Abstract
Purpose
Flavopiridol is primarily a cyclin-dependent kinase (CDK)-9 inhibitor and we performed a dose escalation trial to determine the maximum tolerated dose, safety, and generate a pharmacokinetic profile.
Methods
Patients with a diagnosis of relapsed myeloma after at least two prior treatments were included. Flavopiridol was administered as a bolus then continuous infusion weekly for 4 weeks in a 6 week cycle.
Results
Fifteen patients were treated at three dose levels (30 mg/m2 bolus, 30 mg/m2 CIV to 50 mg/m2 bolus, 50 mg/m2 CIV). Cytopenias were significant and elevated transaminases (grade 4 in 3 patients, grade 3 in 4 patients, and grade 2 in 3 patients) were noted but were transient. Diarrhea (grade 3 in 6 patients, grade 2 in 5 patients) did not lead to hospital admission. There were no confirmed partial responses although one patient with t(4;14) had a decrease in his monoclonal protein greater than 50% percent that did not persist. Pharmacokinetic properties were similar to prior publications and immunohistochemical staining for cyclin D1 and phospho-retinoblastoma did not predict response.
Conclusions
Flavopiridol as a single agent given by bolus then infusion caused significant diarrhea, cytopenias, and transaminase elevation but only achieved marginal responses in relapsed myeloma (ClinicalTrials.gov identifier NCT00112723).
Keywords: Multiple myeloma, flavopiridol
INTRODUCTION
Multiple Myeloma (MM) is a plasma cell neoplasm for which modern therapies have approximately doubled overall survival[17], primarily due to bortezomib, a proteasome inhibitor, and immune modulators (IMiDs) thalidomide and lenalidomide [31] that lead to interferon regulatory factor-4 inhibition[18] and caspase-mediated apoptosis. The vast majority of myeloma patients will still die of progressive disease leading to a search for novel agents.
Cyclin-dependent kinase (CDK) inhibition is an attractive target as MM cells are dependent on cell cycle dysregulation to overcome c-Myc induced apoptosis[26, 28]. Cell cycling is regulated in part by CDK complexes, and therapeutic intervention to prevent their binding to cyclins is of interest in myeloma as most myeloma cells have high levels of cyclin D1, D2 and/or D3[1] that phosphorylate retinoblastoma (Rb), moving cell cycle progression forward. In vitro studies have shown marked decrease in myeloid cell leukemia-1 (MCL-1) and phospho- RNA polymerase II after sustained exposure to Flavopiridol in U266[10], 8226[25], and OPM- 2[13] cell lines, but this could be overcome by overexpression of BCL-XL and BCL-2 and a resistance mechanism was suggested by late MCL-1 overexpression.
Flavopiridol targets the cyclin-dependent kinase (CDK) 9/cyclin T complex (preventing activation of RNA polymerase II)[5, 9], downregulates MCL-1[13], induces mitochondrial permeability changes[15], and interrupts NF-κB pathway by inhibiting IκK[29]. It is highly protein bound when in human serum, requiring a 30-minute intravenous bolus followed by 4-hour intravenous infusion – with this hybrid infusional schedule, significant responses have been observed in patients with refractory chronic lymphocytic leukemia[3].
Previous trials using dosing based on in vitro cytotoxicity were ineffective in patients with treated multiple myeloma[10] thought to be from inadequate AUC levels reached. We designed a phase I dose escalation study to establish the maximum tolerated dose (MTD) and describe toxicities associated with single agent flavopiridol in patients with relapsed myeloma.
Methods
Clinical trial
This study was approved by the Ohio State University Cancer Institutional Review Board and informed consent was obtained from all enrolled patients. Adult patients were required to have symptomatic myeloma using criteria from the International Myeloma Working Group[11] and be seen as outpatients in the myeloma clinic at The Ohio State University Arthur G. James Hospital and Solove Research Institute. This trial was registered on clinicaltrials.gov as NCT00112723.
Patients with a diagnosis of relapsed myeloma after at least two prior treatments with no limit on prior therapies were included. Adequate organ function was required with creatinine less than or equal to 1.5 mg/dL and total bilirubin less than or equal to twice the institutional upper limit of normal. Adequate hematologic parameters were also required with a hemoglobin greater than or equal to 9 g/dL, absolute neutrophil counter greater than or equal to 1500/µL, and platelets greater than or equal to 50,000/µL; however, lower platelet values were allowed if attributable to the patient’s underlying myeloma on screening bone marrow biopsy. Flavopiridol was administered weekly via central venous catheter as a 30 minute intravenous bolus followed by a 4-hour continuous intravenous infusion (CIV) for 4 weeks in a 6 week cycle. Responses were recorded based on International Myeloma Working Group Criteria[12].
Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. until July 31, 2010 and version 4.0 beginning August 1, 2010. Dose limiting toxicity (DLT) was defined as 1) any grade 3–4 non-hematologic toxicity (except leukopenia or neutropenia) that does not resolve or decrease to grade 1–2 within 2 weeks, or 2) any grade 4 hematologic toxicity that causes more than a one week delay in administration of therapy. Granulocyte colony stimulating factor (G-CSF) was used at the discretion of the treating investigator. The MTD was defined as that dose level beneath the dose at which 2 or more of 6 patients experienced DLT.
