Abstract
Vaccines constructed from rare-serotype recombinant adenovirus vectors (rAd) such as rAd serotype 28 (rAd28) and rAd35 are currently being explored as alternatives to rAd5-based vaccines because they circumvent the problems with pre-existing immunity that complicate the effectiveness of rAd5 vaccines. However, previous work has demonstrated that the immunogenicity of rAd28 and rAd35 is substantially lower than rAd5. Here we show that rAd28 and rAd35 increase apoptosis of antigen presenting cells (APCs), such as monocytes, relative to rAd5 and mock infected controls. APCs undergoing apoptosis showed an increased loss of vector-insert expression. Loss of vector-insert expression correlated with activation of NK cells, which resulted in apoptosis of co-cultured monocytes. Finally, we show that activation of NK cells is dependent on IFNα which is produced by exposure to rAd28 or rAd35, but not to rAd5. Taken together, these data demonstrate that IFNα-induced activation of NK cells leads to increased monocyte apoptosis and subsequent vector-insert loss. This may be a possible mechanism that results in reduced immunogenicity of rAd28 and rAd35-based vectors.
Introduction
Adenoviruses (Ad) have been well studied as vectors for recombinant vaccines because of their ability to generate strong, insert-specific memory immune responses [1, 2]. Vectors constructed from Ad serotype 5 (rAd5) are the most well characterized, but their use as a clinical vaccine product is limited by high preexisting immunity [3, 4]. As a result vectors constructed from rarer serotypes, such as rAd28 or rAd35, are under consideration. The differing immunogenicity of vectors constructed from different adenovirus serotypes is well documented, with rAd5 being the most immunogenic, rAd35 being the least immunogenic, and other serotypes, including rAd28, being moderately immunogenic [1, 3–6]. The mechanistic causes of this differential immunogenicity are incompletely understood. The serotypes differ in infectivity, trophism, cellular receptor usage, intracellular trafficking routes, and genome CpG content but these factors have not been conclusively shown to be directly responsible for the differing immunogenicity [1, 7–10].
We have previously shown that rAd5 vectors induce large insert-specific CD8 T cell populations with a high proportion producing both IFNγ and TNF and that the CD8 T cell population resulting from exposure to vectors constructed from rare-serotypes, such as rAd28 and rAd35, is lower in magnitude, but contain a greater frequency of triple positive IFNγ-, TNF, and IL2- producing cells and a higher frequency of long-lived CD127+ cells [4, 11]. These differences are largely attributable to the induction of type I interferon (IFNα) by rAd28 and rAd35, but not by rAd5 [11].
Here we further examined the impact of innate immunity on vector-insert expression. Specifically we examined the impact of cell death and apoptosis on the duration of vector-insert expression and the contribution of NK cells. We show that human CD14+ monocytes are lost during infection with rAd28 or rAd35, but not rAd5. rAd28 and rAd35, but not rAd5, induced IFNα-dependent activation of NK cells and these activated NK cells were capable of inducing monocyte apoptosis. This provides a possible mechanism for the loss of CD14+ monocytes after infection with rAd28 and rAd35, but not rAd5. Collectively, these data suggest that duration of the vector-insert expression, APC apoptosis, and NK cell activation differs greatly between vectors constructed from different Ad serotypes and should be taken into consideration when designing rAd vaccines.
Materials and Methods
Isolation of primary PBMCs, NK cells, monocytes, and DC subsets
PBMCs from healthy donors were obtained by automated leukapheresis and isolated by density gradient centrifugation. Signed informed consent was obtained from all donors in accordance with the Declaration of Helsinki and the study was approved by the relevant Institutional Review Board.
CD14+ microbeads (Miltenyi Biotec) were used with an AutoMACS magnetic cell sorter (Miltenyi Biotec) to isolate CD14+ monocytes from PBMCs (>90% CD14+). NK Cell Isolation Kit (Miltenyi Biotec) was used with LD MACS Separation Columns (Miltenyi Biotec) to negatively select for NK cells (>90% CD56+ CD3-). Isolation of CD11c+ myeloid DCs (mDCs) and CD123+ plasmacytoid DCs (pDCs) were performed from elutriated monocytes using CD1c and BDCA4 isolation kits (Mitenyi Biotec) (>85% CD11c+ and CD123+ respectively) as described earlier [1].
Labeling of monocytes with violet tracking dye
Monocytes were stained violet using CellTrace Violet Cell Proliferation Kit (Invitrogen) by incubating monocytes at the concentration of 1×106 cells/mL with 5 µM CellTrace Violet Solution at 37°C for 7 minutes, in accordance with the manufacturer’s instructions.
rAd vectors
Replication-deficient rAd5, rAd28, and rAd35 vectors expressing eGFP were provided by GenVec Inc. The construction of the E1/E3/E4-deleted rAd5 and rAd35 [12, 13] and the E1-deleted rAd28 [6] has been described previously. The concentration of all vectors was determined by measuring viral particles per milliliter and their activity by measuring their ability to infect HEK293 cells. An MOI of 1000 was used for rAd5 and an MOI of 100 was used for rAd28 and rAd35 to keep the rate of infectivity consistent for each vector [11].
