Abstract
Background
Chlorhexidine is a skin disinfectant that reduces skin and mucous membrane bacterial colonization and inhibits organism growth. Despite numerous studies assessing chlorhexidine safety in term infants, residual concerns have limited its use in hospitalized neonates, especially low birth weight preterm infants. The aim of this study was to assess the potential neurotoxicity of chlorhexidine on the developing central nervous system using a well-established in vitro model of neurite outgrowth that includes laminin and L1 cell adhesion molecule (L1) as neurite outgrowth promoting substrates.
Methods
Cerebellar granule neurons are plated on either poly L-lysine, L1 or laminin. Chlorhexidine, hexachlorophene or their excipients are added to the media. Neurons are grown for 24 h, then fixed and neurite length measured.
Results
Chlorhexidine significantly reduced the length of neurites grown on L1 but not laminin. Chlorhexidine concentrations as low as 125 ng/ml statistically significantly reduced neurite length on L1. Hexachlorophene did not affect neurite length.
Conclusion
Chlorhexidine at concentrations detected in the blood following topical applications in preterm infants specifically inhibited L1 mediated neurite outgrowth of cerebellar granule neurons. It is now vital to determine whether the blood brain barrier is permeable to chlorhexidine in preterm infants.
INTRODUCTION
Chlorhexidine is a skin disinfectant that reduces skin and mucous membrane bacterial colonization and inhibits organism growth. It is frequently used to prevent infections and the spread of antibiotic resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) (1, 2). Chlorhexidine is used to prepare the skin before surgical procedures and reduces the risk of surgical site infections (3). The Centers for Disease Control and Prevention recommend cleaning the skin with chlorhexidine before placement of central venous catheters, because numerous studies have demonstrated reduced catheter infection rates following chlorhexidine skin preparation compared with alternatives such as povidone iodine. However, “no recommendation can be made for the safety or efficacy of chlorhexidine in infants aged <2 months” (4).
Trace amounts of chlorhexidine can be absorbed through the skin after a single bath in adults and term neonates. In 1976, DE Case showed that, in adults, chlorhexidine “is absorbed through intact human skin to an extraordinarily small degree, if at all” (5). This report only detected chlorhexidine in feces of adults following a handwash with 5% chlorhexidine digluconate. No chlorhexidine was detected in blood, indicating minimal absorption. In term neonates given a 1 or 2% chlorhexidine bath, chlorhexidine was detected in 4 out of 20 neonates (range 13.5 – 26. 7 ng/ml) (6). However, the interval between the bath and blood sampling was not reported nor is it known when the chlorhexidine concentration may peak in the blood after topical exposure. There are no reports of immediate adverse consequences as a result of chlorhexidine absorption in studies of term newborns or adults, no data to suggest that detectable serum concentrations have clinical importance (7), and no data that chlorhexidine can cross the blood brain barrier. Another broad spectrum, organopolychlorinated antiseptic, hexachlorophene (HEX) was found to penetrate intact human skin and cause vacuolar encephalopathy in newborns in the 1970s (8, 9), thus showing that antiseptics used without harm in adults can cause devastating neurological injury in infants. As a result, chlorhexidine has been scrutinized for its potential to be absorbed through the skin. Despite numerous studies assessing short term outcomes from the use of chlorhexidine in term infants, residual concerns have limited its use in hospitalized neonates, especially low birth weight preterm infants (10). No developmental neurotoxicity studies have been performed to assess the safety of chlorhexidine exposure in term or preterm neonates.
Our goal is to begin studies of the impact of chlorhexidine exposure on the developing central nervous system. In the current study, our objective was to assess the potential neurotoxicity of chlorhexidine using a well-established in vitro model of neurotoxicity, that of neurite outgrowth (11, 12) using both laminin and L1 cell adhesion molecule (L1) as outgrowth promoting substrates. L1, but not laminin, mediated neurite outgrowth is dependent on lipid rafts, portions of the plasma membrane similar to bacterial membranes disrupted by chlorhexidine (13). Thus, L1 mediated neurite outgrowth may be a sensitive indicator of chlorhexidine neurotoxicity. HEX has been shown to cause axonopathy in vitro. While chemically distinct, both chlorhexidine and HEX contain the lipophilic moieties of polychlorinated phenol rings, making them both lipophilic. Measurement of neurite outgrowth may be one way to demonstrate the potential neurotoxicity of chlorhexidine and HEX (14).
Due to concerns that chlorhexidine may cause neurotoxicity to third trimester equivalent preterm infants, cerebellar granule neurons (CGN) are an ideal in vitro model, as neurite outgrowth of these neurons occurs at this age of development. Neurite outgrowth is a sensitive measure for nonlethal neurotoxicity and provides an excellent model to assess the potential cellular toxicity of chlorhexidine (11, 12). Neurite outgrowth occurs through different mechanisms depending on environmental cues. These cues include proteins either on the surface of other cells or in the extracellular matrix. L1 cell adhesion molecule (L1) is one such environmental cue. It binds homophilically to itself, initiating the processes of protein trafficking, phosphorylation/dephosphorylation and signal transduction in the growth cone. Laminin promotes neurite outgrowth by binding to the integrin receptor, and hence represents a different mechanism by which neurite outgrowth is promoted. Together these cells lines provide an opportunity to investigate the potential neurotoxicity of chlorhexidine.