Pharmacokinetic (PK) analysis
Plasma samples were obtained on days 1 and 22 of the first cycle. Sodium heparinized blood was obtained at the following time points: prior to dosing (t=0), at 0.5, 1, 3, 4.5, 6, 8 and 24 hours after initiation of infusion on day 1 and day 22. Blood samples were centrifuged, and plasma was stored at (−70)°C until analysis. Flavopiridol quantification in plasma samples was achieved using a validated liquid chromatography-tandem mass spectrometry assay as previously described[23]. Plasma flavopiridol concentration-time data were analyzed using standard non-compartmental methods in WinNonlin Professional v 5.2.1 (Pharsight, Mountain View, CA).
Immunohistochemical analysis
We hypothesized that cyclin D1 overexpression would sensitize myeloma cells to flavopiridol[8]. Immunohistochemical staining (IHC) for Cyclin D1 (BCL-1) (Neomarkers) and Retinoblastoma (RB-358, Leica), and phospho-RB (pRB, Leica) was performed on the 4 micron (µm) sections of formalin-fixed, paraffin-embedded (FFPE) bone marrow biopsy or clot sections. Briefly, sections were placed in a 60°C oven for one hour, cooled, and deparaffinized and rehydrated through xylenes and graded ethanol solutions to water. All slides were quenched for 5 minutes in 3% hydrogen peroxide (v/v) for endogenous peroxidase. Antigen retrieval was performed by a heat method in which the specimens were placed in a citric acid solution (pH 6.1) for 25 minutes at 94°C and cooled for 15 minutes. Slides were then placed on an autostainer (Dako Immunostaining) for immunohistochemistry. The antibodies for Cyclin D1, RB, and pRB were used at a dilution of 1:100, 1:50, and 1:100, respectively and incubated for 30 minutes at room temperature. The Envision Plus horseradish peroxidase (HRP) with 3,3'-diaminobenzidine (DAB) chromogen (Dako) was used to produce a brown precipitate. Slides were then counterstained in Richard Allen hematoxylin. A scoring system was developed from a single observer (author WZ) based on intensity for the majority of the stained plasma cells with 0–1 negative, 2 as moderate, and 3 strong.
Statistical analysis
This protocol is a standard 3×3 phase I dose escalation study of single agent flavopiridol to determine the maximum tolerable dose of the agent to be used in a phase II evaluation of response. Separate, parallel phase I studies were conducted in each of six disease groups (indolent B-cell NHL, mantle cell lymphoma, intermediate grade B-cell NHL, T/NK-cell NHL, Hodgkin’s lymphoma, multiple myeloma) in order to determine disease-specific DLT, MTD and recommended phase II doses.
Spearman correlation analysis (two-tailed p-values) was performed between clinical response and immunohistochemical staining results for BCL-1, RB, and pRB using Prism v 5.0F (GraphPad Software, San Diego, CA).
Statistical analyses for PK parameters were performed on all enrolled patients with evaluable PK data (n=15); PK profiles that yielded ≥ 30% estimated area under the concentration versus time curve from zero to infinity (AUC(0-∞)) extrapolation were omitted from final analyses. Paired t-tests were used to evaluate differences in AUC(0-∞) and Cmax between days 1 and 22 of cycle 1. The associations between PK parameter estimates and dose level were tested using one-way analysis of variance (ANOVA) and 2-sample t-tests or nonparametric tests when appropriate. Data are described with means ± standard deviations and/or medians with ranges. These data were analyzed using SigmaPlot v11 (Systat Software Inc.).
Results
Patients
Fifteen patients (ages 49–81 years) with relapsed myeloma were treated. The median number of prior therapies was 7 (3–12). At the time of study entry, 10 patients displayed a complex karyotype, 2 patients demonstrated 17p deletion by CD138-selected fluorescence in situ hybridization (FISH; Rosette Sep), and one patient showed t(4;14) fusion by FISH. There were 3 patients with karyotypic chromosome 13 deletion and 9 patients with deleted LAMP or D13S319 probes by FISH. At study entry, 8 patients had International Staging System (ISS) stage 3 disease, 3 with stage 2, and 4 with stage 1 disease. Five patients were treated in cohort 1 (30 mg/m2 bolus, 30 mg/m2 CIV; 30/30), 3 patients in cohort 2 (30 mg/m2 bolus, 50 mg/m2 CIV; 30/50), and 7 patients in cohort 3 (50 mg/m2 bolus, 50 mg/m2 CIV; 50/50). The median number of cycles received was one. Two patients at the 30/30 level and one patient at 50/50 had to be replaced as they did not finish the first cycle.