Sorting of insert-positive cells
eGFP+ cells from infected violet-labeled monocytes were sorted 24 hours post-exposure to rAd vectors using a FACSAria cell sorter (BD Biosciences) (>99% eGFP+).
Measurements of cell apoptosis
rAd and mock-infected PBMCs were incubated with a viability marker and FcR blocking reagent (Miltenyi Biotec). Cells were than washed with buffer containing 0.5% BSA and 25µM CaCl and stained with fluorescently labeled annexin V (Invitrogen) and antibodies to CD3, CD11c, CD14, CD123, HLA-DR, and active caspase 3. Samples were then fixed with 1% PFA containing 25µM CaCl and analyzed using a LSR II flow cytometer (BD Biosciences).
Activation of NK cells and NK cell/monocyte co-cultures
The processes for co-culturing NK cells with monocytes have been described previously [14]. Briefly, sorted NK cells were incubated with media, conditioned media, or recombinant IFNα at 37°C and 5% CO2 for 18 hours. The NK cells were then washed, added to sorted monocytes cultures at a 1:1 or 1:10 effector to target ratio, and incubated at 37°C and 5% CO2 for 6 hours. Cells were then stained for flow cytometery as described. For experiments blocking IFNα-signaling, NK cells were pretreated with 10µg/mL of IFNα/β receptor neutralizing antibody (PBL Interferon Source).
Measurements of NK cell activation and monocyte apoptosis
rAd and mock-infected PBMCs were incubated with a viability marker and FcR blocking reagent (Miltenyi Biotec) and then stained with fluorescently labeled annexin V and antibodies to CD3, CD11c, CD14, CD56, CD69 and HLA-DR. Samples were then analyzed using a LSR II flow cytometer.
Statistical analyses
Data analysis was performed using FlowJo version 9.3.2 (Tree Star). Statistical analyses were performed using the Wilcoxon’s signed-rank test or the Mann-Whitney U-test with GraphPad Prism version 5.0c software (GraphPad Software). Error bars shown represent standard deviation in all cases. Measurements were considered statistically different at p<.05.
Results
APCs undergo apoptosis after infection with rAd28 and rAd35, but not rAd5
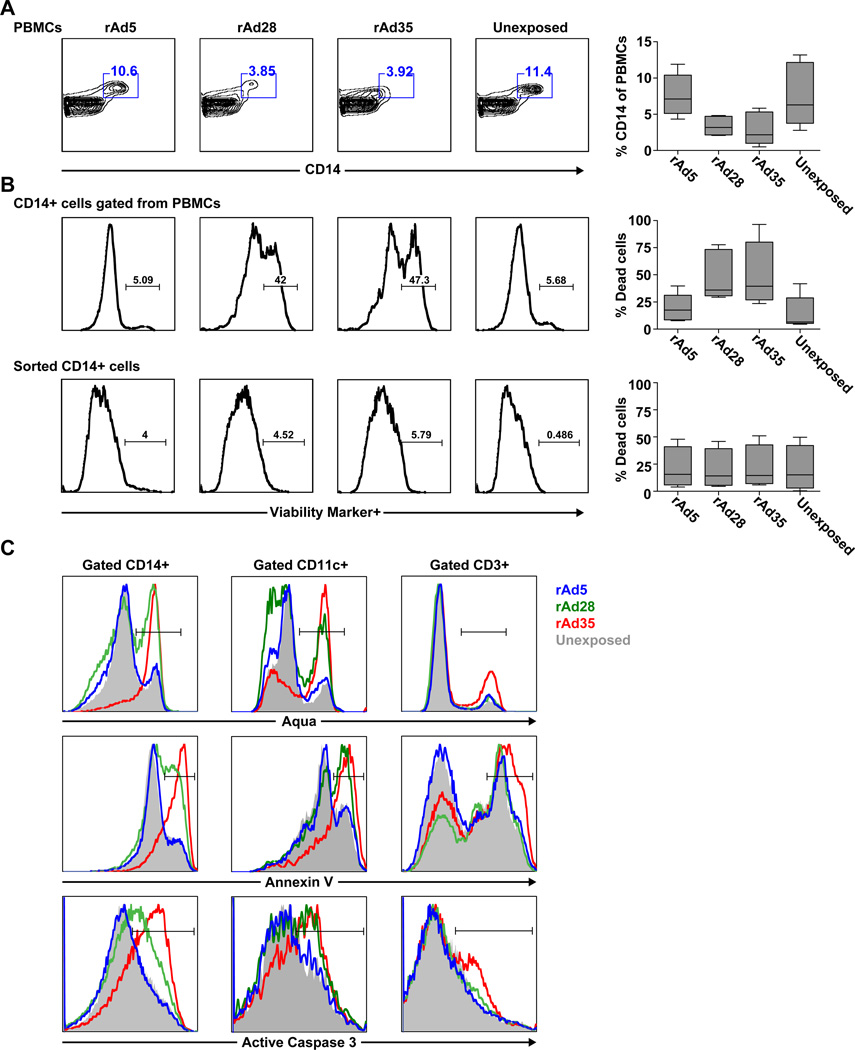
We compared the size and viability of the CD14+ monocyte population within PBMCs exposed to rAd5, rAd28, or rAd35 for 48 hours. The CD14+ monocyte population was greatly reduced in frequency in samples exposed to rAd28 and rAd35, but not rAd5 or the unexposed control (Figure 1A). Viability staining using Aqua cell viability dye revealed that the remaining CD14+ population in the rAd28 and rAd35 exposed samples had a much larger fraction of dead or apoptotic cells than the rAd5 samples or unexposed controls (Figure 1B, top panels). In contrast, only low levels of cell death were detected in isolated CD14+ cells alone that were exposed to any of the rAd vectors for 48 hours and no difference was found between the vectors (Figure 1B, bottom panels).
