inhibitory effect of P53 on EMT involves the transactivation of miR-200; miR-200 repressing ZEBs is responsible for P53 regulated EMT [97]. In addition, P63 and P73, the members of the P53 transcription factor family, can also directly regulate transcription of miR-200 through binding with the p53/p63/p73 binding sites within the promoter regions of both miR-200 clusters [98]. Ahn et al. recently reported that Smad3 can transcriptionally induce miR-200 in a transforming growth factor β (TGF-β) independent manner, accounting for its suppressive effect on EMT in gastric cancer cells [99].
As discussed earlier, the transcription factors c-Myc, Oct4, and Sox2, which are largely essential for maintenance of pluripotency in stem cells, also play critical roles in regulating miR-200 family expression. In ES cells, c-Myc can upregulate miR-200b that leads to attenuation of ES differentiation [72], whereas in the endometrial cancer cells treated with tamoxifen, miR-200 repressed by elevated c-Myc and associated with the onset of EMT [100]. During iPSC reprogramming from fibroblasts, exogenous Oct4 and Sox2 can bind the promoter regions of the miR-200ba/429 and miR-200c/141 clusters, respectively, to activate their transcription [73], suggesting that miR-200 is involved in reprogramming and stem cell self-renewal, whereas Sox2 and E2F3 can activate the transcription of miR-200c/141 cluster in vivo [74]. In addition, the TGF-β pathway ligand, bone morphogenetic protein (BMP7), was shown to synergize with the Yamanaka factors (Oct4, Klf, c-Myc, and Sox2) to induce miR-200 expression during the initial reprogramming phase of iPSC generation involving MET [75]. As such, many different molecular factors have been documented to control the regulated expression of miR-200, which in turn shows variable downstream effects on human diseases.
3.2 Epigenetic modification
Aside from the regulated expression conferred by the aforementioned transcription factors, epigenetic modification in the form of DNA methylation has been determined to control miR-200 family expression in both normal and cancer cells [101]. Substantial cytosine methylation, usually in CpG islands located within the gene's promoter region, has been documented as an epigenetic marker of gene repression. Studies have reported that the regulatory regions of both miR-200 clusters contain CpG-rich sequences. Li et al. reported the epigenetic regulation of miR-200 expression when they showed that miR-200a and miR-200b were hypomethylated and overexpressed in pancreatic cancer cells [52]. Vrba et al. found that in miR-200c/141-negative normal and tumor cells, its CpG island was heavily methylated, whereas in miR-200c/141-expressing cells, the CpG island was unmethylated [101], indicating a role for DNA methylation in miR-200c/141 regulation in both normal and tumor cells. In addition, mouse cells showed a similar correlation between DNA methylation and miR-200c expression. Wiklund et al. found that in muscle invasive bladder tumors and undifferentiated bladder cell lines, both miR-200 clusters were silenced concomitant with DNA hypermethylation, while in oral squamous cell carcinoma, miR-200 was epigenetically activated [29, 45]. More recently, the focal adhesion protein Kindlin 2 was found to form a complex with DNA (cytosine-5-)-methyltransferase 3 alpha (DNMT3A) in the breast cancer cell nucleus to induce CpG island hypermethylation of the miR-200 promoter in order to downregulate the expression of the miR-200 family [102]. It is of note that the aforementioned cases in which DNA methylation was used to downregulate miR-200 expression seemed to correlate with increased tumor formation and/or cell invasion.
In addition to DNA methylation, histone modification is another major class of epigenetic modulation used to regulate expression of the miR-200 family. Tellez et al. reported that a 4-week exposure of immortalized human bronchial epithelial cells to tobacco carcinogens could induce EMT, most likely due to the silencing of miR-200b, miR-200c and miR-205,which occurred due to methylation initiated at histone H3 (H3K27me3) and later by increasing DNA methylation [103]. Recently, Lim et al. found that in the stem-like phenotype of immortalized human mammary epithelial cells, the miR-200ba/429 cluster was silenced primarily through histone modifications, whereas the miR-200c/141 cluster was repressed via DNA methylation [71]. More recently and interestingly, Zhang et al. found that the H19 long non-coding RNA (lncRNA) is associated with the hnRNPU/PCAF/RNA Pol II complex, which is able to activate the miR-200 family via histone acetylation [104]. Treatment of an endocrine-resistant breast cancer cell line with both a demethylating agent as well as a deacetylating agent resulted in an increase in expression of both miR-200b and miR-200c, leading to a repression of ZEB1 and showing more epithelial-like characteristics [105]. Thus, it is clear that epigenetic modulation, including DNA methylation as well as histone methylation and acetylation, plays an essential role in the regulated expression of the miR-200 family.
