Abstract
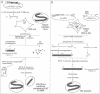
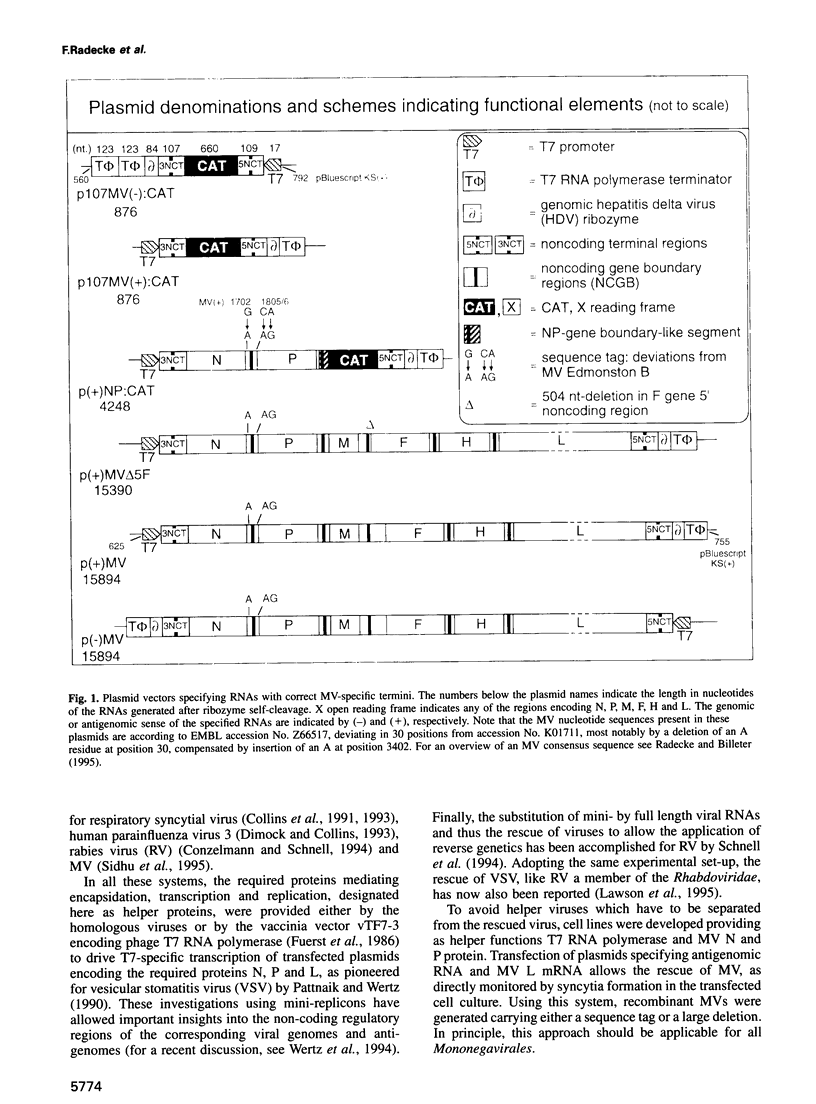
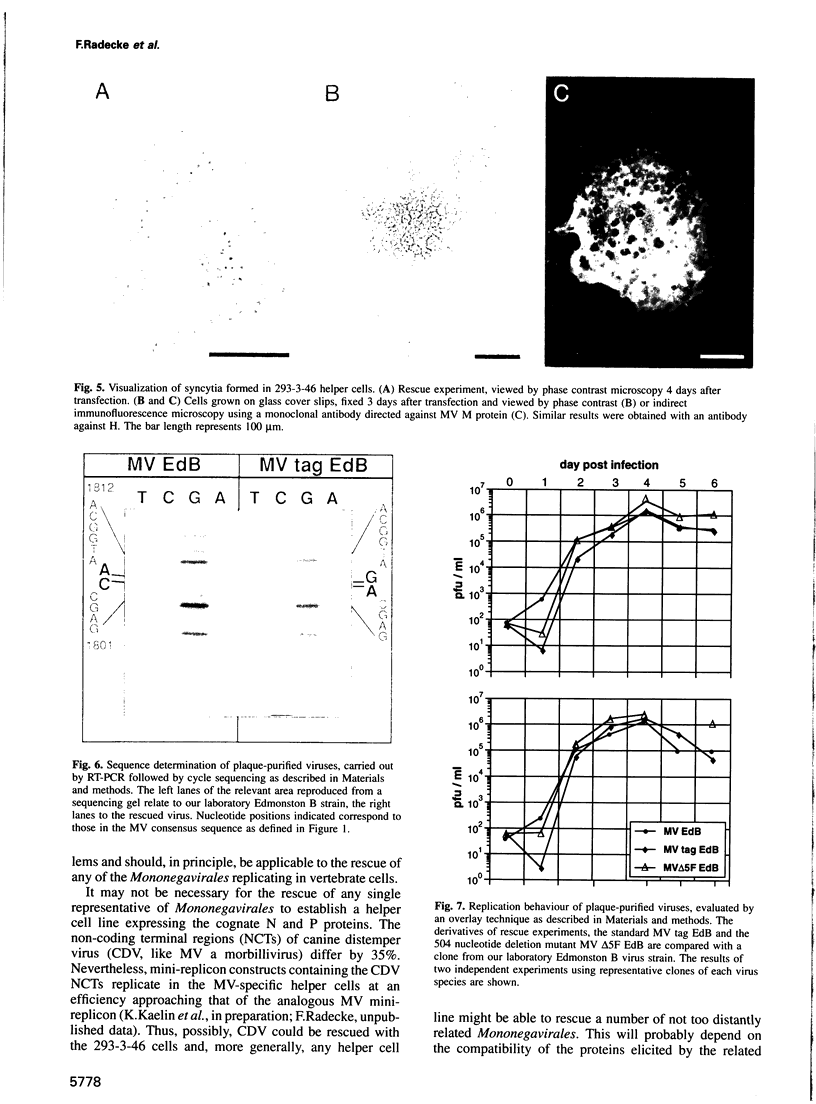
A system has been established allowing the rescue of replicating measles viruses (MVs) from cloned DNA. On one hand, plasmids were constructed from which MV antigenomic RNAs with the correct termini are transcribed by phage T7 RNA polymerase. On the other hand, helper cells derived from the human embryonic kidney 293 cell line were generated constitutively expressing T7 RNA polymerase together with MV nucleocapsid protein and phosphoprotein. Simultaneous transfection of the helper cells with the MV antigenomic plasmid and with a plasmid encoding the MV polymerase under direction of a T7 promoter led to formation of syncytia from which MVs were easily recovered. A genetic tag comprising three nucleotide changes was present in the progeny virus. As a first application of reverse genetics, a segment of 504 nucleotides from the 5' non-coding region of the fusion gene was deleted, leading to an MV variant whose replication behaviour in Vero cells was indistinguishable from that of the laboratory Edmonston B strain. Since no helper virus is involved, this system, in principle, should be applicable to the rescue of any member of the large virus order Mononegavirales, i.e. viruses with a nonsegmented negative-strand RNA genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Silvera D., Suggett S. D., Achacoso P. L., Miller C. J., Baltimore D., Feinberg M. B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994 Sep 2;265(5177):1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- Ballart I., Eschle D., Cattaneo R., Schmid A., Metzler M., Chan J., Pifko-Hirst S., Udem S. A., Billeter M. A. Infectious measles virus from cloned cDNA. EMBO J. 1990 Feb;9(2):379–384. doi: 10.1002/j.1460-2075.1990.tb08121.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Billeter M. A., Cattaneo R., Spielhofer P., Kaelin K., Huber M., Schmid A., Baczko K., ter Meulen V. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. Ann N Y Acad Sci. 1994 Jun 6;724:367–377. doi: 10.1111/j.1749-6632.1994.tb38934.x. [DOI] [PubMed] [Google Scholar]
- Boyer J. C., Haenni A. L. Infectious transcripts and cDNA clones of RNA viruses. Virology. 1994 Feb;198(2):415–426. doi: 10.1006/viro.1994.1053. [DOI] [PubMed] [Google Scholar]
