Abstract
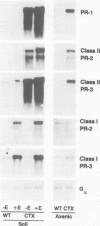
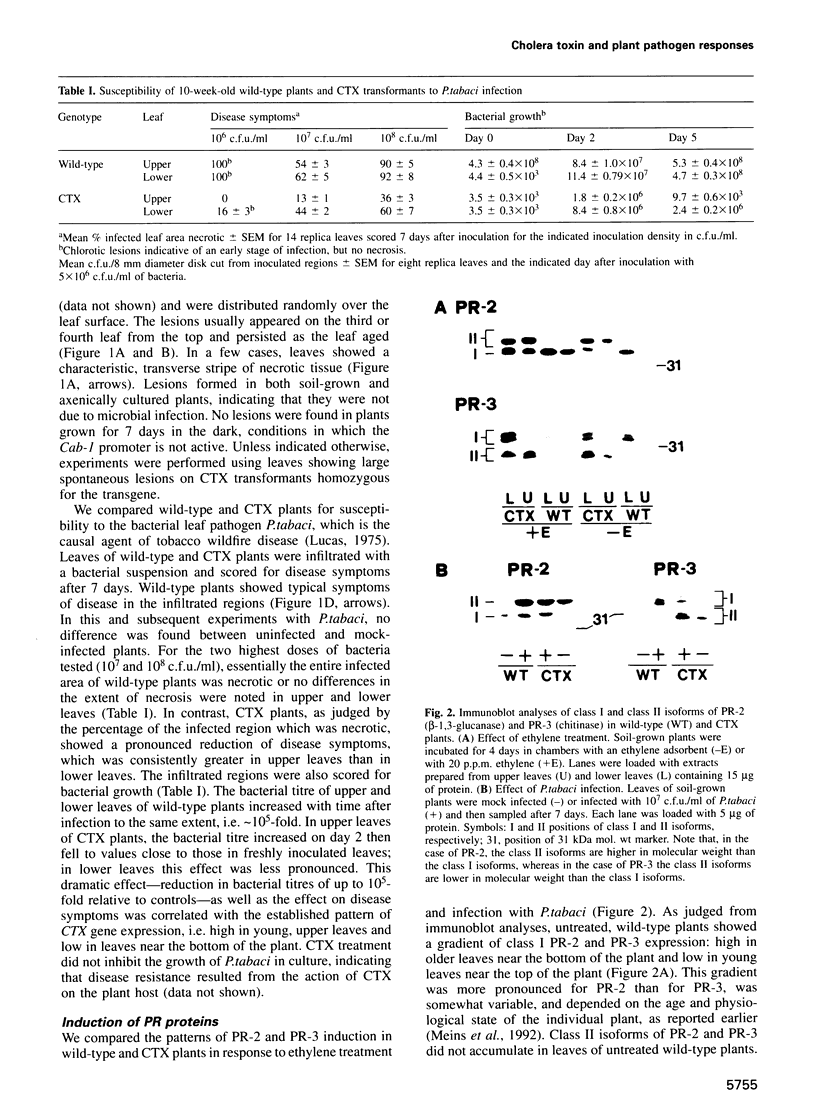
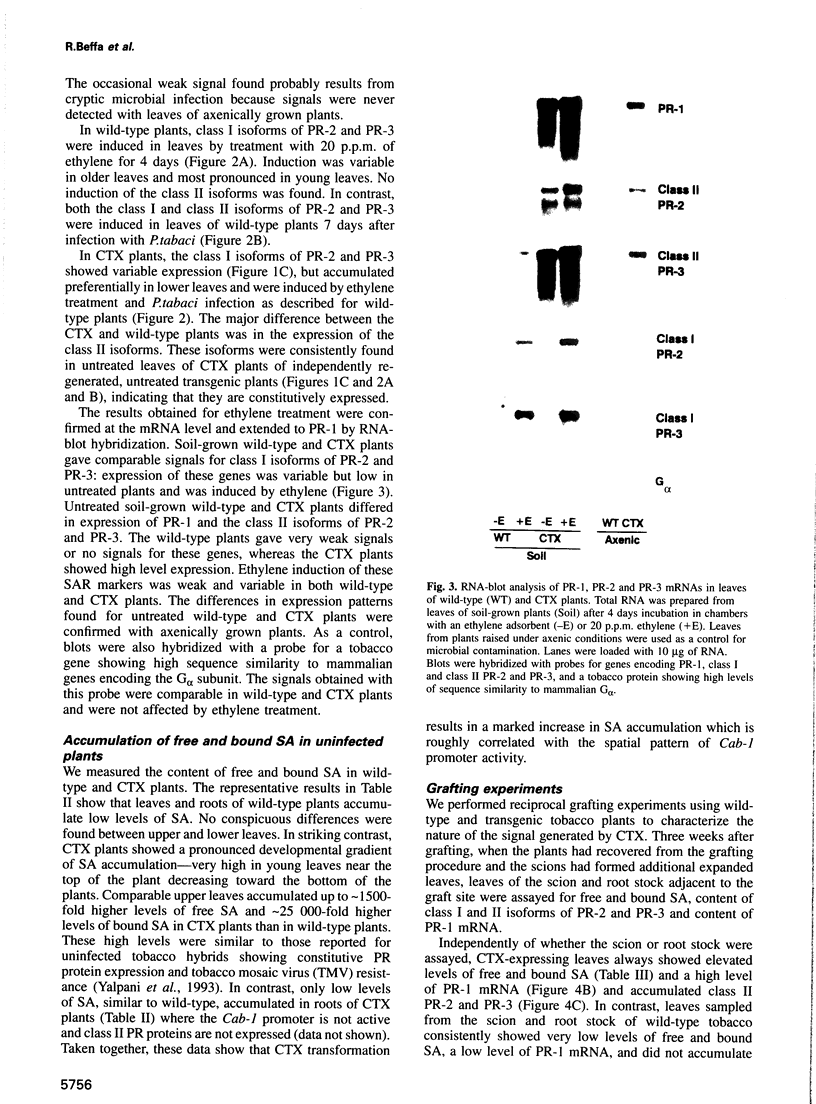
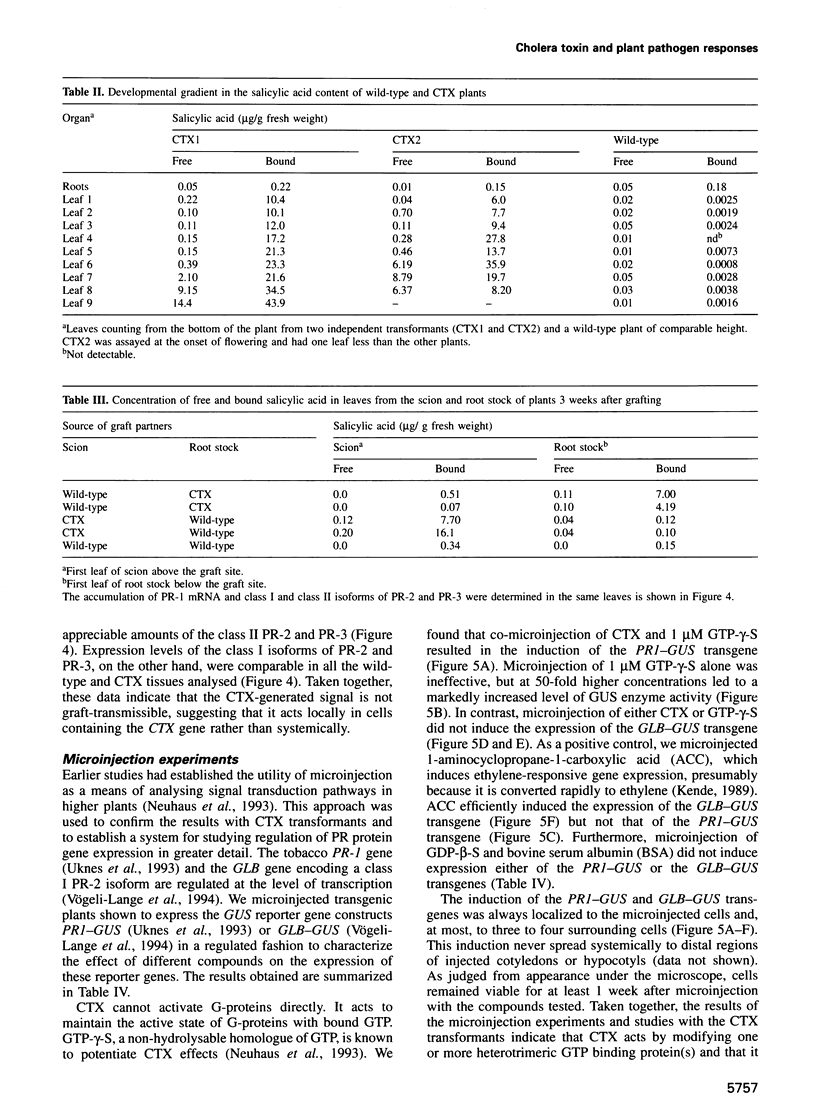
In animals, plants and fungi, cholera toxin (CTX) can activate signalling pathways dependent on heterotrimeric GTP binding proteins (G-proteins). We transformed tobacco plants with a chimeric gene encoding the A1 subunit of CTX regulated by a light-inducible wheat Cab-1 promoter. Tissues of transgenic plants expressing CTX showed greatly reduced susceptibility to the bacterial pathogen Pseudomonas tabaci, accumulated high levels of salicylic acid (SA) and constitutively expressed pathogenesis-related (PR) protein genes encoding PR-1 and the class II isoforms of PR-2 and PR-3. In contrast, the class I isoforms of PR-2 and PR-3 known to be induced in tobacco by stress, by ethylene treatment and as part of the hypersensitive response to infection, were not induced and displayed normal regulation. In good agreement with these results, microinjection experiments demonstrated that CTX or GTP-gamma-S induced the expression of a PR1-GUS reporter gene but not that of a GLB-GUS reporter gene containing the promoter region of a gene encoding the class I isoform of PR-2. Microinjection and grafting experiments strongly suggest that CTX-sensitive G-proteins are important in inducing the expression of a subset of PR genes and that these G-proteins act locally rather than systemically upstream of SA induction.
Full text
PDF
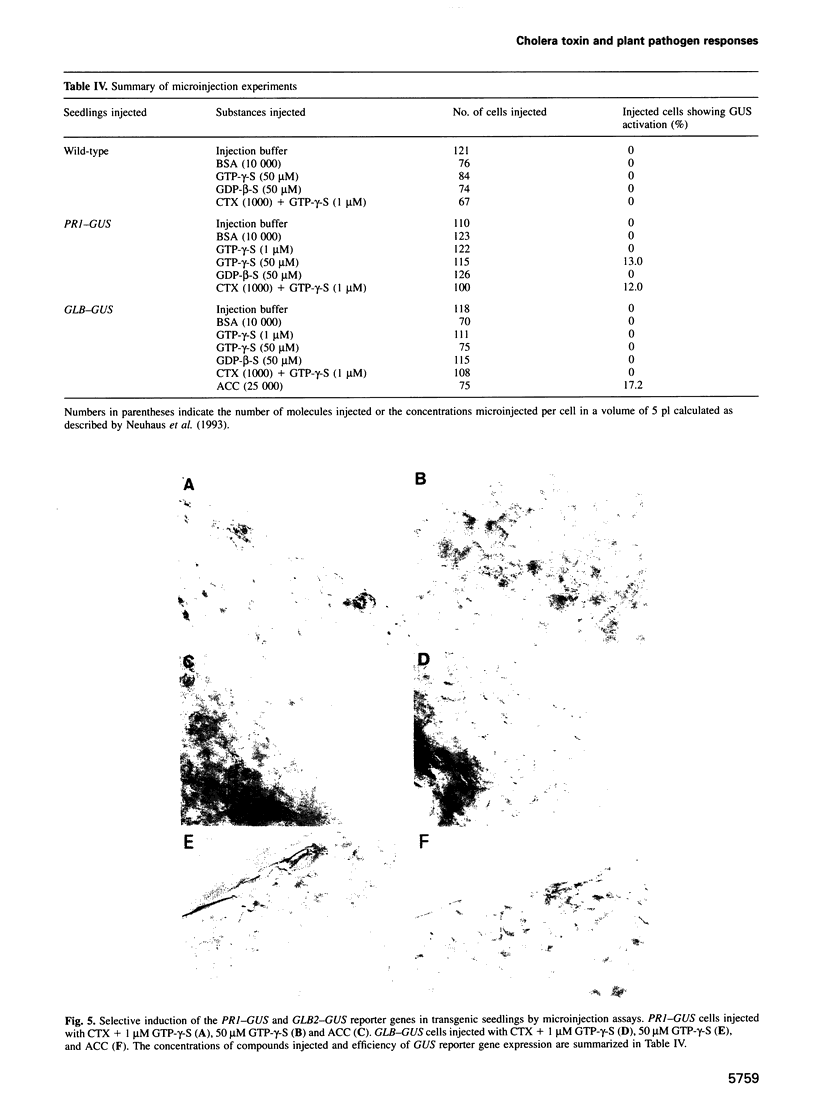