The molecular mechanisms and related factors are mapped on Figure 2, illustrating the transcriptional regulation of miR-200 expression. In addition to the transcription factor involved regulation and epigenetic modification, other mechanisms/factors have also been studied. For example, Iliopoulos et al. reported that miR-200 was differentially regulated by two of the serine/threonine kinase Akt isoforms in breast cancer cells [106]. As they found that miR-200 family expression was decreased in either cells bearing Akt2 or cells of Akt 1 knockdown, but unaltered in cells of Akt2 knockdown or both Akt1 and Akt2 knockdown, it was inferred that miR-200 expression may be dependent on the balance between Akt1 and Akt2[106]. However, in prostate cancer, silencing of Akt2 is able to inversely induce the expression of miR-200 [107]. These findings suggest that Akt is involved in the regulation of miR-200 expression, although the mechanism of action has not been uncovered.
Figure 2.
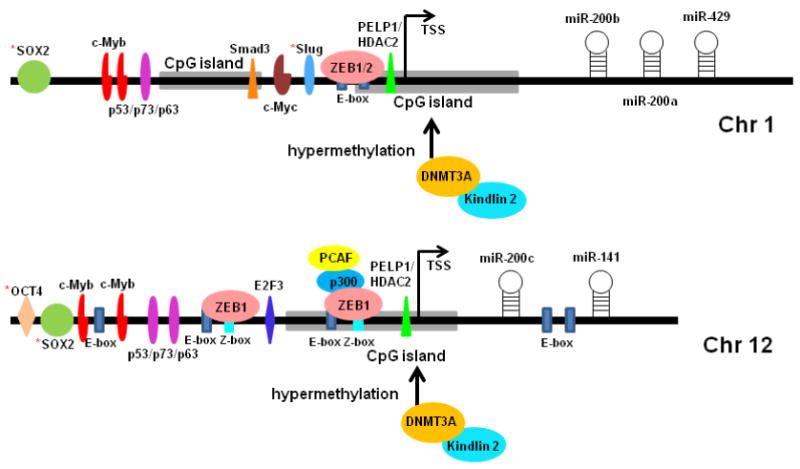
Regulation of miR-200 expression involving multiple transcription factors (TFs) and epigenetic modification. TSS represents transcription starting site. * TFs determined in mouse models.
4. Conclusion and Perspective
The inhibitory effect of miR-200 on EMT by targeting ZEBs is the benchmark achievement to understand the tumor suppressor roles of miR-200 in human cancer. As discussed earlier, the promoter regions of both miR-200 clusters have binding sites for ZEB1 and ZEB2 transcription factors. Interestingly, both ZEBs can be targeted by miR-200, reciprocally, hinting at a possible unknown mechanism by which a feedback inhibition loop can be broken and cells can be switched between an epithelial and mesenchymal phenotype. Given the miR-200 family functioning as a potential suppressor of EMT to prevent malignant tumor progression, future work may improve our understanding of miR-200's role in cancer progression by identification and functional characterization of additional downstream targets of miR-200 that might be involved in multiple cellular events. Moreover, the newly identified tumor suppressor roles of miR-200, such as inhibition of CSC self-renewal and differentiation, modulation of cell division and apoptosis, and reversal of chemoresistance, are of significance to support that miR-200 is a new star miRNA in cancer research. In addition to the updats of new knowledge with regard to the tumor suppressive signatures of miR-200, we sort out the latest findings and summarize the mechanisms involved in the regulation of miR-200 expression. Given the non-coding nature of miRNA, transcription regulation involving multiple transcription factors and epigenetic modulation including DNA methylation and histone modification are major forms accounting for the regulatory basis of miR-200 expression. The understanding of these regulatory mechanisms can not only deepen our understanding of miR-200 anticancer activity, but also aid us in development of novel drugs to target these small molecules.