METHODS
Antibodies and materials
Antibodies used in this research are mouse monoclonal anti-beta III tubulin obtained from Sigma (St. Louis, MO), and goat anti mouse IgG (heavy and light chain) conjugated to Alexa 488 which is obtained from Invitrogen (Grand Island, NY). The substrates used for these experiments are 0.1% poly L-lysine (PLL) (Sigma), laminin (Invitrogen), and L1-Fc, a chimeric protein consisting of the extracellular domain of L1 cell adhesion molecule and the Fc domain of IgG, is purchased from R&D system (Minneapolis, MN). Chlorhexidine gluconate ((CHG) in a proprietary vehicle (V)) and V alone were supplied by Sage Products (Carey, IL). Cells are viewed on a Zeiss Observer Z1 fluorescence inverted microscope (Carl Zeiss Microscopy, LLC). Images are captured using Axiovision camera software (Carl Zeiss Oberkochen, Germany), and neurite outgrowth is measured using Image J software (National Institutes of Health, Bethesda, MD).
Preparation of coverslips
Cover slips obtained from Fisher Scientific (Hanover Park, IL) are cleaned and placed in the bottom of each of a 24 well Costar tissue culture flat bottom plate. One ml ice cold 0.1% PLL is placed into each of the 24 wells. They are then sealed and placed in the refrigerator at 4 °C over night. Laminin containing wells are prepared as follows: The PLL is removed from the wells, each well is washed in ice cold phosphate buffered saline (PBS) three times, then 1 ml of a 2 mg/ml laminin solution in PBS is added to each well. The plate is again sealed and placed in the refrigerator overnight at 4 °C. L1 containing wells are prepared as follows: Just prior to the addition of CGN, the PLL is removed from the wells and each well is washed in ice cold phosphate buffered saline (PBS) three times. CGN are added to the well in a final volume of 1 ml. L1-Fc, a chimeric protein consisting of the extracellular domain of L1 and the Fc domain of IgG is added following CGN to give a final concentration of 0.2 μg/ml.
Cell Cultures
CGN from 6 day-old Sprague-Dawley rat pups are prepared as previously described (11, 15). To minimize pain and discomfort, the pups are rapidly decapitated as approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Viability of CGN is assessed with trypan blue and is routinely >90 %. CGN are plated on prepared glass coverslips coated with PLL, laminin or L1-Fc in Neurobasal media (Gibco, Rockville, MD) with the following additions: 2% B27 supplement (Gibco), 20 mM L-glutamine, 6 g/L glucose, 20 mM HEPES, Ph 7.2, penicillin/streptomycin. CGN cultures are incubated for 2 h at 37 °C in 10 % CO2 to allow for cell adhesion. 10 μl aliquots of either dimethyl sulfoxide ( final concentration 1% DMSO), DMSO with 40.7 mg/ml (100 mM) HEX, the vehicle for CHG (V) either alone (1% V) or containing 100 μg/ml CHG is added to 1 ml of CGN cultures to give a final concentration of 407 μg/ml (1 mM) HEX or 1.0 μg/ml (0.002 mM) CHG. For the CHG dilution curve, the stock solution is serially diluted in vehicle 1:2. Aliquots of 10 μl of the stock and each dilution are added to the wells. Cells are grown for 24 h in a humid atmosphere of 90% air, 10% CO2 at 37 °C. After 24 h the media is removed and cells are washed with ice cold PBS three times. The cells are then fixed in 4% paraformaldehyde for 30 min at room temperature, followed by 3 more washes of PBS. Blocking solution (3% BSA/0.2%Triton X-100/PBS) is added to each coverslip for 1 h at 37 °C, or overnight at 4 °C. After blocking cells are immunostained.
Immunostaining
Blocked cells are exposed to mouse monoclonal anti-tubulin beta III for 1 h 37 °C and washed 3 times with PBS followed by Alexa 488 anti-mouse IgG for one h at 37 °C, washed 3 times with PBS, and mounted on glass slides.
Neurite outgrowth
Eligible neurites are identified by a masked investigator in an a priori design. The eligible neurons are photographed and neurite length measured. Only neurons containing neurites that meet the following criteria are measured: 1) The neurite is as long as the width of soma; 2) The neurite is not synapsing on another neuron; 3) The neuron must be single and not in a cluster (11). Images of neurites are measured using Image J (NIH). At least 30 neurites from each coverslip are measured.