Response
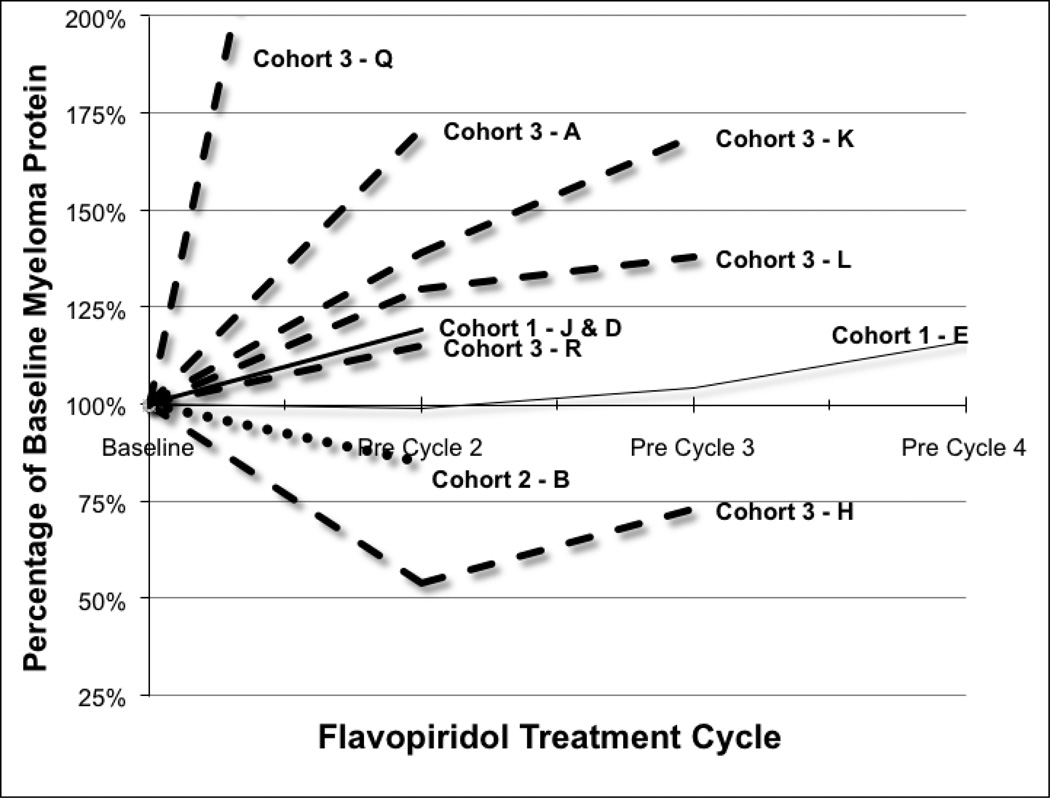
Immunoglobulins, free light chains, and 24-hour urine samples were obtained at screening and then the appropriate myeloma proteins were obtained on the first day of each subsequent cycle to follow response. There were no confirmed partial responses although one patient had a decrease in his monoclonal protein greater than 50% percent which was not quite maintained with the second cycle and hence qualifies as a confirmed MR. Overall there was one minor response, one patient with stable disease for three cycles, and the remainder suffered either progressive disease after the first cycle (4 patients) or did not continue on study treatment (9 patients).
The one patient with a minor response had an IgA myeloma with a t(4;14), deletion chromosome 13, and tetraploid cytogenetics by FISH that had been treated with vincristine, doxorubicin, and high dose dexamethasone (VAD) with progression, complete response with salvage bortezomib and dexamethasone, then an autologous transplant from which he remained in a remission off maintenance therapy for three years until relapse then treatment on this clinical protocol.
Toxicities
Grade 3/4 toxicities were significant (table 2). Cytopenias were considerable with grade 4 neutropenia (11 patients), grade 4 anemia (7 patients), and grade 4 thrombocytopenia (3 patients). Elevated transaminases (grade 4 in 3 patients, grade 3 in 4 patients, and grade 2 in 3 patients) were frequent but was asymptomatic and resolved in the periods between flavopiridol exposure. Diarrhea (grade 3 in 6 patients, grade 2 in 5 patients) was common but did not lead to hospitalization. There were two patients with neutropenic pneumonia and one patient with neutropenic fever.
Table 2.
Toxicities
| Toxicity Grade (n) | |||
|---|---|---|---|
| Adverse Events | 2 | 3 | 4 |
| Cytopenias | |||
| Neutropenia | 1 | 11 | |
| Anemia | 2 | 7 | |
| Thrombocytopenia | 3 | 2 | 3 |
| Lymphopenia | 5 | ||
| Gastrointestinal | |||
| ALT/AST | 3 | 4 | 3 |
| Dehydration | 2 | ||
| Diarrhea | 5 | 6 | |
| Nausea | 3 | ||
| Vomiting | 1 | ||
| Constitutional | |||
| Fatigue | 2 | 3 | |
| Dizziness | 1 | ||
| Diaphoresis | 1 | ||
| Electrolyte | |||
| Hyperglycemia | 4 | ||
| Hyperkalemia | 2 | ||
| Hypocalcemia | 3 | ||
| Hypophosphatemia | 2 | 1 | |
| Infection | |||
| Neutropenic pneumonia | 2 | ||
| Neutropenic fever | 1 | ||
| 32 | 22 | 29 | |
Adverse events (grades 2–4) with an attribution of possible, probable, or definitely related to flavopiridol therapy with the highest grade of all toxicities per patient tabulated above. Leukopenia was ignored in favor of more specific toxicities of lymphopenia and/or neutropenia. Toxicites were graded according to CTCAE version 3.0. Small cohort sizes prevented a comparison of toxicities between cohorts.