Figure 1. Monocytes begin apoptosis and lose surface CD14 expression after exposure to rAd28 or rAd35.
(A) Frequency of CD14+ monocytes from PBMCs exposed to rAd5, rAd28, or rAd35 for 48 hours and an unexposed control (n=5). (B) Viability staining of CD14+ monocytes gated from PBMCs (upper panels) and sorted CD14 monocytes (lower panels) exposed to rAd5, rAd28, or rAd35 for 48 hours and an unexposed control (n=6–8). (C) Staining of Aqua cell viability dye (top panels) and the apoptotic markers annexin V (middle panels) and active caspase 3 (lower panels) on CD14+ cells (left panels), CD11c+ HLADR+ CD14− cells (middle panels), and CD3+ cells (right panels) gated from PBMCs exposed to rAd5, rAd28, or rAd35 for 48 hours and an unexposed control (representative of 5 independent experiments).
Further viability staining with Aqua, annexin V or with active-caspase 3 antibody confirmed that CD14+ monocytes and CD11c+ CD14− HLADR+ mDCs, but not CD3+ T cells, within PBMCs exposed to rAd28 and rAd35 underwent a much higher degree of apoptotic death than cells exposed to rAd5 and unexposed controls (Figure 1C, left and center panels; Supplemental Table 1).
Infected monocytes rapidly undergo apoptosis and lose vector-insert expression
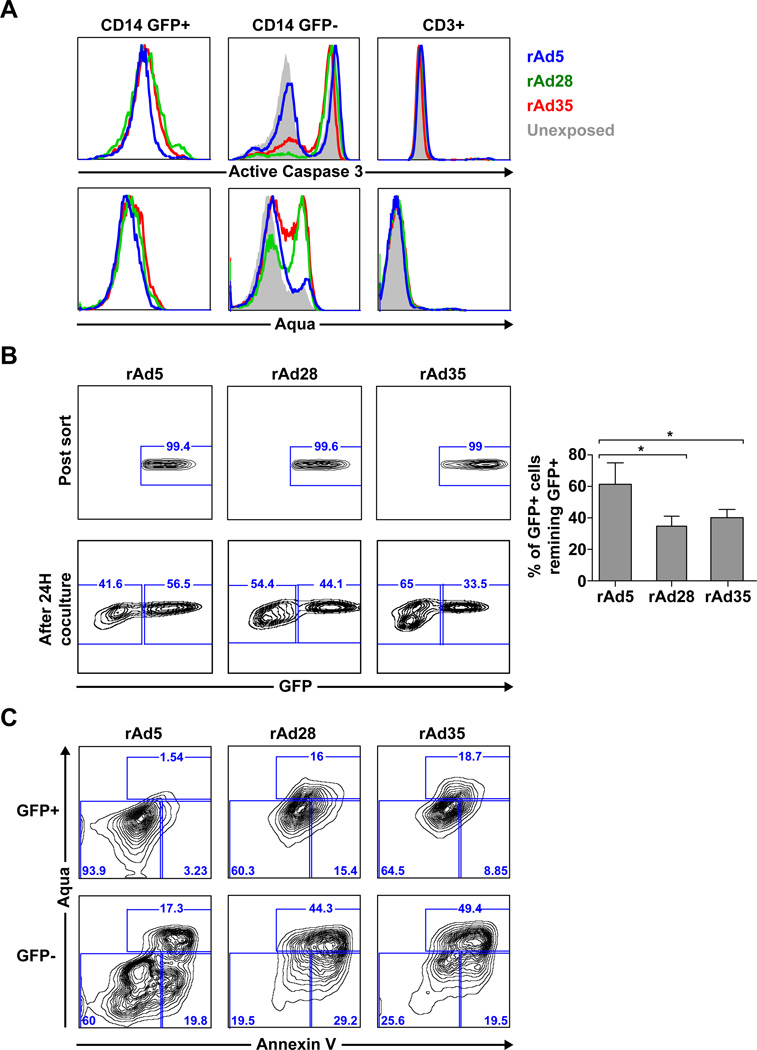
To investigate the impact of vector infection on apoptosis we first exposed PBMCs to rAd5, rAd28 or rAd35 expressing eGFP and compared the expression of viability and apoptosis markers along with presence or absence of eGFP in monocytes. We chose to focus on the monocyte population because they are the largest populations of cell infected by rAd5 vectors, both by frequency and by infectivity, containing the bulk of insert positive cells, and for their ease of access. Aqua dye and active-caspase 3 antibody staining of eGFP+ monocytes showed limited or absent apoptosis in samples exposed to any rAd vectors (Figure 2A, left panels). GFP-monocytes, however, showed increased apoptosis in cells exposed to rAd28 or rAd35 compared to those exposed to rAd5 (Figure 2A, center panels). In contrast, CD3+ T cells, which were not infected by any rAd vectors, did not up-regulate any apoptotic markers after exposure (Figure 2A, right panels).