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beffa R. S., Neuhaus J. M., Meins F., Jr Physiological compensation in antisense transformants: specific induction of an "ersatz" glucan endo-1,3-beta-glucosidase in plants infected with necrotizing viruses. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8792–8796. doi: 10.1073/pnas.90.19.8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Neuhaus G., Yamagata H., Chua N. H. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994 Apr 8;77(1):73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Bowling S. A., Guo A., Cao H., Gordon A. S., Klessig D. F., Dong X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell. 1994 Dec;6(12):1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton F. H., Hasel K. W., Bloom F. E., Sutcliffe J. G. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991 Mar 7;350(6313):74–77. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- Dallmann G., Sticher L., Marshallsay C., Nagy F. Molecular characterization of tobacco cDNAs encoding two small GTP-binding proteins. Plant Mol Biol. 1992 Aug;19(5):847–857. doi: 10.1007/BF00027080. [DOI] [PubMed] [Google Scholar]
- Dietrich R. A., Delaney T. P., Uknes S. J., Ward E. R., Ryals J. A., Dangl J. L. Arabidopsis mutants simulating disease resistance response. Cell. 1994 May 20;77(4):565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejes E., Pay A., Kanevsky I., Szell M., Adam E., Kay S., Nagy F. A 268 bp upstream sequence mediates the circadian clock-regulated transcription of the wheat Cab-1 gene in transgenic plants. Plant Mol Biol. 1990 Dec;15(6):921–932. doi: 10.1007/BF00039431. [DOI] [PubMed] [Google Scholar]
- Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., Ward E., Kessmann H., Ryals J. Requirement of salicylic Acid for the induction of systemic acquired resistance. Science. 1993 Aug 6;261(5122):754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Godiard L., Grant M. R., Dietrich R. A., Kiedrowski S., Dangl J. L. Perception and response in plant disease resistance. Curr Opin Genet Dev. 1994 Oct;4(5):662–671. doi: 10.1016/0959-437x(94)90132-m. [DOI] [PubMed] [Google Scholar]
- Green R., Fluhr R. UV-B-Induced PR-1 Accumulation Is Mediated by Active Oxygen Species. Plant Cell. 1995 Feb;7(2):203–212. doi: 10.1105/tpc.7.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Ausubel F. M. Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J. 1993 Aug;4(2):327–341. doi: 10.1046/j.1365-313x.1993.04020327.x. [DOI] [PubMed] [Google Scholar]
- Hennig J., Dewey R. E., Cutt J. R., Klessig D. F. Pathogen, salicylic acid and developmental dependent expression of a beta-1,3-glucanase/GUS gene fusion in transgenic tobacco plants. Plant J. 1993 Sep;4(3):481–493. doi: 10.1046/j.1365-313x.1993.04030481.x. [DOI] [PubMed] [Google Scholar]
- Hennig J., Malamy J., Grynkiewicz G., Indulski J., Klessig D. F. Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J. 1993 Oct;4(4):593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Enzymes of ethylene biosynthesis. Plant Physiol. 1989 Sep;91(1):1–4. doi: 10.1104/pp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K. A., Potter S. L., Uknes S., Ryals J. Acquired Resistance Signal Transduction in Arabidopsis Is Ethylene Independent. Plant Cell. 1994 May;6(5):581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Meuwly P., Métraux J. P. Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem. 1993 Nov 1;214(2):500–505. doi: 10.1006/abio.1993.1529. [DOI] [PubMed] [Google Scholar]