There were eight serious adverse events related primarily to pancytopenia with or without infection, asymptomatic elevated transaminases, or progressive myeloma. Patient E (30/50) became febrile with grade 3 neutropenia after her initial infusion for which 20 mg intravenous dexamethasone was added to subsequent treatments. However, on day 20 of cycle 1, she presented septic due to citrobacter bacteremia. Patient B (30/50) became infected day 2 of cycle 1 with pseudomonas bacteremia, and patient P (50/50) became pancytopenic and septic during cycle 1. Patients K (50/50) and J (30/50) both developed asymptomatic grade 4 elevated transaminases. On day 1 of cycle 2, Patient C (30/50) developed mental status changes from hypercalcemia attributed to progressive disease. Patient G (30/50) developed grade 4 neutropenia and thrombocytopenia with cycle 1, and suffered a pathologic fracture just prior to treatment on cycle 2 day 1 consistent with progressive disease. After progressing on therapy and receiving infusional DCEP (Dexamethasone-Cyclophosphamide-Etoposide-Platinum), patient R (50/50) became septic with grade 4 neutropenia and died within 30 days of his last dose of flavopiridol.
Immunohistochemistry
Pretreatment biopsy or aspirate samples were stained for BCL-1 (Cyclin D1, CCND1), RB, and pRB (table 3). A single reviewer scored the intensity of staining in plasma cells. RB staining was seen in all but one specimen. The intensity of BCL-1 and RB staining could not be statistically associated with response (p=0.84 and p=0.12 respectively), but intense staining was seen in two of the patients with stable disease. The staining intensity of pRB showed no correlation with response to flavopiridol (p=0.88).
Table 3.
Plasma cell pretreatment staining
| ID | Best response | BCL-1 | RB | pRB |
|---|---|---|---|---|
| H | MR | 0 | 2+ | 3+ |
| B | SD | 3+ | 1+ | 1+ (cytoplasmic) |
| E | SD | 3+ | 3+ | 3+ |
| R | SD | 0 | 3+ | 3+ (nuclear) |
| J | SD | 1+ | 2+ | 3+ (cytoplasmic) |
| D | SD | 0 | 3+ | 2+ |
| K | PD | 0 | 3+ | 3+ |
| A | PD | 0 | 3+ | 2+ (cytoplasmic) |
| Q | PD | 1 | 3+ | 3+ (cytoplasmic) |
| C | -- | 0 | 3+ | 3+ (nuclear and cytoplasmic) |
| F | -- | 1+ | 3+ | 2+ |
| G | -- | 1+ | 2+ | 0 (cytoplasm low) |
| M | -- | 0 | 2+ | 0 |
| O | -- | 0 | 3+ | 3+ |
Immunohistochemistry on paraffin-embedded bone marrow core tissue was performed for BCL-1, retinoblastoma (RB), and phospho-RB (pRB) and patient IDs were sorted from best response to worst response when possible. A score was assigned which represents the intensity for the majority of stained plasma cells. 0–1+ will be considered as negative, 2+ as moderate, and 3+ will be strong. The majority of the pRB stains are in agreement with RB, but patients G and M were not. Patient’s I, L, and P had inadequate plasma cells for staining and are not listed. Best response per IMWG was listed whenever possible.
Pharmacokinetics
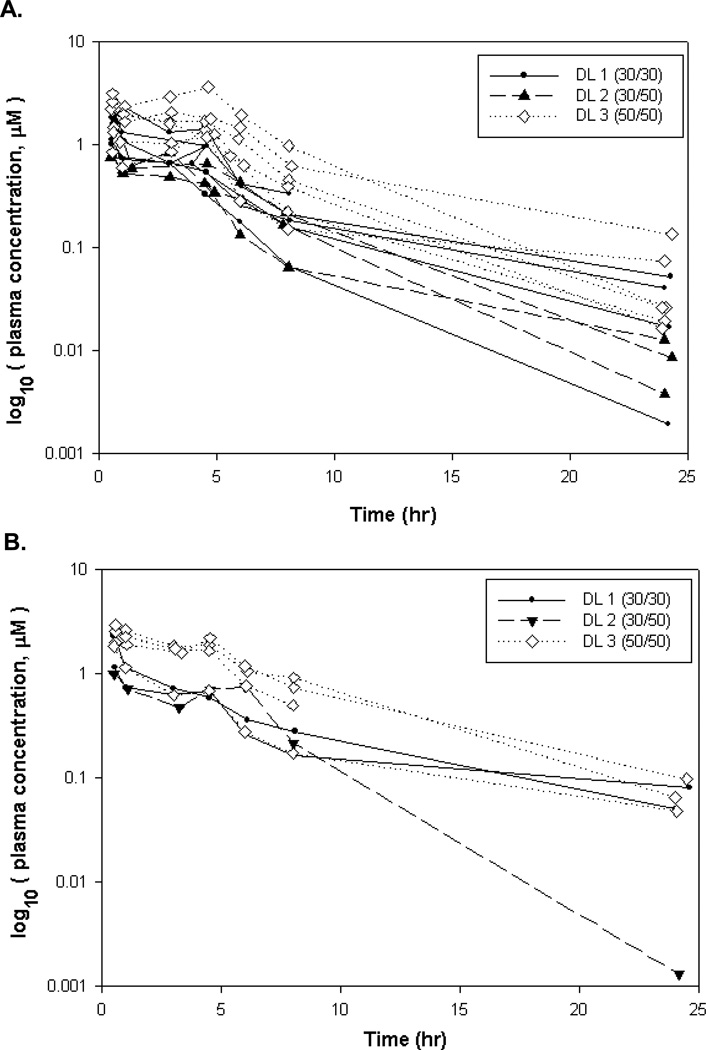
Plasma samples from 15 patients (a total of 24 concentration-time profiles with 187 plasma concentration observations) were available for analysis. Three PK profiles for 3 patients were omitted due to ≥ 30% AUC(0-∞) extrapolation, resulting in 14 plasma flavopiridol concentration-time profiles on day 1 and 7 profiles on day 22 (Figure 2). Data points for determining the terminal elimination phase (λz) were selected automatically in WinNonlin and resulting λz ranges were determined to be adequate by manual review. Three dose levels, ranging from total dose (bolus + maintenance) of 60 to 100 mg/m2, were administered in this study. Among those with PK data on both days 1 and 22 (n=6), the mean differences in AUC(0-∞) and Cmax for day 22 versus day 1 were not significant (P=0.53 and P=0.57, respectively), where the average difference in AUC(0-∞) was 1.00 hr*µM, (95% CI −2.78 to 4.78 hr*µM) and the average difference in Cmax was 0.153 µM (95% CI: −0.50 to 0.80 µM). There was no statistically significant difference between the apparent volume of distribution based on terminal phase (Vz), total body clearance (CL) and terminal phase elimination half-life (T1/2) for the 2 dosing days in these same individuals. PK parameter data from cycle 1 days 1 and 22 combined are summarized in Table 4.