To date, there are 236 publications collected in PUBMED with regard to miR-200 and cancer; however, more than 75% of these publications are published over the past three years (from September 2010 to August 2013), which indicates the growing interest in the study of miR-200. Due to the tumor suppressive signature of miR-200, a proposal for restoring miR-200 expression may be taken into consideration as a novel therapeutic to treat human cancer, although the development of such a strategy still depends on a better understanding of the mechanistic basis of miR-200 anticancer activity. In the near future, studying the relationship between miR-200 and CSCs, coupled with improvements of drug delivery systems, may also become a promising area to discover novel therapeutics for treating human cancer efficaciously.
Acknowledgments
This study is supported by the American Cancer Society Research Scholar Grant (RSG-13-265-01-RMC, to Xi) and the NIH/NCI R21 Grant (5R21CA160280, to Xi). We sincerely apologize to those whose work was not cited due to time and space constraints.
Footnotes
Conflict of Interest Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 6.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 7.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 10.Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression o the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 11.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 12.Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight SW, Bass BL. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science. 2001;293:2269–2271. doi: 10.1126/science.1062039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 16.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Uhlmann S, Zhang JD, Schwager A, Mannsperger H, Riazalhosseini Y, Burmester S, Ward A, Korf U, Wiemann S, Sahin O. miR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene. 2010;29:4297–4306. doi: 10.1038/onc.2010.201. [DOI] [PubMed] [Google Scholar]
- 18.Choi PS, Zakhary L, Choi WY, Caron S, Alvarez-Saavedra E, Miska EA, McManus M, Harfe B, Giraldez AJ, Horvitz HR, Schier AF, Dulac C. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cano A, Nieto MA. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. doi: 10.1016/j.tcb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 21.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219–222. doi: 10.4161/cbt.10.6312548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: at the crossroads of signal transduction in cancer. Int J Cancer. 2013;132:745–754. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- 25.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 27.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browne G, Sayan AE, Tulchinsky E. ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle. 2010;9:886–891. doi: 10.4161/cc.9.5.10839. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund ED, Bramsen JB, Hulf T, Dyrskjot L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, Peter ME, Orntoft TF, Kjems J, Clark SJ. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 30.Aydogdu E, Katchy A, Tsouko E, Lin CY, Haldosen LA, Helguero L, Williams C. MicroRNA-regulated gene networks during mammary cell differentiation are associated with breast cancer. Carcinogenesis. 2012;33:1502–1511. doi: 10.1093/carcin/bgs161. [DOI] [PubMed] [Google Scholar]
- 31.Castilla MA, Diaz-Martin J, Sarrio D, Romero-Perez L, Lopez-Garcia MA, Vieites B, Biscuola M, Ramiro-Fuentes S, Isacke CM, Palacios J. MicroRNA-200 family modulation in distinct breast cancer phenotypes. PLoS One. 2012;7:e47709. doi: 10.1371/journal.pone.0047709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gravgaard KH, Lyng MB, Laenkholm AV, Sokilde R, Nielsen BS, Litman T, Ditzel HJ. The miRNA-200 family and miRNA-9 exhibit differential expression in primary versus corresponding metastatic tissue in breast cancer. Breast Cancer Res Treat. 2012;134:207–217. doi: 10.1007/s10549-012-1969-9. [DOI] [PubMed] [Google Scholar]