- Mittler R., Shulaev V., Lam E. Coordinated Activation of Programmed Cell Death and Defense Mechanisms in Transgenic Tobacco Plants Expressing a Bacterial Proton Pump. Plant Cell. 1995 Jan;7(1):29–42. doi: 10.1105/tpc.7.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Boutry M., Hsu M. Y., Wong M., Chua N. H. The 5'-proximal region of the wheat Cab-1 gene contains a 268-bp enhancer-like sequence for phytochrome response. EMBO J. 1987 Sep;6(9):2537–2542. doi: 10.1002/j.1460-2075.1987.tb02541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990 Jul;2(7):673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus G., Bowler C., Kern R., Chua N. H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993 Jun 4;73(5):937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Payne G., Ahl P., Moyer M., Harper A., Beck J., Meins F., Jr, Ryals J. Isolation of complementary DNA clones encoding pathogenesis-related proteins P and Q, two acidic chitinases from tobacco. Proc Natl Acad Sci U S A. 1990 Jan;87(1):98–102. doi: 10.1073/pnas.87.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. B., Hammerschmidt R., Zook M. N. Systemic Induction of Salicylic Acid Accumulation in Cucumber after Inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 1991 Dec;97(4):1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J., Uknes S., Ward E. Systemic Acquired Resistance. Plant Physiol. 1994 Apr;104(4):1109–1112. doi: 10.1104/pp.104.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Seo S., Orudgev E., Youssefian S., Ishizuka K. Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10556–10560. doi: 10.1073/pnas.91.22.10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M., Ernst D., Langebartels C., Sandermann H. Biochemical Plant Responses to Ozone : III. Activation of the Defense-Related Proteins beta-1,3-Glucanase and Chitinase in Tobacco Leaves. Plant Physiol. 1992 Aug;99(4):1321–1328. doi: 10.1104/pp.99.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Uknes S., Dincher S., Friedrich L., Negrotto D., Williams S., Thompson-Taylor H., Potter S., Ward E., Ryals J. Regulation of pathogenesis-related protein-1a gene expression in tobacco. Plant Cell. 1993 Feb;5(2):159–169. doi: 10.1105/tpc.5.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B., Friedrich L., Morse A., Reist R., Kolditz-Jawhar R., Ward E., Uknes S., Kessmann H., Ryals J. Salicylic Acid Is Not the Translocated Signal Responsible for Inducing Systemic Acquired Resistance but Is Required in Signal Transduction. Plant Cell. 1994 Jul;6(7):959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli-Lange R., Fründt C., Hart C. M., Nagy F., Meins F., Jr Developmental, hormonal, and pathogenesis-related regulation of the tobacco class I beta-1,3-glucanase B promoter. Plant Mol Biol. 1994 May;25(2):299–311. doi: 10.1007/BF00023245. [DOI] [PubMed] [Google Scholar]
- Ward E. R., Uknes S. J., Williams S. C., Dincher S. S., Wiederhold D. L., Alexander D. C., Ahl-Goy P., Metraux J. P., Ryals J. A. Coordinate Gene Activity in Response to Agents That Induce Systemic Acquired Resistance. Plant Cell. 1991 Oct;3(10):1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]