Cell death
All photomicrographs from each condition were counted for cell bodies present. The number of cell bodies per high powered field was averaged. Each value was divided by average cell number per high powered field for PLL alone to give a relative cell number. The relative cell number was averaged across four experiments.
Statistical analysis
The mean neurite length is determined for each condition from each cell preparation. The mean neurite length for each cell preparation was calculated. Descriptive statistics determined the mean +/− SD of the mean neurite length from multiple cell preparations (11, 15). The data was analyzed by two-tailed paired t test to determine significance. P< 0.05 was set as significant.
RESULTS
We first tested whether CHG inhibited L1 mediated neurite outgrowth by plating CGN on a substrate of L1 (Figure 1, 2). Ethanol was used as a positive control for inhibition of L1 mediated neurite outgrowth. We chose the highest concentration of chlorhexidine detected in the serum of a newborn following topical exposure (1021 ng/ml) (16). As can be seen in Figure 1 and 2, compared to L1 control, CHG reduced neurite length of CGN plated on L1 (p<0.05). The vehicle (V) in which it is solubilized had no effect on neurite length. There was no apparent affect on neurite branching under any of these conditions.
Figure 1.
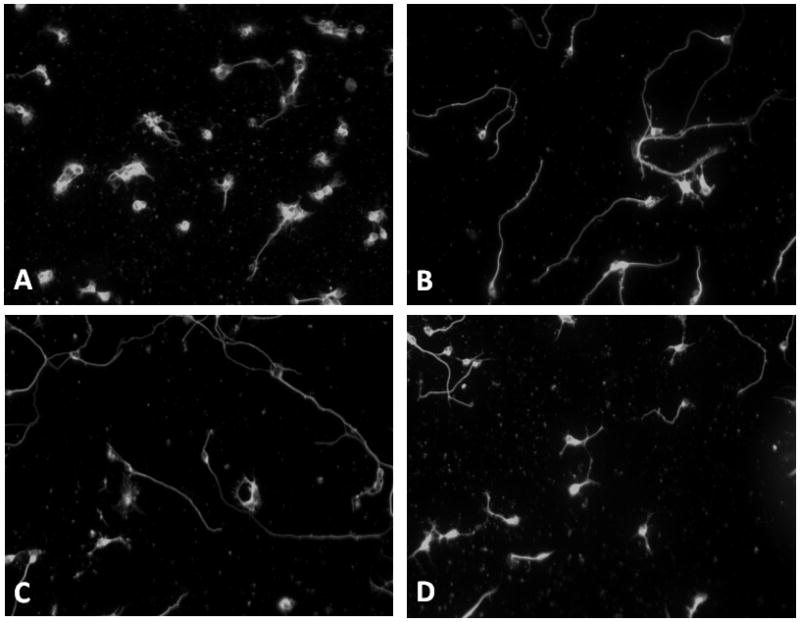
Chlorhexidine inhibits L1 mediated neurite outgrowth. Cerebellar granule neurons are plated on either poly L-lysine (PLL) alone or PLL plus L1-Fc (L1), then ethanol (E, final concentration 25 mM), chlorhexidine vehicle (V, final concentration 1%)) or chlorhexidine in vehicle (CHG, final concentration 1.0 μg/ml CHG, 1% vehicle) are added to the media. The cells are grown for 24 h, fixed and immunostained. Representative photomicrographs are shown: A) PLL alone; B) L1; C) L1 + V; D) L1+V+CHG. Magnification 20X.
Figure 2.
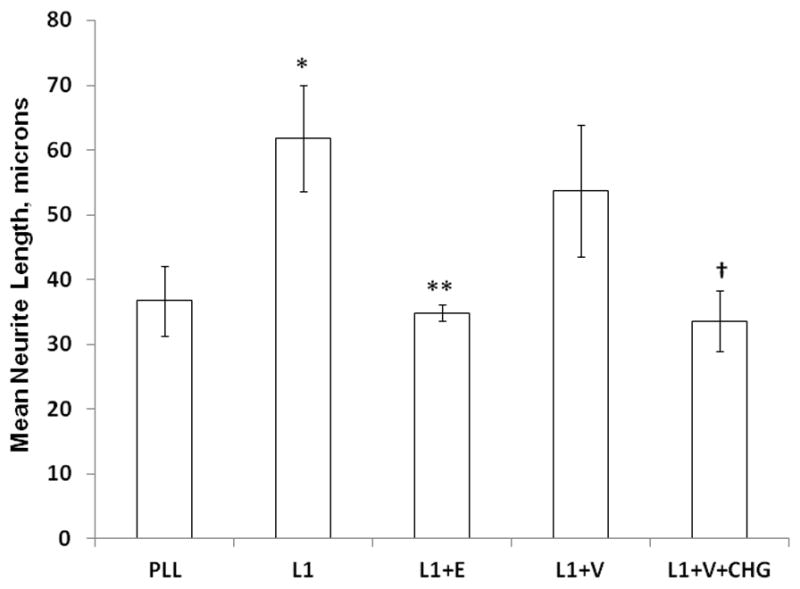
Chlorhexidine inhibits L1 mediated neurite outgrowth. Cerebellar granule neurons are plated on either poly L-lysine (PLL) alone or PLL plus L1-Fc (L1), then ethanol (E, final concentration 25 mM), chlorhexidine vehicle (V, final concentration 1%)) or chlorhexidine in vehicle (CHG, final concentration 1.0 μg/ml CHG, 1% vehicle) are added to the media. The cells are grown for 24 h, fixed and neurite length measured. The mean of the neurite lengths for each condition is calculated, then the mean +/− SD is determined for 4 separate experiments and shown here. *p<0.001, PLL vs L1; **p<0.01, L1 vs L1+E; †p<0.05, L1+V vs L1+V+CHG. L1 vs L1+V is not significant (p=0.29).