- Calain P., Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993 Aug;67(8):4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Schmid A., Baczko K., ter Meulen V., Billeter M. A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987 Mar;6(3):681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Hill M. G., 3rd, Camargo E., Grosfeld H., Stec D. S. Rescue of a 7502-nucleotide (49.3% of full-length) synthetic analog of respiratory syncytial virus genomic RNA. Virology. 1993 Jul;195(1):252–256. doi: 10.1006/viro.1993.1368. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Stec D. S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann K. K., Schnell M. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J Virol. 1994 Feb;68(2):713–719. doi: 10.1128/jvi.68.2.713-719.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Kolakofsky D. Rescue of a Sendai virus DI genome by other parainfluenza viruses: implications for genome replication. Virology. 1991 May;182(1):168–176. doi: 10.1016/0042-6822(91)90660-4. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Wang C., Acsadi G., Wolff J. A. High-efficiency protein synthesis from T7 RNA polymerase transcripts in 3T3 fibroblasts. Gene. 1991 Dec 30;109(2):193–201. doi: 10.1016/0378-1119(91)90609-f. [DOI] [PubMed] [Google Scholar]
- Dimock K., Collins P. L. Rescue of synthetic analogs of genomic RNA and replicative-intermediate RNA of human parainfluenza virus type 3. J Virol. 1993 May;67(5):2772–2778. doi: 10.1128/jvi.67.5.2772-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991 May;65(5):2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschle D., Cattaneo R., Schmid A., Metzler M., Chan J., Pifko-Hirst S., Udem S. A., Billeter M. A. Retraction. Infectious measles virus from cloned cDNA. EMBO J. 1991 Nov;10(11):3558–3558. doi: 10.1002/j.1460-2075.1991.tb04920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bonin A. L., Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993 Dec;18(12):471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Huber M., Cattaneo R., Spielhofer P., Orvell C., Norrby E., Messerli M., Perriard J. C., Billeter M. A. Measles virus phosphoprotein retains the nucleocapsid protein in the cytoplasm. Virology. 1991 Nov;185(1):299–308. doi: 10.1016/0042-6822(91)90777-9. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981 Feb;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Stillman E. A., Whitt M. A., Rose J. K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E. The paradigms of measles vaccinology. Curr Top Microbiol Immunol. 1995;191:167–180. doi: 10.1007/978-3-642-78621-1_11. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E. Immunological relationships between homologous structural polypeptides of measles and canine distemper virus. J Gen Virol. 1980 Oct;50(2):231–245. doi: 10.1099/0022-1317-50-2-231. [DOI] [PubMed] [Google Scholar]