- 33.Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–1326. doi: 10.1136/gutjnl-2011-301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paterson EL, Kazenwadel J, Bert AG, Khew-Goodall Y, Ruszkiewicz A, Goodall GJ. Down-regulation of the miRNA-200 family at the invasive front of colorectal cancers with degraded basement membrane indicates EMT is involved in cancer progression. Neoplasia. 2013;15:180–191. doi: 10.1593/neo.121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kempen LC, van den Hurk K, Lazar V, Michiels S, Winnepenninckx V, Stas M, Spatz A, van den Oord JJ. Loss of microRNA-200a and c, and microRNA-203 expression at the invasive front of primary cutaneous melanoma is associated with increased thickness and disease progression. Virchows Arch. 2012;461:441–448. doi: 10.1007/s00428-012-1309-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Park YA, Choi JJ, Lee YY, Kim CJ, Choi C, Kim TJ, Lee NW, Kim BG, Bae DS. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol Oncol. 2011;120:56–62. doi: 10.1016/j.ygyno.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Snowdon J, Zhang X, Childs T, Tron VA, Feilotter H. The microRNA-200 family is upregulated in endometrial carcinoma. PLoS One. 2011;6:e22828. doi: 10.1371/journal.pone.0022828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T, Si J. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J Gastroenterol. 2009;44:556–561. doi: 10.1007/s00535-009-0037-7. [DOI] [PubMed] [Google Scholar]
- 39.Kurashige J, Kamohara H, Watanabe M, Hiyoshi Y, Iwatsuki M, Tanaka Y, Kinoshita K, Saito S, Baba Y, Baba H. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19(Suppl 3):S656–664. doi: 10.1245/s10434-012-2217-6. [DOI] [PubMed] [Google Scholar]
- 40.Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, Uozaki H, Takada K, Fukayama M. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70:4719–4727. doi: 10.1158/0008-5472.CAN-09-4620. [DOI] [PubMed] [Google Scholar]
- 41.Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Diaz P, Lorenzo-Patino MJ, Haz M, Santamarina I, Blanco M, Fernandez-Tajes J, Quindos M, Carral A, Figueroa A, Anton-Aparicio LM, Calvo L. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 43.Pacurari M, Addison JB, Bondalapati N, Wan YW, Luo D, Qian Y, Castranova V, Ivanov AV, Guo NL. The microRNA-200 family targets multiple non-small cell lung cancer prognostic markers in H1299 cells and BEAS-2B cells. Int J Oncol. 2013;43:548–560. doi: 10.3892/ijo.2013.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia H, Ng SS, Jiang S, Cheung WK, Sze J, Bian XW, Kung HF, Lin MC. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem Biophys Res Commun. 2010;391:535–541. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 45.Wiklund ED, Gao S, Hulf T, Sibbritt T, Nair S, Costea DE, Villadsen SB, Bakholdt V, Bramsen JB, Sorensen JA, Krogdahl A, Clark SJ, Kjems J. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One. 2011;6:e27840. doi: 10.1371/journal.pone.0027840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendoraite A, Knouf EC, Garg KS, Parkin RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY, Drescher CW, Urban N, Knudsen BS, Tewari M. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010;116:117–125. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN, Wang X. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Leskela S, Leandro-Garcia LJ, Mendiola M, Barriuso J, Inglada-Perez L, Munoz I, Martinez-Delgado B, Redondo A, de Santiago J, Robledo M, Hardisson D, Rodriguez-Antona C. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 49.Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P, Sastre-Garau X, Mechta-Grigoriou F. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- 50.Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 51.Prislei S, Martinelli E, Mariani M, Raspaglio G, Sieber S, Ferrandina G, Shahabi S, Scambia G, Ferlini C. MiR-200c and HuR in ovarian cancer. BMC Cancer. 2013;13:72. doi: 10.1186/1471-2407-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, Borges M, Goggins M. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee GV, Koestler DC, Christensen BC, Sugarbaker DJ, Ugolini D, Ivaldi GP, Resnick MB, Houseman EA, Kelsey KT, Marsit CJ. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127:2859–2869. doi: 10.1002/ijc.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barron N, Keenan J, Gammell P, Martinez VG, Freeman A, Masters JR, Clynes M. Biochemical relapse following radical prostatectomy and miR-200a levels in prostate cancer. Prostate. 2012;72:1193–1199. doi: 10.1002/pros.22469. [DOI] [PubMed] [Google Scholar]