Figure 2. Monocytes exposed to rAd28 or rAd35 lose vector-insert expression during apoptosis.
(A) Viability dye (upper panels) and active caspase 3 (lower panels) staining of PBMCs gated on CD14+ eGFP+ cells, CD14+ eGFP− cells, or CD3+ cells after exposure to rAd5, rAd28 or rAd35 and an uninfected control after 48 hour culture (representative of four independent experiments). (B) eGFP+ cells sorted from violet-labeled CD14+ cells after 24-hour exposure to rAd5, rAd28, or rAd35 (top panels). The percentage of those eGFP+ violet-labeled CD14+ cells remaining eGFP+ after 24-hour co-culture with PBMCs (bottom panels) (n=4) (* p<0.05). (C) Viability staining of cells remaining eGFP+ (upper panels) and cells that lose eGFP expression (lower panels) (representative of four independent experiments).
We speculated that the low frequency of Aqua+ and active-caspase 3+ cells in the eGFP+ CD14+ cell population might be caused by increased survival of vector-infected cells or by preferential loss of eGFP-expression during apoptosis. To determine this we sorted CD14+ cells from PBMC cultures, labeled the cells with a violet tracking dye, and then exposed them to rAd5, rAd28 or rAd35 for 24 hours. We then sorted the eGFP+ cells from the violet-labeled monocytes (Figure 2B, top panels) and co-cultured them with autologous PBMCs for an additional 24 hours. The ratio of violet-labeled monocytes retaining eGFP expression was then determined for each vector (Figure 2B, lower panels). Monocytes exposed to rAd28 or rAd35 lost a higher proportion of eGFP+ cells than samples exposed to rAd5 (Figure 2B, right panel).
Moreover, monocytes that lost eGFP expression (Figure 2C, bottom panels) showed a much higher level of apoptosis than their counterparts that retained eGFP expression (Figure 2C, top panels). This was particularly evident in cultures exposed to rAd28 or rAd35. These results demonstrate that vector-insert+ monocytes undergoing apoptosis rapidly lose vector-insert expression.
Impact of apoptosis on vector-insert loss
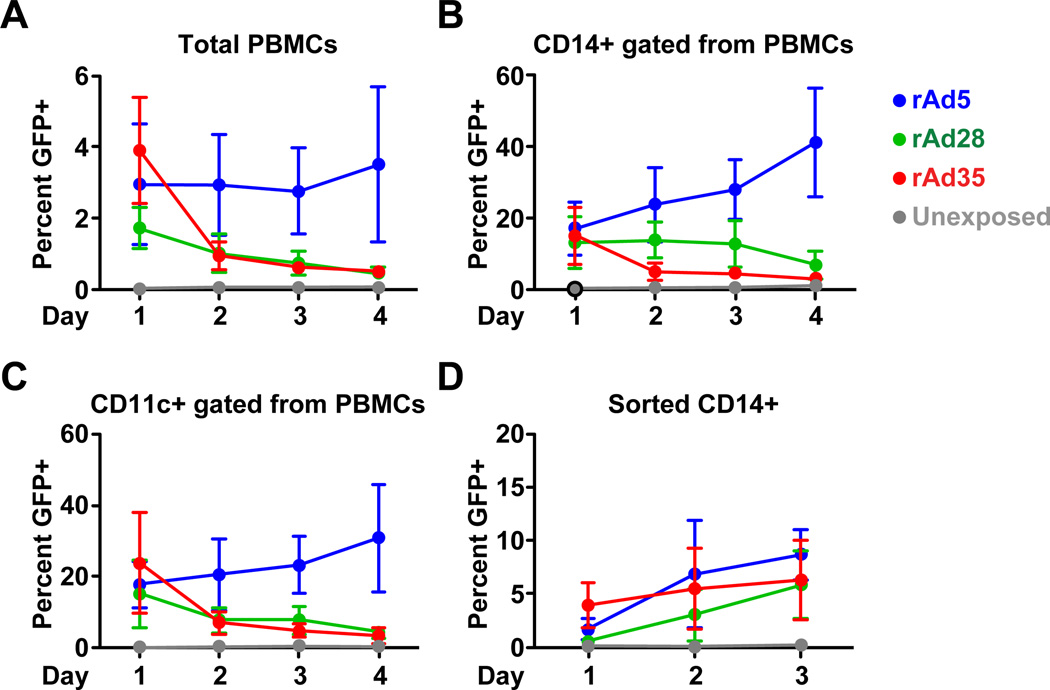
To determine the impact of vector-insert loss during apoptosis on overall vector-insert expression we tracked the expression of eGFP in PBMCs exposed to rAd5, rAd28, or rAd35 for 4 days after rAd exposure. eGFP expression was rapidly lost in PBMCs exposed to rAd35 and rAd28 after the first day, and continued to decline for the rest of the experiment (Figure 3A). In contrast rAd5 infected cultures maintained consistently high levels of eGFP expression. We then tracked the rate of eGFP expression in CD14+ cells or CD11c+ CD14− HLADR+ cells within the PBMCs cultures. These populations were pre-labeled with violet dye to ensure that losses in eGFP expression were not caused by downregulation of cell surface markers due to infection, activation, or apoptosis. We observed that the loss of eGFP expression associated with rAd28, or rAd35, but not rAd5 was apparent in both the CD14+ (Figure 3B) and the CD11c+ CD14− HLADR+ cell populations (Figure 3C). Purified CD14+ monocyte cultures did not lose eGFP expression after infection with any rAd vector (Figure 3D).