- Park K. H., Huang T., Correia F. F., Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Wertz G. W. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J Virol. 1990 Jun;64(6):2948–2957. doi: 10.1128/jvi.64.6.2948-2957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. The self-cleaving domain from the genomic RNA of hepatitis delta virus: sequence requirements and the effects of denaturant. Nucleic Acids Res. 1990 Dec 11;18(23):6821–6827. doi: 10.1093/nar/18.23.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Radecke F., Billeter M. A. Appendix: measles virus antigenome and protein consensus sequences. Curr Top Microbiol Immunol. 1995;191:181–192. doi: 10.1007/978-3-642-78621-1_12. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Koch T., Pinhasi O., Bratosin S. Infective substructures of measles virus from acutely and persistently infected cells. J Virol. 1979 Oct;32(1):329–333. doi: 10.1128/jvi.32.1.329-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M. J., Mebatsion T., Conzelmann K. K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994 Sep 15;13(18):4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Richardson C. D., Meier E. Expression of a cDNA encoding a functional 241-kilodalton vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7984–7988. doi: 10.1073/pnas.82.23.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Sjöberg E. M., Suomalainen M., Garoff H. A significantly improved Semliki Forest virus expression system based on translation enhancer segments from the viral capsid gene. Biotechnology (N Y) 1994 Nov;12(11):1127–1131. doi: 10.1038/nbt1194-1127. [DOI] [PubMed] [Google Scholar]
- Skerra A. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. 1992 Jul 25;20(14):3551–3554. doi: 10.1093/nar/20.14.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Spehner D., Kirn A., Drillien R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991 Nov;65(11):6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G., Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Whelan S., LeGrone A., Ball L. A. Extent of terminal complementarity modulates the balance between transcription and replication of vesicular stomatitis virus RNA. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8587–8591. doi: 10.1073/pnas.91.18.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrink W., Neubert W. J. Long-term replication of Sendai virus defective interfering particle nucleocapsids in stable helper cell lines. J Virol. 1994 Dec;68(12):8413–8417. doi: 10.1128/jvi.68.12.8413-8417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]