To determine if these reagents reduce cell viability, we counted the numbers of cells per high powered field. Nonviable cells lose adhesion and lift off the plates. Thus, decreasing number of adherent cells would indicate increased cell death. There was no statistically significant difference in the numbers of cells adherent to the plate compared to PLL, and no significant difference between the number of cells adherent to the plate in V alone or V with CHG (Figure 3).
Figure 3.
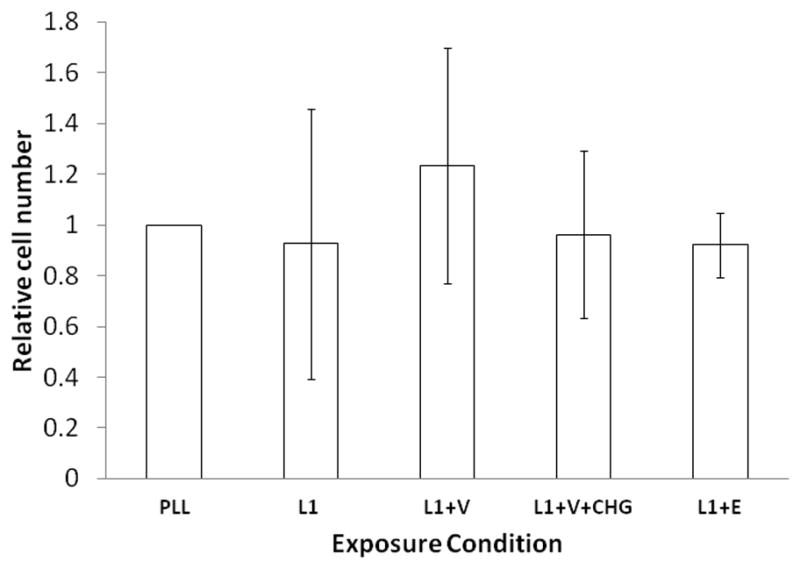
Chlorhexidine at 1.0 μg/ml does not increase cell death at 24 h. Photomicrographs used to determine neurite length in Figure 2, were re examined to determine number of cells per high powered field. Dead cells detach from the plate. Therefore a decrease in cell number compared to PLL alone would indicate increased cell death. The average number of cells in each photomicrograph obtained for each condition were counted and averaged for total cell number per high powered field, and divided by average cell number per high powered field in PLL alone to obtain a relative cell number. Four separate experiments were counted and the average relative cell number +/− SD is shown. No significant change in cell number compared to PLL alone was determined.
HEX is a known developmental neurotoxicant (8, 9, 17). The ability of HEX to inhibit L1 mediated neurite outgrowth was tested. The concentration of HEX used was 100X that in the serum of a newborn with liver disease (4350 ng/ml) (18). We found no significant effect of either DMSO alone or DMSO with 1 mM HEX on L1 mediated neurite outgrowth (Figure 4).
Figure 4.
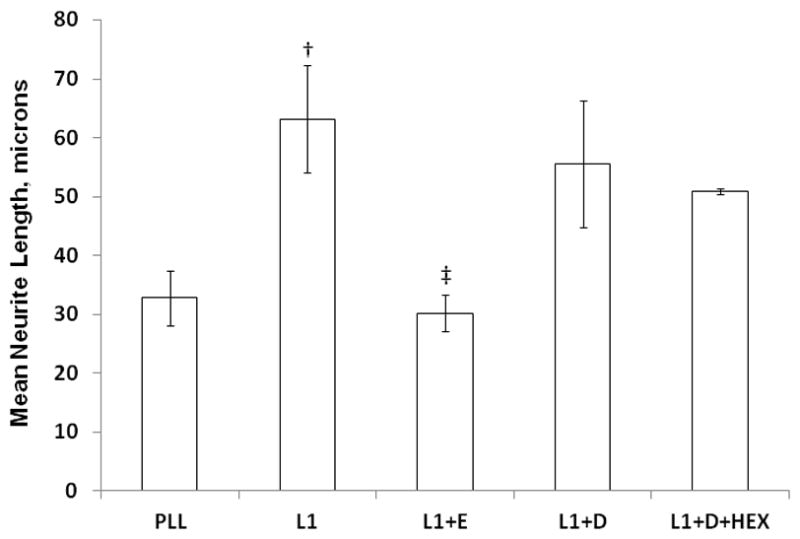
Hexachlorophene does not inhibit L1 mediated neurite outgrowth. Cerebellar granule neurons are prepared as in Figure 1. Additions to the media are 25 mM ethanol (L1+E), 1% DMSO (L1+D), or 1% DMSO with 1mM hexachlorophene (L1+D+HEX). The mean of the neurite lengths for each condition is calculated, then the mean +/− SD is determined for 4 separate experiments and shown here. †p<0.05, PLL vs L1; ‡p<0.02, L1 vs L1+E. L1 vs L1+D and L1+D vs L1+D+HEX are not significant.