Figure 2. Plasma flavopiridol concentration versus time.
Flavopiridol plasma concentration versus post-infusion initiation time semi-log plots for patients with evaluable PK profiles. A. Consists of Cycle 1 Day 1 PK profiles (14) from all dose levels; B. Consists of cycle 1 day 22 PK profiles (7) from all dose levels.
Table 4.
Flavopiridol pharmacokinetic parameters for patients with evaluable PK profiles
| Dose level (mg/m2) |
n | PK | Mean AUC0-∞ ± SD Median [range], hr*µM |
Mean CL ± SD Median [range], L/hr |
Mean Cmax ± SD Median [range], µM |
Mean Vz ± SD Median [range], L |
Mean T1/2 ± SD Median [range], hr |
Mean Tmax ± SD Median [range], hr |
|---|---|---|---|---|---|---|---|---|
| 30 + 30 | 5 | Day 1: 5 | 7.78 ± 2.75 | 39.97 ± 20.87 | 1.85 ± 0.80 | 367.9 ± 210.1 | 6.87 ± 3.24 | 0.52 ± 0.03 |
| Day 22: 2 | 7.64 [3.69–11.59] | 35.11 [20.22–80.18] | 1.83 [1.01–3.09] | 265.1 [211.6–764.0] | 6.44 [3.03–13.36] | 0.50 [0.50–0.57] | ||
| 30+50 | 3 | Day 1: 3 | 4.75 ± 1.06 | 77.05 ± 12.31 | 1.09 ± 0.43 | 417.2 ± 242.2 | 3.65 ± 1.75 | 1.18 ± 1.22 |
| Day 22: 1 | 4.92 [3.39–5.77] | 77.23 [64.81–88.91] | 0.92 [0.80–1.72] | 346.7 [209.6–765.5] | 3.16 [2.13–6.15] | 0.61 [0.50–3.00] | ||
| 50+50 | 7 | Day 1: 6 | 14.18 ± 5.34 | 38.56 ± 14.26 | 2.41 ± 0.74 | 316.6 ± 254.9 | 5.35 ± 2.83 | 1.40 ± 1.67 |
| Day 22: 4 | 13.82 [7.10–22.56] | 35.31 [22.08–59.96] | 2.55 [1.31–3.62] | 214.4 [100.8–886.5] | 4.24 [3.00–12.06] | 0.58 [0.50–4.63] | ||
Summary of non-compartmental PK parameter estimates by dose levels for cycle 1 day 1 and cycle 1 day 22 combined. N is the number of treated patients per dose level while PK is the number of evaluable PK profiles by day included in the mean and standard deviation (SD) calculations. Data is presented as mean ± SD (1st line) and median [range] (2nd line). Abbreviations: AUC 0-∞, estimated area under the flavopiridol concentration vs. time curve from zero to infinity; CL, total body clearance; Cmax, maximum observed concentration; Vz, apparent volume of distribution based on terminal phase; T1/2, terminal phase elimination half-life; Tmax, time corresponding to Cmax; SD, standard deviation.