- 55.Castro-Vega LJ, Jouravleva K, Liu WY, Martinez C, Gestraud P, Hupe P, Servant N, Albaud B, Gentien D, Gad S, Richard S, Bacchetti S, Londono-Vallejo A. Telomere crisis in kidney epithelial cells promotes the acquisition of a microRNA signature retrieved in aggressive renal cell carcinomas. Carcinogenesis. 2013;34:1173–1180. doi: 10.1093/carcin/bgt029. [DOI] [PubMed] [Google Scholar]
- 56.Zidar N, Bostjancic E, Gale N, Kojc N, Poljak M, Glavac D, Cardesa A. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck--hallmark of epithelial-mesenchymal transition. Hum Pathol. 2011;42:482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 58.Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR, Zhang W. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ, Wang YK, Zeng F, Zhou JH, Zhang YK. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29:618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, Wang L, Matyunina LV, Hill CG, McDonald JF. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecol Oncol. 2011;121:200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 64.Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, McCue PA, Quong AA, Lisanti MP, Pestell RG. microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci U S A. 2010;107:8231–8236. doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallejo DM, Caparros E, Dominguez M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011;30:756–769. doi: 10.1038/emboj.2010.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, Goodall GJ, Kurie JM. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121:1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, Martinvalet D, Song E, Lim B, Lieberman J. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim YY, Wright JA, Attema JL, Gregory PA, Bert AG, Smith E, Thomas D, Lopez AF, Drew PA, Khew-Goodall Y, Goodall GJ. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci. 2013;126:2256–2266. doi: 10.1242/jcs.122275. [DOI] [PubMed] [Google Scholar]
- 72.Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J. 2009;28:3157–3170. doi: 10.1038/emboj.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang G, Guo X, Hong W, Liu Q, Wei T, Lu C, Gao L, Ye D, Zhou Y, Chen J, Wang J, Wu M, Liu H, Kang J. Critical regulation of miR-200/ZEB2 pathway in Oct4/Sox2-induced mesenchymal-to-epithelial transition and induced pluripotent stem cell generation. Proc Natl Acad Sci U S A. 2013;110:2858–2863. doi: 10.1073/pnas.1212769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng C, Li N, Ng YK, Zhang J, Meier F, Theis FJ, Merkenschlager M, Chen W, Wurst W, Prakash N. A unilateral negative feedback loop between miR-200 microRNAs and Sox2/E2F3 controls neural progenitor cell-cycle exit and differentiation. J Neurosci. 2012;32:13292–13308. doi: 10.1523/JNEUROSCI.2124-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Xia W, Li J, Chen L, Huang B, Li S, Yang G, Ding H, Wang F, Liu N, Zhao Q, Fang T, Song T, Wang T, Shao N. MicroRNA-200b regulates cyclin D1 expression and promotes S-phase entry by targeting RND3 in HeLa cells. Mol Cell Biochem. 2010;344:261–266. doi: 10.1007/s11010-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 77.Yao CX, Wei QX, Zhang YY, Wang WP, Xue LX, Yang F, Zhang SF, Xiong CJ, Li WY, Wei ZR, Zou Y, Zang MX. miR-200b targets GATA-4 during cell growth and differentiation. RNA Biol. 2013;10:465–480. doi: 10.4161/rna.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–915. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 81.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: A Marker of Aggressiveness and Chemoresistance in Female Reproductive Cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 84.Chen J, Tian W, Cai H, He H, Deng Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med Oncol. 2012;29:2527–2534. doi: 10.1007/s12032-011-0117-4. [DOI] [PubMed] [Google Scholar]