To determine if the inhibition by CHG of neurite outgrowth is specific to L1 or is a general effect on neurite outgrowth, we plated CGN on laminin. Laminin is another cell adhesion molecule that binds to integrin receptors. As can be seen in Figure 5, neither the vehicles nor CHG nor HEX had any effect on the length of neurites of CGN grown on laminin.
Figure 5.
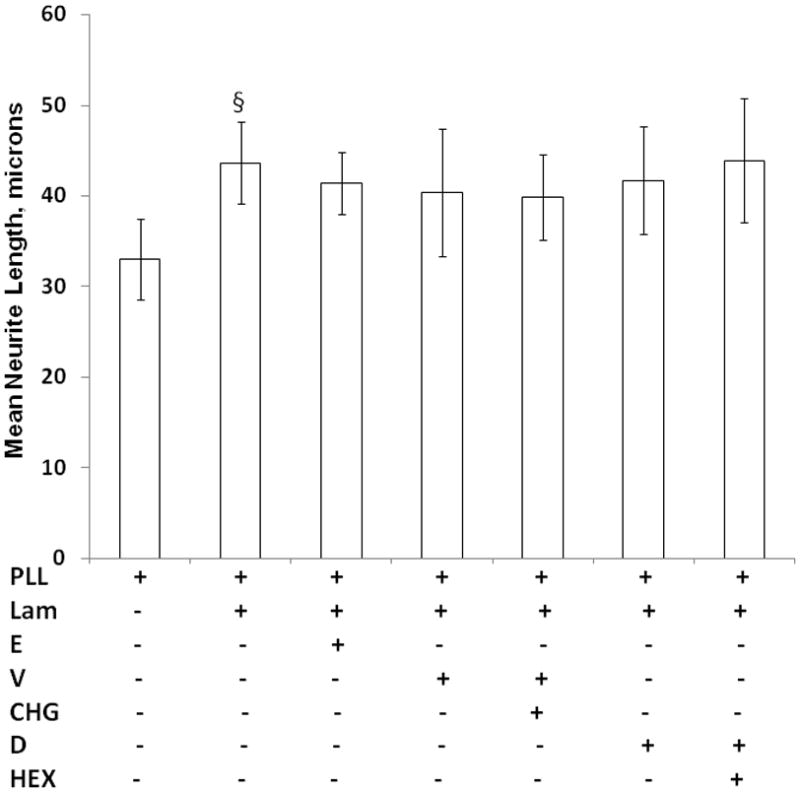
Neither chlorhexidine nor hexachlorophene inhibit laminin mediated neurite outgrowth. Cerebellar granule neurons are plated on either PLL alone or PLL plus laminin (lam). The following are added to the media to give final concentrations of: ethanol (E, 25 mM), chlorhexidine vehicle (V, 1%), Chlorhexidine (CHG,1.0 μg/ml), DMSO (D, 1%) or hexachlorophene (HEX, 1 mM). Neurite length is determined as described in Figure 2. The mean of the neurite lengths for each condition is calculated, then the mean +/− SD is determined for 4 separate experiments and shown here. §p<0.005, PLL vs L1.
To determine at what concentrations CHG is capable of inhibiting L1 mediated neurite outgrowth, we examined the impact of CHG concentration on neurite length. The results from 4 experiments are shown in Figure 6. Vehicle alone did not have a significant effect on L1 mediated neurite outgrowth. CHG at a concentration of 31 ng/ml did not inhibit neurite outgrowth compared to cultures with L1 and V alone. However, 62 ng/ml CHG showed a trend of reduced neurite length (p= 0.08). Concentrations of CHG of 125 ng/ml or greater all significantly inhibited neurite growth similar to ethanol, the positive control. There is no significant difference in neurite length of CGN grown in 62, 125, 250, 500 or 1000 ng/ml CHG and PLL alone.
Figure 6.