Figure 3. Vector-insert expression is lost in cultures infected with rAd28 or rAd35 but not rAd5.
Percentage of cells expressing eGFP in PBMC cultures infected with rAd5, rAd28, or rAd35 and an uninfected control gated on total PBMCs (upper left panel), CD14+ monocytes (upper right panel), CD11c+ CD14− HLADR+ mDCs, (lower left panel) (n=6). The percentage of eGFP in sorted CD14+ monocyte cultures infected with rAd5, rAd28, or rAd35 and an uninfected control (lower right panel) (n=6).
NK cells activated by rAd-exposed cells induce apoptosis of monocytes
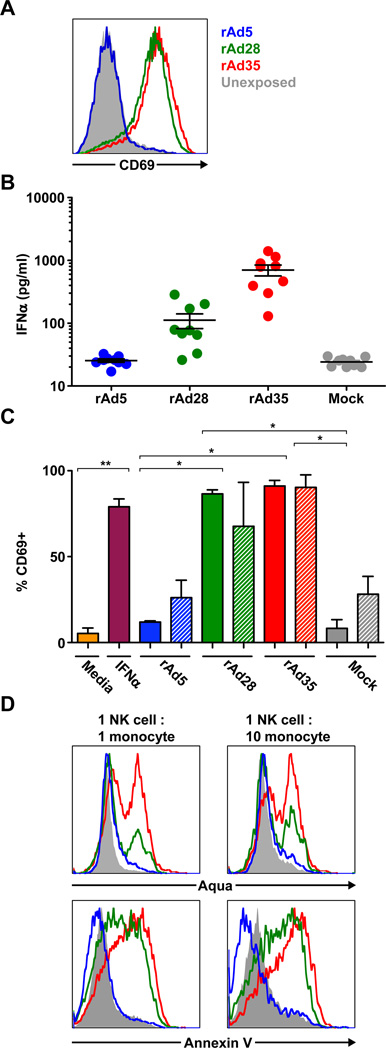
Previous studies have shown NK cells, while not infected by rAd vectors [11], may be involved in the clearance of rAd-insert expressing cells in vitro [15] and in vivo [16]. To determine the involvement of NK cells in the clearance of vector-insert positive cells in rAd28 or rAd35 exposed PBMCs we first measured the activation state of NK cells by staining for the NK cell activation marker CD69 [17]. NK cells in PBMC cultures exposed to rAd28 or rAd35 upregulated CD69 expression while NK cells in PBMC cultures exposed to rAd5 or unexposed controls did not (Figure 4A).
Figure 4. NK cells are activated by supernatants from PBMCs exposed to rAd28 or rAd35, but not from unexposed or rAd5 cells.
(A) The level of CD69 expression on CD3− CD56+ NK cells following exposure of PBMCs to rAd5, rAd28, or rAd35 for 24 hours and an unexposed control (representative of 6 different experiments). (B) Level of IFNα in the supernatant of PBMC cultures exposed to rA5, rAd28, or rAd35 for 24 hours and mock infected controls. (C) Level of CD69 positivity on NK cells following 18-hour exposure to media, media plus IFNα, or indicated supernatant and 6-hour co-culture with monocytes (n=4–8) (* p<0.05, **p<0.001). (D) Aqua cell viability dye (top panels) and annexin V staining (bottom panels) of CD14+ monocytes following 5-hour co-culture with NK cells activated by supernatants from PBMCs exposed to rAd5, rAd5, rAd28, or rAd35 and an unexposed control (representative of 3 different experiments).
We have previously identified the induction of IFNα by exposure to rAd28 or rAd35 as a major factor limiting their immunogenicity [11]. We therefore examined the role of IFNα in NK cell activation using supernatant collected from cells exposed to rAd5, rAd28, or rA35 as well as unexposed PBMCs. Supernatants from bulk PBMCs or isolated pDCs exposed to rAd28 or rAd35 showed increased levels of IFNα, while those exposed to rAd5 were similar to the unexposed controls (Figure 4B). Notably rAd35 induced much greater IFNα production than rAd28, contrasting with observations in mouse models [4, 6, 11].