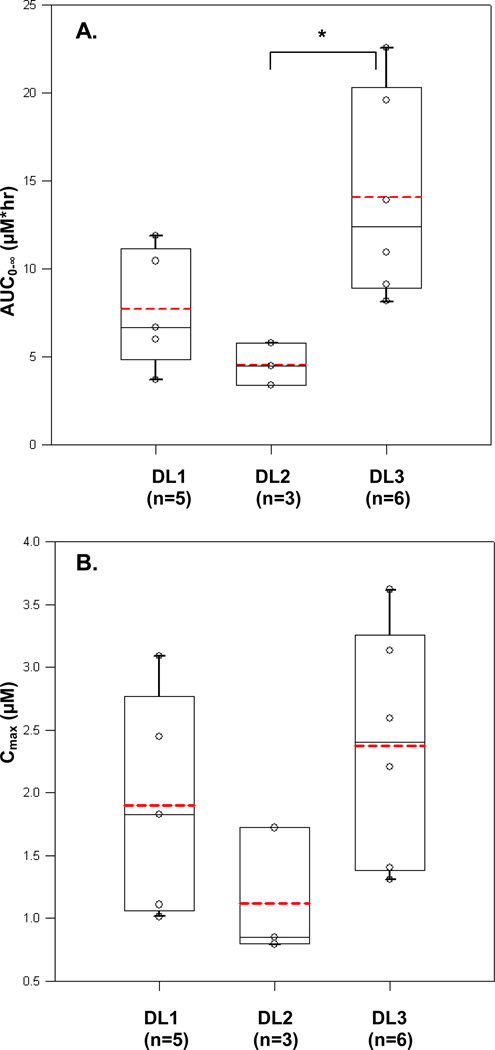
When the PK parameter estimates for cycle 1 day 1 were compared between the 3 dose levels, a significantly higher mean AUC(0-∞) was observed for dose level 3 compared to dose level 2 (P<0.05; Figure 3a). The mean AUC(0-∞) for dose level 2 was unexpectedly lower than that of dose level 1, likely because there were only 3 concentration profiles available for evaluation for this dose level. The mean AUC(0-∞) for dose level 3 was higher when compared to dose level 1 but not statistically significant. The differences in mean Cmax of all dose levels were insignificant (Figure 3b). The mean Tmax was longer in the 30/50 and 50/50 mg/m2 dose level groups compared to 30/30 mg/m2 (1.18±1.22; 1.40±1.67 hr and 0.52±0.03, respectively). However, it should be noted that there was one outlier in the 30/50 mg/m2 dose level group (3.0 hr), and 2 outliers (4.6 and 4.5 hr) in the latter group. The medians Tmax were comparable among the 3 dose levels.
Figure 3. Comparison of AUC by dose level.
A. Relationship between C1D1 AUC0-∞ and dose levels (p=0.024, ANOVA). B. Relationship between C1D1 Cmax and dose levels (p=0.16, ANOVA). Abbreviations: DL1, Dose level 1= 30/30 mg; DL2, dose level 2=30/50 mg; and DL3, dose level 3=50/50 mg. Solid line within the box represents the median, the lower and upper box borders represent the first and third quartiles, and the whiskers extend to the minimum and maximum values. The mean is marked with a dotted line. *p<0.05
There were also no large differences in Vz, and T1/2 of flavopiridol among the three dose levels. The clearance parameters estimated here, except for the 30/50 mg/m2 dose level, were very comparable to the previously published data[2]. Based on a two compartment population pharmacokinetic model with first-order elimination, we previously reported flavopiridol clearance of 31.4 ± 5.4 L/Hr[22]. In this study, the mean CL for the 30/30 and 50/50 mg/m2 dose levels were 39.97±20.87 and 38.56 ± 14.26 L/Hr, respectively. The CL estimated from the 30/50 mg/m2 group was significantly higher than the other 2 groups (p<0.005). This is consistent with the lower mean AUC estimated as presented above, and this unexpected result should be interpreted cautiously and may be attributed to the small sample size. Overall, pharmacokinetics appear comparable to that reported for flavopiridol in other hematologic malignancies[2, 3, 22].
Discussion
In this phase I trial of flavopiridol in relapsed multiple myeloma, we determined the MTD at 50 mg/m2 30-minute bolus followed by 50 mg/m2 4-hour CIV. With only one documented marginal response, we did not feel that there was adequate single agent activity to continue into the phase II portion of the trial.
CDK inhibition is a tempting therapeutic target because increased expression of at least one of the three CCND genes is a near universal event in plasma cell dyscrasias[1]; CCND1 is expressed in hyperdiploid myeloma, while CCND2 is expressed by most of the remaining tumors. D-type cyclins are critical regulators of the cell cycle that act in a complex with cyclin-dependent kinases (CDKs) -4 or -6 to promote the phosphorylation of the retinoblastoma protein (Rb) to initiate cellular transition from G1 to S phase[6, 7, 20, 27]. Focal amplification of cyclin D1 may be required for CDK inhibitors to keep p21CIP1 level low and inactivate NF-κB[16], while RB1 mutations or deletions may lead to resistance to Flavopiridol as with other CDK inhibitors, or perhaps resistance could be mediated by autophagy as it is in CLL[19]. Notably in our trial immunohistochemical staining for BCL-1 and pRB was unable to demonstrate a correlation between the staining and response – this staining was exploratory as a phosphorylated antibody in bone marrow samples has not been validated and immunohistochemical analysis is semi-quantitative at best. In a phase Ib study of Flavopiridol in combination with bortezomib[14], responses were seen in bortezomib naïve myeloma patients, confirming the molecular studies[21] demonstrating that resistance for bortezomib and CDK inhibitors overlap.
Flavopiridol led to considerable adverse events in this patient population and responses were generally short-lived. It is possible that the higher doses used in this patient population in the final cohort represent responses that we would see more commonly at even higher doses, but unfortunately off target effects of neutropenia and diarrhea would prevent further dose escalation. Flavopiridol is no longer being pursued in lymphoproliferative diseases but ongoing trials are accruing in combination in myeloid neoplasms in combination with standard cytotoxic agents. Preclinical evaluation of the CDK inhibitor AT7519 has demonstrated the importance of glycogen synthase kinase-3β for apoptosis[24], testing that has not been performed with most other CDK inhibitors including flavopiridol. The precise anti-neoplastic mechanism of action of CDK inhibitors in myeloma remains controversial.