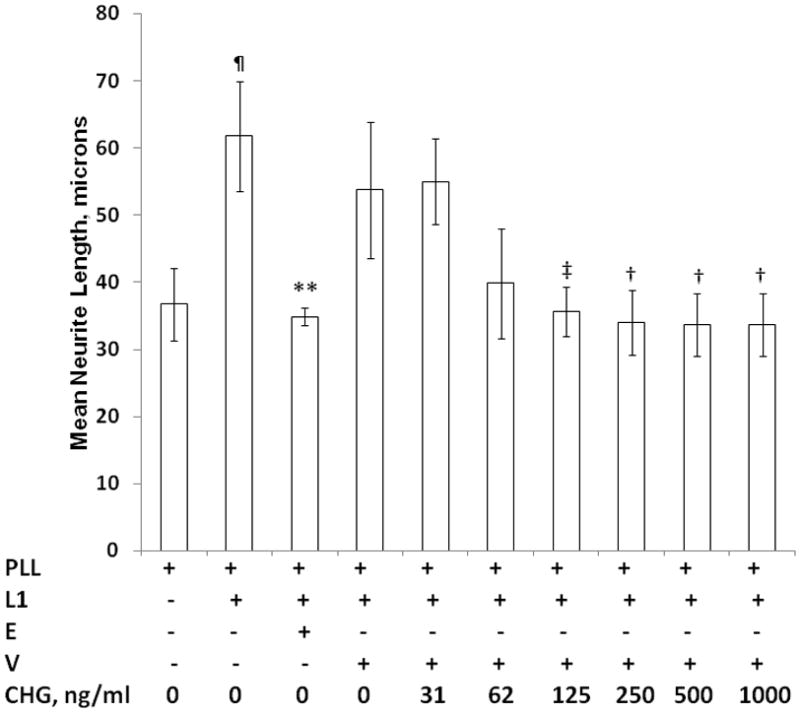
Chlorhexidine inhibits L1 mediated neurite outgrowth at pharmacologic concentrations. Cerebellar granule neurons are prepared as described in Figure 1 and plated on poly L-lysine alone (PLL), or with L1 (L1). Additions to the media are: Ethanol, E (25 mM); chlorhexidine vehicle, V (1%); and increasing concentrations of chlorhexidine (CHG) as indicated. Mean neurite lengths from 4 separate experiments are calculated then the mean +/− SD are calculated and shown here. ¶p<0.001, PLL vs L1; **p<0.01, L1 vs L1+E; ‡p<0.02, L1+V vs L1+V+CHG at 125 μg/ml; †p<0.05, L1+V vs L1+V+CHG at 250, 500, and 1000 μg/ml.
DISCUSSION
The recent demonstration that chlorhexidine is systemically absorbed and can be detected in the blood of preterm infants days after exposure (19) raises concerns about the possible consequences of exposure in preterm infants. To begin to determine if chlorhexidine concentrations detected in preterm infants are capable of causing developmental neurotoxicity, we examined the effects of similar concentrations on neurite outgrowth. The model of neurite outgrowth reported here is dependent on lipid rafts, microdomains of the plasma membrane which may be susceptible to the chemical properties of chlorhexidine that provide bactericidal activity.
Our results suggest that chlorhexidine at concentrations observed in the blood of preterm infants (19) specifically inhibited L1 mediated neurite outgrowth of cerebellar granule neurons. The effect of chlorhexidine is found to be specific to neurite outgrowth of neurons plated on L1. It is interesting to speculate on the differences in susceptibility of laminin mediated neurite outgrowth versus that of L1. L1 mediated neurite outgrowth is dependent on lipid rafts whereas laminin mediated neurite outgrowth is not (20). Lipid rafts are microdomains of the plasma membrane important for protein-protein interaction and signal transduction. Disruption of the lipid raft by photobleaching (20) or cholesterol chelation (15) reduces neurite outgrowth of neurons plated on L1 but not laminin. The developmental neurotoxicant ethanol has been shown to disrupt lipid raft function (15, 21–25). Recently, data show that chlorhexidine’s bactericidal action occurs through effects on the plasma membrane (13). Using model membranes of 1,2 dimyristroyl-sn-glycero-3-phosphocholine and neutron diffraction, chlorhexidine is shown to insert itself into the lipid bilayer with the phenols stacked forming a wedge, and the hexamethylene linker interaction with the headgroup, allowing cytoplasmic elements to leak out. Of note, chlorhexidine is only active on viruses possessing lipid envelopes. We speculate that a sensitive function of the plasma membrane in growth cones is the dynamic nature of lipid rafts. A perturbation of the plasma membrane may impede the trafficking of critical proteins, such as L1, through the lipid raft. Further studies are needed to explore this potential mechanism.