We then co-cultured NK cells and uninfected monocytes with or without supernatants from PBMCs (Figure 4C, solid bars) or pDCs (Figure 4C, striped bars) exposed to rAd5, rAd28, or rAd35 or unexposed. NK cells cultured with supernatants from rAd5 exposed PBMCs or pDCs showed no upregulation of CD69 expression compared to NK cells cultured with supernatants from unexposed PBMCs or PDCs. However, supernatants from PBMCs and pDCs exposed to rAd28 or rAd35 strongly upregulated CD69 expression which was comparable to the levels induced by exogenous IFNα which was used as a control (Figure 4C).
Finally, NK cells, activated by supernatants from PBMCs and pDCs exposed to rAd28 or rAd35, had an increased ability to induce apoptosis in unexposed monocytes during co-culture as measured by Aqua dye and annexin V staining. NK cells treated with supernatants from rAd5 exposed PBMCs showed no changes compared to NK cells treated with supernatants from unexposed PBMCs (Figure 4D). Taken together, these data suggest that NK cells, activated by factors secreted by accessory cells after exposure to rAd28 or rAd35, but not rAd5, can enhance apoptosis of monocytes.
NK cell activation and vector-insert clearance is dependent on IFNα
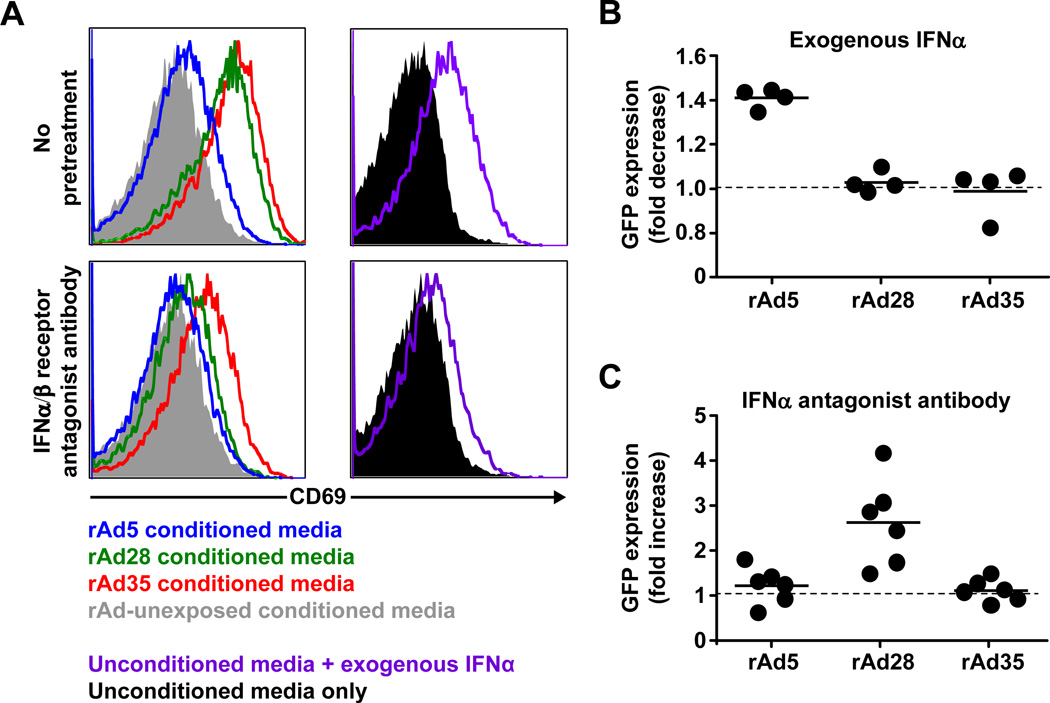
NK cells were then treated with supernatants from PBMCs exposed to rAd5, rAd28 or rAd35 or supernatants from unexposed PBMCs, media supplemented with exogenous IFNα, or media alone in the presence or absence of IFNα/β receptor antagonist antibody before co-culture (Figure 5A). As expected, NK cells upregulated CD69 expression when treated with supernatants from rAd28 or rAd35 exposed PBMCs or to media supplemented with IFNα, but did not upregulate CD69 when treated with supernatant from rAd5 exposed PBMCs, unexposed PBMCs, or media alone. Pretreatment with the IFNα/β receptor antagonist antibody reduced the level of CD69 activation on NK cells found with supernatants from PBMCs infected with rAd28 or rAd35, or with media supplemented with IFNα. However, although this reduction was consistent in multiple experiments it was incomplete. This may be caused by insufficient neutralization of IFNα-signaling by the IFNα/β receptor antagonist antibody or that the NK cells can be partially activated by alternative cytokines or factors other than IFNα/β. Pretreatment with IFNα/β receptor antagonist had no effect on NK cells treated with supernatant from PBMCs exposed to rAd5 or unexposed PBMCS, or media only (Figure 5A).
Figure 5. NK cell activation and vector clearance is IFNα-dependent.