In summary this phase I trial established the MTD of single agent flavopiridol MTD to be 50 mg/m2 30-minute bolus followed by 50 mg/m2 4-hour CIV. The clinical evaluation of CDK inhibitors in MM has been hampered by tight therapeutic indices[4, 30] and lack of efficacy in early phase studies. Novel agents with broader CDK inhibition[21, 24] and wider therapeutic indices relative to flavopiridol are needed with compounds such as SCH-72765 and TG-02 in phase 3 and 1 clinical trials, targeted towards patients most likely to respond with p16 or p18 deletions.
Figure 1. Myeloma clinical response.
At baseline and on day 1 of each subsequent cycle, myeloma clinical labs were repeated and response determined as determined by the International Myeloma Working Group Criteria (IMWG). Patient G had serum free light chain disease only. Patients C, F, G, M, N, O, P had only baseline laboratories drawn – patients F, O, P were replaced during cycle 1 and the remainder had non-measurable disease per IMWG criteria.
Table 1.
Treated patients
| Age | Protein | ANC | ISS | β2 | Priors | FISH | Karyotype | |
|---|---|---|---|---|---|---|---|---|
| 1. 30 mg/m2 bolus / 30 mg/m2 CIV | ||||||||
| Patient E | 58 | IgG-L | 1900 | 2 | 3 | 3 | Unsuccessful | Normal |
| Patient F | 58 | IgG-K | 3300 | 3 | 9.3 | 9 | Trisomies/Tetrasomies, 13q− | Complex |
| Patient O | 60 | IgG-L | 2200 | 2 | 1.4 | 9 | Unsuccessful | Normal |
| Patient J | 78 | IgG-L | 1300 | 3 | 7.7 | 6 | Trisomies/Tetrasomies, 13− | Complex |
| Patient D | 49 | IgG-L | 1900 | 3 | 6.7 | 3 | Tetrasomies, 13−, 17p− | Complex |
| 2. 30 mg/m2 bolus / 50 mg/m2 CIV | ||||||||
| Patient B | 49 | IgG-K | 1200 | 3 | 6.9 | 9 | 13q−, t(11;14) | Complex |
| Patient G | 65 | IgG-K | 3300 | 3 | 6.9 | 10 | Unsuccessful | Complex |
| Patient C | 69 | IgG-K | 1700 | 3 | 6.2 | 6 | Trisomies, 13− | Failed |
| 3. 50 mg/m2 bolus / 50 mg/m2 CIV | ||||||||
| Patient Q | 60 | IgG-K | 2300 | 1 | 2.9 | 4 | Trisomies/Tetrasomies, 17p−, 13q−, 12p− | Complex |
| Patient K | 52 | IgA-L | 1900 | 1 | 2.5 | 11 | Trisomies, 1q21+, 1q23−, | Complex |
| Patient P | 81 | LLC | 1600 | 2 | 2.7 | 5 | Trisomies/Tetrasomies, 1q+ | Failed |
| Patient M | 66 | IgA-L | 1200 | 3 | 6.3 | 12 | Tetraploid, 13q−, 1q+ | Failed |
| Patient A | 64 | IgG-K | 5900 | 1 | 2 | 6 | Trisomies, 1p−, 13q− | Complex |
| Patient H | 72 | IgA-K | 2100 | 1 | 1.7 | 3 | Tetraploid, 13−, t(4;14) | Complex |
| Patient R | 64 | IgG-K | 4300 | 3 | 6.8 | 9 | Unsuccessful | Complex |
Patients F, O, and P were replaced as they did not complete the first cycle due to progression or toxicity. Patient E was the only patient that did not have CD138-selected FISH. Patients with 13q deletion showed one copy of 13q14.3 (D13S319) while those with 13- deletion had only one copy of the LAMP probe. Abbreviations Protein is the paraprotein secreted with lambda light chains represented as LLC. International Staging System (ISS) and β2-microglobulin represent values obtained during screening. Priors are the number of prior treatments received, not lines of therapy. Karyotype was listed as failed when there were insufficient metaphases.
Acknowledgments
Grant Support
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA076576 (PI Michael Grever). CCH was a Paul Calabresi scholar on K12CA133250 from the National Cancer Institute. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
None of the authors have a relevant conflict of interest to report.
Authorship roles: CCH: Designed the study, enrolled patients, analyzed the data, wrote the manuscript. MP: Analyzed the data, wrote the manuscript. MAB, MAP, WZ: Analyzed data. SF, DMB, EHK, WJH: Enrolled patients. TSL, JCB, SF: Designed the study. All authors approved the final manuscript.