The present study confirms findings from previous studies investigating the cytotoxic effect of chlorhexidine in vitro. Because chlorhexidine is frequently used for oral care to prevent dental plaque and gingivitis, many studies have examined and identified in vitro toxicity of chlorhexidine after direct application on gingival epithelial cells, odontoblast –like cells, and periodontal ligament cells (26–29). Similar cytotoxicity has been seen with HeLa cells and human newborn fibroblasts (30). These studies have focused on chlorhexidine concentrations used in topical preparations. A recent study exposed human-derived neuroblastoma cells and rat Schwann cells to diluted chlorhexidine (20% stock solution with density of 1.06 g/ml diluted to lowest concentration of 1:2,000 or 500 μg/ml) and found evidence of cytotoxicity (31). The chlorhexidine concentrations used in that study (500 μg/ml) were much higher than both concentrations reported in human serum following topical exposure and concentrations used in the present study.
This study also demonstrates that chlorhexidine and HEX have different toxic effects on neurite outgrowth. In vivo studies have found differences in the toxic effects of chlorhexidine and HEX. Studies in baboons have found that topical daily HEX application induced changes in myelin (32). Studies in macaques found that daily chlorhexidine bathing did not produce any histopathological changes in the brain following 90 days of exposure (33). However, chlorhexidine was undetectable in the blood of these monkeys, so no chlorhexidine could have possibly gained access to the brain. There are no studies assessing whether chlorhexidine can cross the blood brain barrier. Overall, many studies demonstrate that chlorhexidine can cause cytotoxicity, but the clinical correlation of these in vitro studies remains unknown. Specifically for our study, it is not known if chlorhexidine in the blood can cross the blood-brain-barrier and cause direct exposure to neurons.
Limitations of this study include the uncertainly of extrapolating media concentrations of chlorhexidine and HEX to blood concentrations of exposed premature infants. The permeability of the blood brain barrier in premature infants is affected by many factors, including developmental maturation and disruption caused by disease (34–36). Thus, the local concentration of chlorhexidine next to neuronal membranes in humans is unknown. More research is needed in this area. A second limitation of the study is the relationship between inhibition of neurite outgrowth and clinical neurodevelopmental effects. More research is needed linking behavior to chlorhexidine exposure. The current study provides rationale for examining behavior known to be disrupted in L1 knock out animals, such as cage activity, rotarod, open field test, social exploration and Morris water maze (37).
In summary, chlorhexidine at concentrations reported following topical applications in infants specifically inhibited L1 mediated neurite outgrowth of cerebellar granule neurons. Chlorhexidine has lifesaving applications for neonates around the world, including umbilical cord care to reduce neonatal mortality (38). As chlorhexidine use increases in hospitalized patients, further studies are needed to determine the clinical relevance of these in vitro findings. It is now vital to determine whether the blood brain barrier is permeable to chlorhexidine in premature infants.
Acknowledgments
This study was funded by UL1 RR 025005 (AM) from the National Center for Research Resources (NCRR), Bethesda, MD, a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH, The Cobey Endowment, Baltimore, MD (CB), The Munro Fund, Baltimore, MD (CB), and NIH R01AA016398, Rockville, MD (CB).
Footnotes
The authors have disclosures to declare.
References
- 1.Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant staphylococcus aureus, vancomycin-resistant enterococcus, and healthcare-associated bloodstream infections: Results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–65. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 2.O’Horo JC, Silva GL, Munoz-Price LS, et al. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: A meta-analysis. Infect Control Hosp Epidemiol. 2012;33:257–67. doi: 10.1086/664496. [DOI] [PubMed] [Google Scholar]
- 3.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46:274–81. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 4.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Case DE. Chlorhexidine: Attempts to detect percutaneous absorption in man. Proceedings of the International Congress of Chemotherapy. 1976;3:367–374. [Google Scholar]
- 6.Wilson CM, Gray G, Read JS, et al. Tolerance and safety of different concentrations of chlorhexidine for peripartum vaginal and infant washes: HIVNET 025. J Acquir Immune Defic Syndr. 2004;35:138–43. doi: 10.1097/00126334-200402010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullany LC, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25:665–75. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curley A, Kimbrough RD, Hawk RE, et al. Dermal absorption of hexochlorophane in infants. Lancet. 1971;2:296–7. doi: 10.1016/s0140-6736(71)91337-7. [DOI] [PubMed] [Google Scholar]
- 9.Kopelman AE. Cutaneous absorption of hexachlorophene in low-birth-weight infants. J Pediatr. 1973;82:972–5. doi: 10.1016/s0022-3476(73)80427-5. [DOI] [PubMed] [Google Scholar]