(A) Activation of NK cells as measured by CD69 expression after exposure to supernatants from PBMCs exposed to rAd5, rAd28, or rAd35, media supplemented with exogenous IFNα, or media in the presence (lower panels) or absence (upper panels) of interferon α/β receptor antagonist antibody (representative of 3 independent experiments). (B) Fold decrease (upper panel) of eGFP+ cells in PBMC cultures exposed to rAd5, rAd28, or rAd35 after pretreatment with exogenous IFNα compared to donor matched, untreated rAd-exposed cultures (n=4). (C) Fold increase (lower panel) of eGFP+ cells in PBMC cultures exposed to rAd5, rAd28, or rAd35 after pretreatment with anti-IFNα antagonist antibody compared to donor matched, untreated rAd-exposed cultures (n=6).
We also examined the effects of IFNα on vector-insert expression by pretreating PBMCs with exogenous IFNα or anti-IFNα/β receptor antagonist antibody before exposure to rAd5, rAd28 or rAd35 and comparing the level of eGFP-expressing cells to controls. Consistent with our previous findings in mice [11], PBMCs pretreated with exogenous IFNα and exposed to rAd5 showed a reduction in the percentage of cells expressing eGFP (Figure 5B). Additionally, PBMCs pretreated with exogenous IFNα and exposed to rAd28 or rAd35 showed no reduction in eGFP expression (Figure 5B). Furthermore, PBMCs pretreated with anti-IFNα antagonist antibody and exposed to rAd5 showed no increase in eGFP+ frequency whereas exposure to rAd28 after pretreatment showed a strong increase in eGFP+ frequency (Figure 5C). In contrast with our previous findings in mice, samples pretreated with anti-IFNα/β receptor antagonist antibody and exposed to rAd35 did not show an increase in eGFP-expressing cells. However, this is consistent with the incomplete reduction of CD69 levels we observe on NK cells after pretreatment with anti-IFNα/β receptor antagonist antibody (Figure 5A).
Collectively, these results show that rAd28 and rAd35, but not rAd5 induce IFNα leading to activation of NK cells that results in increased killing of monocytes. Activated NK cells with increased killing ability correlate with reduced duration of vector-insert expression in rAd28 or rAd35 exposed cultures as compared with rAd5-exposed cultures.
Discussion
While examining the ability of rAd5, rAd28, and rAd35 to infect PBMCs we observed a consistent loss of the CD14+ monocytes in cultures exposed to rAd28 or rAd35, which concurrent with increased apoptosis. We found that vector-insert expression was rapidly lost in vector-insert positive cells undergoing apoptotic cell death with all rAd vectors. However, vector-insert positive monocytes infected with rAd28 or rAd35 showed a greater frequency of loss of vector-insert expression than those infected with rAd5 as a result of increased apoptosis. PBMCs infected with rAd28 or rAd35 had a rapid loss of vector-insert expression after 48 hours of infection while PBMCs infected with rAd5 maintained a consistently high level of vector-insert expression throughout the experiment. Interestingly, rAd28-exposed samples had reduced loss of vector-insert as compared to rAd35-exposed samples. This could potentially explain why rAd28 is generally more immunogenic than rAd35, despite having similar levels of DC maturation and infectivity.
Previous work has shown that rAd28 and rAd35 are only immunogenic at high inocula, whereas rAd5 remains immunogenic at low inocula [11]. This observation is consistent with the concept that increased apoptosis leads to reduced insert expression, which, in turn, may lead to decreased immunogenicity. At low doses, the expression of inserts from rAd28 and rAd35 may be reduced by IFNα-induced apoptosis while the insert from rAd5 remains unaffected. At high doses inserts from rAd28 and rAd35 may be expressed at a high enough level to compensate for their loss due to IFNα-induced apoptosis.
It is not known why rAd28 and rAd35 induce IFNα, but rAd5 does not. It has been suggested that differences in trophism, cellular receptor usage, intracellar trafficking routes, or genome CpG content could be responsible, but none of these potential mechanisms has been shown conclusively [1, 7–10].
There have been a limited number of studies examining the role of NK cell activation and function during exposure to rAd vectors. One such study found that rAd35, but not rAd5 inducted the activation of NK cells in vitro through induction of IFNα production by pDCs in a TLR9 dependent manner [15]. However this study did not address the effects of NK cell activation on immunogenicity, vector-insert expression, or vector clearance. A second study determined that NK cells, activated by IFNα, are responsible for the in vivo clearance of insert-antigen from mice vaccinated with rAd5, but did not examine rare serotype rAd vectors such as rAd28 or rAd35 [16]. Here we show that increased IFNα production induced by rAd28 and rAd35, but not rAd5 leads to increased NK cell activation, which correlates with increased vector loss and APC apoptosis.
The effects of vector-induced apoptosis and vector-insert loss on the immunogenicity of vaccines constructed from rare serotype adenoviruses are unknown. Notably, we and others have reported that although rAd5 induces a strong T cell response, the smaller CD8 T cell responses induced by rAd28 and rAd35 are of higher quality with increased CD127 expression, proliferative capacity, and polyfunctionality [11, 18, 19]. How these qualitative differences affect protective efficacy remains to be determined.