References
- 1.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni W, Albanese KA, Rovin B, Kefauver C, Devine SM, Lucas DM, Johnson A, Schaaf LJ, Byrd JC, Marcucci G, Grever MR. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95:1098–1105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, Moran M, Blum KA, Rovin B, Brooker-McEldowney M, Broering S, Schaaf LJ, Johnson AJ, Lucas DM, Heerema NA, Lozanski G, Young DC, Suarez JR, Colevas AD, Grever MR. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, Moran M, Blum KA, Rovin B, Brooker-McEldowney M, Broering S, Schaaf LJ, Johnson AJ, Lucas DM, Heerema NA, Lozanski G, Young DC, Suerez JR, Colevas AD, Grever MR. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2006 doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 6.Classon M, Dyson N. p107 and p130: versatile proteins with interesting pockets. Exp Cell Res. 2001;264:135–147. doi: 10.1006/excr.2000.5135. [DOI] [PubMed] [Google Scholar]
- 7.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Hamm TE, Dent P, Grant S. Cyclin D1 overexpression increases the susceptibility of human U266 myeloma cells to CDK inhibitors through a process involving p130- , p107- and E2F-dependent S phase entry. Cell Cycle. 2006;5:437–446. doi: 10.4161/cc.5.4.2441. [DOI] [PubMed] [Google Scholar]
- 9.de Azevedo WF, Jr, Canduri F, da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293:566–571. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 10.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Fitch TR, Fenton RG, Fonseca R, Isham CR, Ziesmer SC, Erlichman C, Bible KC. Flavopiridol in patients with relapsed or refractory multiple myeloma: a phase 2 trial with clinical and pharmacodynamic end-points. Haematologica. 2006;91:390–393. [PubMed] [Google Scholar]
- 11.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 12.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 13.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and downregulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 14.Holkova B, Perkins EB, Ramakrishnan V, Tombes MB, Shrader E, Talreja N, Wellons MD, Hogan KT, Roodman GD, Coppola D, Kang L, Dawson J, Stuart RK, Peer C, Figg WD, Sr, Kolla S, Doyle A, Wright J, Sullivan DM, Roberts JD, Grant S. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin Cancer Res. 2011;17:3388–3397. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain SR, Lucas DM, Johnson AJ, Lin TS, Bakaletz AP, Dang VX, Viatchenko-Karpinski S, Ruppert AS, Byrd JC, Kuppusamy P, Crouser ED, Grever MR. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer A, Schultheis B, Bergmann J, Willer A, Hegenbart U, Ho AD, Goldschmidt H, Hehlmann R. Alterations of the cyclin D1/pRb/p16(INK4A) pathway in multiple myeloma. Leukemia. 2002;16:1844–1851. doi: 10.1038/sj.leu.2402609. [DOI] [PubMed] [Google Scholar]
- 17.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Girona A, Heintel D, Zhang LH, Mendy D, Gaidarova S, Brady H, Bartlett JB, Schafer PH, Schreder M, Bolomsky A, Hilgarth B, Zojer N, Gisslinger H, Ludwig H, Daniel T, Jager U, Chopra R. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154:325–336. doi: 10.1111/j.1365-2141.2011.08689.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney E, Lucas DM, Gupta SV, Wagner AJ, Herman SEM, Smith LL, Yeh Y-Y, Andritsos L, Jones JA, Flynn JM, Blum KA, Zhang X, Lehman A, Kong H, Gurcan M, Grever MR, Johnson AJ, Byrd JC. ER stress and autophagy: new players in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood. 2012 doi: 10.1182/blood-2011-12-400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 21.McMillin DW, Delmore J, Negri J, Buon L, Jacobs HM, Laubach J, Jakubikova J, Ooi M, Hayden P, Schlossman R, Munshi NC, Lengauer C, Richardson PG, Anderson KC, Mitsiades CS. Molecular and cellular effects of multi-targeted cyclin-dependent kinase inhibition in myeloma: biological and clinical implications. Br J Haematol. 2011;152:420–432. doi: 10.1111/j.1365-2141.2010.08427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, Wu D, Blum KA, Fischer B, Mitchell SM, Moran ME, Brooker-McEldowney M, Heerema NA, Jarjoura D, Schaaf LJ, Byrd JC, Grever MR, Dalton JT. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phelps MA, Rozewski DM, Johnston JS, Farley KL, Albanese KA, Byrd JC, Lin TS, Grever MR, Dalton JT. Development and validation of a sensitive liquid chromatography/mass spectrometry method for quantitation of flavopiridol in plasma enables accurate estimation of pharmacokinetic parameters with a clinically active dosing schedule. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;868:110–115. doi: 10.1016/j.jchromb.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santo L, Vallet S, Hideshima T, Cirstea D, Ikeda H, Pozzi S, Patel K, Okawa Y, Gorgun G, Perrone G, Calabrese E, Yule M, Squires M, Ladetto M, Boccadoro M, Richardson PG, Munshi NC, Anderson KC, Raje N. AT7519, A novel small molecule multi-cyclindependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3beta activation and RNA polymerase II inhibition. Oncogene. 2010;29:2325–2336. doi: 10.1038/onc.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenov I, Akyuz C, Roginskaya V, Chauhan D, Corey SJ. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk Res. 2002;26:271–280. doi: 10.1016/s0145-2126(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, Zeng Y, Chen B, Epstein J, Staudt LM. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 28.Shou Y, Martelli ML, Gabrea A, Qi Y, Brents LA, Roschke A, Dewald G, Kirsch IR, Bergsagel PL, Kuehl WM. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma. Proc Natl Acad Sci U S A. 2000;97:228–233. doi: 10.1073/pnas.97.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–4759. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 30.Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL, Chow C, Brahim JS, Steinberg SM, Figg WD, Swain SM. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:5038–5047. doi: 10.1158/1078-0432.CCR-04-0025. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]