- 85.Rui W, Bing F, Hai-Zhu S, Wei D, Long-Bang C. Identification of microRNA profiles in docetaxel-resistant human non-small cell lung carcinoma cells (SPC-A1) J Cell Mol Med. 2010;14:206–214. doi: 10.1111/j.1582-4934.2009.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- 87.Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, Bar-Eli M, Dinney C. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 89.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 91.Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene. 2013;32:296–306. doi: 10.1038/onc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gill JG, Langer EM, Lindsley RC, Cai M, Murphy TL, Kyba M, Murphy KM. Snail and the microRNA-200 family act in opposition to regulate epithelial-to-mesenchymal transition and germ layer fate restriction in differentiating ESCs. Stem Cells. 2011;29:764–776. doi: 10.1002/stem.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mizuguchi Y, Specht S, Lunz JG, 3rd, Isse K, Corbitt N, Takizawa T, Demetris AJ. Cooperation of p300 and PCAF in the control of microRNA 200c/141 transcription and epithelial characteristics. PLoS One. 2012;7:e32449. doi: 10.1371/journal.pone.0032449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pieraccioli M, Imbastari F, Antonov A, Melino G, Raschella G. Activation of miR200 by c-Myb depends on ZEB1 expression and miR200 promoter methylation. Cell Cycle. 2013;12:2309–2320. doi: 10.4161/cc.25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cesi V, Casciati A, Sesti F, Tanno B, Calabretta B, Raschella G. TGFbeta-induced c-Myb affects the expression of EMT-associated genes and promotes invasion of ER+ breast cancer cells. Cell Cycle. 2011;10:4149–4161. doi: 10.4161/cc.10.23.18346. [DOI] [PubMed] [Google Scholar]
- 96.Roy SS, Gonugunta VK, Bandyopadhyay A, Rao MK, Goodall GJ, Sun LZ, Tekmal RR, Vadlamudi RK. Significance of PELP1/HDAC2/miR-200 regulatory network in EMT and metastasis of breast cancer. Oncogene. 2013 doi: 10.1038/onc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–883. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knouf EC, Garg K, Arroyo JD, Correa Y, Sarkar D, Parkin RK, Wurz K, O'Briant KC, Godwin AK, Urban ND, Ruzzo WL, Gentleman R, Drescher CW, Swisher EM, Tewari M. An integrative genomic approach identifies p73 and p63 as activators of miR-200 microRNA family transcription. Nucleic Acids Res. 2012;40:499–510. doi: 10.1093/nar/gkr731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahn SM, Cha JY, Kim J, Kim D, Trang HT, Kim YM, Cho YH, Park D, Hong S. Smad3 regulates E-cadherin via miRNA-200 pathway. Oncogene. 2012;31:3051–3059. doi: 10.1038/onc.2011.484. [DOI] [PubMed] [Google Scholar]
- 100.Bai JX, Yan B, Zhao ZN, Xiao X, Qin WW, Zhang R, Jia LT, Meng YL, Jin BQ, Fan DM, Wang T, Yang AG. Tamoxifen represses miR-200 microRNAs and promotes epithelial-to-mesenchymal transition by up-regulating c-Myc in endometrial carcinoma cell lines. Endocrinology. 2013;154:635–645. doi: 10.1210/en.2012-1607. [DOI] [PubMed] [Google Scholar]
- 101.Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, Stampfer MR, Futscher BW. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One. 2010;5:e8697. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y, Wu J, Guan L, Qi L, Tang Y, Ma B, Zhan J, Wang Y, Fang W, Zhang H. Kindlin 2 promotes breast cancer invasion via epigenetic silencing of the microRNA200 gene family. Int J Cancer. 2013;133:1368–1379. doi: 10.1002/ijc.28151. [DOI] [PubMed] [Google Scholar]
- 103.Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, Tessema M, Leng S, Belinsky SA. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L, Yang F, Yuan JH, Yuan SX, Zhou WP, Huo XS, Xu D, Bi HS, Wang F, Sun SH. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- 105.Manavalan TT, Teng Y, Litchfield LM, Muluhngwi P, Al-Rayyan N, Klinge CM. Reduced expression of miR-200 family members contributes to antiestrogen resistance in LY2 human breast cancer cells. PLoS One. 2013;8:e62334. doi: 10.1371/journal.pone.0062334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Virtakoivu R, Pellinen T, Rantala JK, Perala M, Ivaska J. Distinct roles of AKT isoforms in regulating beta1-integrin activity, migration, and invasion in prostate cancer. Mol Biol Cell. 2012;23:3357–3369. doi: 10.1091/mbc.E12-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]