- 10.Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: Results from a national survey. Infect Control Hosp Epidemiol. 2010;31:846–9. doi: 10.1086/655017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bearer CF, Swick AR, O’Riordan MA, et al. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–70. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe H, Ymazaki M, Miyazaki H, et al. Phospholipase D2 functions as a downstream signaling molecule of MAP kinase pathway in L1-stimulated neurite outgrowth of cerebellar granule neurons. J Neurochem. 2004;89:142–51. doi: 10.1111/j.1471-4159.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- 13.Komljenovic I, Marquardt D, Harroun TA, et al. Location of chlorhexidine in DMPC model membranes: A neutron diffraction study. Chem Phys Lipids. 2010;163:480–7. doi: 10.1016/j.chemphyslip.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Schmuck G, Schluter G. An in vitro model for toxicological investigations of environmental neurotoxins in primary neuronal cell cultures. Toxicol Ind Health. 1996;12:683–96. doi: 10.1177/074823379601200507. [DOI] [PubMed] [Google Scholar]
- 15.Tang N, Farah B, He M, et al. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J Neurochem. 2011;119:859–67. doi: 10.1111/j.1471-4159.2011.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowen J, Ellis SH, McAinsh J. Absorption of chlorhexidine from the intact skin of newborn infants. Arch Dis Child. 1979;54:379–83. doi: 10.1136/adc.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuman RM, Leech RW, Alvord EC., Jr Neurotoxicity of hexachlorophene in the human: I. A clinicopathologic study of 248 children. Pediatrics. 1974;54:689–95. [PubMed] [Google Scholar]
- 18.Tyrala EE, Hillman LS, Hillman RE, et al. Clinical pharmacology of hexachlorophene in newborn infants. J Pediatr. 1977;91:481–6. doi: 10.1016/s0022-3476(77)81330-9. [DOI] [PubMed] [Google Scholar]
- 19.Chapman A, Aucott S, Gilmore M, et al. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol. 2013 doi: 10.1038/jp.2013.61. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J Cell Biol. 2002;159:1097–108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolganiuc A, Bakis G, Kodys K, et al. Acute ethanol treatment modulates toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 22.Littner Y, Tang N, He M, et al. L1 cell adhesion molecule signaling is inhibited by ethanol in vivo. Alcohol Clin Exp Res. 2013;37:383–9. doi: 10.1111/j.1530-0277.2012.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghare S, Patil M, Hote P, et al. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: Potential mechanism of alcohol-induced immune suppression. Alcohol Clin Exp Res. 2011;35:1435–44. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, et al. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–39. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 25.Szabo G, Dolganiuc A, Dai Q, et al. TLR4, ethanol, and lipid rafts: A new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–9. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- 26.Babich H, Wurzburger BJ, Rubin YL, et al. An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell Biol Toxicol. 1995;11:79–88. doi: 10.1007/BF00767493. [DOI] [PubMed] [Google Scholar]
- 27.Lessa FC, Aranha AM, Nogueira I, et al. Toxicity of chlorhexidine on odontoblast-like cells. J Appl Oral Sci. 2010;18:50–8. doi: 10.1590/S1678-77572010000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang YC, Huang FM, Tai KW, et al. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 29.de Souza LB, de Aquino SG, de Souza PP, et al. Cytotoxic effects of different concentrations of chlorhexidine. Am J Dent. 2007;20:400–4. [PubMed] [Google Scholar]
- 30.Goldschmidt P, Cogen R, Taubman S. Cytopathologic effects of chlorhexidine on human cells. J Periodontol. 1977;48:212–5. doi: 10.1902/jop.1977.48.4.212. [DOI] [PubMed] [Google Scholar]
- 31.Doan L, Piskoun B, Rosenberg AD, et al. In vitro antiseptic effects on viability of neuronal and schwann cells. Reg Anesth Pain Med. 2012;37:131–8. doi: 10.1097/AAP.0b013e31823cdd96. [DOI] [PubMed] [Google Scholar]
- 32.Tripier MF, Berard M, Toga M, et al. Hexachlorophene and the central nervous system. toxic effects in mice and baboons. Acta Neuropathol. 1981;53:65–74. doi: 10.1007/BF00697186. [DOI] [PubMed] [Google Scholar]
- 33.Gongwer LE, Hubben K, Lenkiewicz RS, et al. The effects of daily bathing of neonatal rhesus monkeys with an antimicrobial skin cleanser containing chlorhexidine gluconate. Toxicol Appl Pharmacol. 1980;52:255–61. doi: 10.1016/0041-008x(80)90112-x. [DOI] [PubMed] [Google Scholar]
- 34.Kronstein R, Seebach J, Grossklaus S, et al. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res. 2012;93:130–40. doi: 10.1093/cvr/cvr256. [DOI] [PubMed] [Google Scholar]
- 35.Wennberg RP. The blood-brain barrier and bilirubin encephalopathy. Cell Mol Neurobiol. 2000;20:97–109. doi: 10.1023/A:1006900111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int J Dev Biol. 2011;55:467–76. doi: 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 37.Fransen E, D’Hooge R, Van Camp G, Verhoye M, Sijbers J, Reyniers E, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum Mol Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- 38.Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR, et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: A community-based, cluster-randomised trial. Lancet. 2012;379:1022–8. doi: 10.1016/S0140-6736(11)61848-5. [DOI] [PubMed] [Google Scholar]