The presence of apoptotic cells has been proven to have a strong immunosuppressive effect on cytokine and chemokine production and the development of immunological memory [20–23] that could be responsible for the reduced immunogenicity of rAd28 and rAd35. Additionally, the effects of antigen dose on adaptive immunity are well established [10, 24, 25]. In our system the initial antigen dose is consistent between rAd vectors by titrating the MOI for each vector individually [11]. Here we show that despite a similar initial antigen dose the duration that antigen remains available before clearance by innate immune mechanisms can vary greatly between rAd vectors. A previous study, using a doxycycline-regulated rAd5 vector capable of premature termination of vector-insert expression, found that shorter periods of antigen duration lead to reduced memory CD8+ T cell responses following vaccination [19]. This suggests that the shorter duration of antigen availability after infection with rAd28 or rAd35 may ablate their immunogenicity and reduce their effectiveness as vaccines.
Future exploration is needed to fully understand the impact of vector-induced apoptosis and vector-insert loss on rAd vaccination strategies. Given that recent HIV vaccine trials incorporating rAd5 vectors have failed to show efficacy [26] studies which elucidate the impact of vector-induced apoptosis and vector-insert loss on rAd vaccination strategies are warranted.
Supplementary Material
Quantification of the upregulation of the cell death and apoptotic markers presented in Figure 1C.
Highlights.
rAd28 and rAd35 vectors, but not rAd5 vectors, induce monocyte apoptosis.
Monocyte apoptosis leads to rapid loss of vector-insert expression.
Vector-induced monocytes apoptosis is, at least in part, due to NK cell activation and killing.
NK cells are activated by vector-induced interferon alpha production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smed-Sorensen A, Loré K, Vasudevan J, Louder MK, Andersson J, Mascola JR, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79(14):8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 3.Abbink P, Lemckert AAC, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RWB, Darrah PA, et al. Comparative analysis of the magnitude, phenotype and protective capacity of SIV gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenovirus vector immunisation. J Immunol. 2013;190(6):2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18(8):1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 6.Kahl CA, Bonnell J, Hiriyanna S, Fultz M, Nyberg-Hoffman C, Chen P, et al. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine. 2010;28(35):5691–5702. doi: 10.1016/j.vaccine.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowlkes DM, Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980;22(2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- 8.Shayakhmetov DM, Li ZY, Ternovoi V, Gaggar A, Gharwan H, Lieber A. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J Virol. 2003;77(6):3712–3723. doi: 10.1128/JVI.77.6.3712-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol. 2009;19(3):165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Jin B, Henrickson SE, Perelson AS, Von Andrian UH, Chakraborty AK. How antigen quantity and quality determine T-cell decisions in lymphoid tissue. Mol Cell Biol. 2008;28(12):4040–4051. doi: 10.1128/MCB.00136-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RWB, Loré K, Gall JGD, et al. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J Immunol. 2012;188(12):6109–6118. doi: 10.4049/jimmunol.1103717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton MM, Byrnes GA, Gall JG, Brough DE, King CR, Wei LL. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol Vis. 2008;14:2535–2546. [PMC free article] [PubMed] [Google Scholar]
- 13.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70(9):6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryceson YT, Fauriat C, Nunes JM, Wood SM, Björkström NK, Long EO, et al. Functional analysis of human NK cells by flow cytometry. Methods Mol Biol. 2010;612:17. doi: 10.1007/978-1-60761-362-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahl JHW, Verhoeven DHJ, Kwappenberg K, Vellinga J, Lankester AC, van Tol MJD, et al. Adenovirus type 35, but not type 5, stimulates NK cell activation via plasmacytoid dendritic cells and TLR9 signaling. Mol Immunol. 2012 doi: 10.1016/j.molimm.2012.02.119. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Huang X, Yang Y. A critical role for type I IFN-dependent NK Cell activation in innate immune elimination of adenoviral vectors in vivo. Mol Ther. 2008;16(7):1300–1307. doi: 10.1038/mt.2008.88. [DOI] [PubMed] [Google Scholar]
- 17.Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 18.Loré K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201(12):2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn JD, Bassett J, Millar JB, Grinshtein N, Yang TC, Parsons R, et al. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J Virol. 2009;83(23):12027–12036. doi: 10.1128/JVI.00593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, et al. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181(5):3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- 21.Tassiulas I, Park-Min KH, Hu Y, Kellerman L, Mevorach D, Ivashkiv LB. Apoptotic cells inhibit LPS-induced cytokine and chemokine production and IFN responses in macrophages. Hum Immunol. 2007;68(3):156–164. doi: 10.1016/j.humimm.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. Opsonization of apoptotic cells and its effect on macrophage and T cell immune responses. Ann N Y Acad Sci. 2006;987(1):68–78. doi: 10.1111/j.1749-6632.2003.tb06034.x. [DOI] [PubMed] [Google Scholar]
- 23.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 24.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9(3):282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leignadier J, Labrecque N. Epitope density influences CD8+ memory T cell differentiation. PloS one. 2010;5(10):e13740. doi: 10.1371/journal.pone.0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. The Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification of the upregulation of the cell death and apoptotic markers presented in Figure 1C.