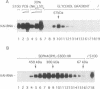
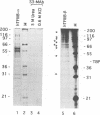
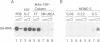Abstract
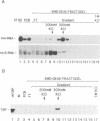
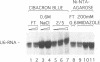
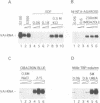
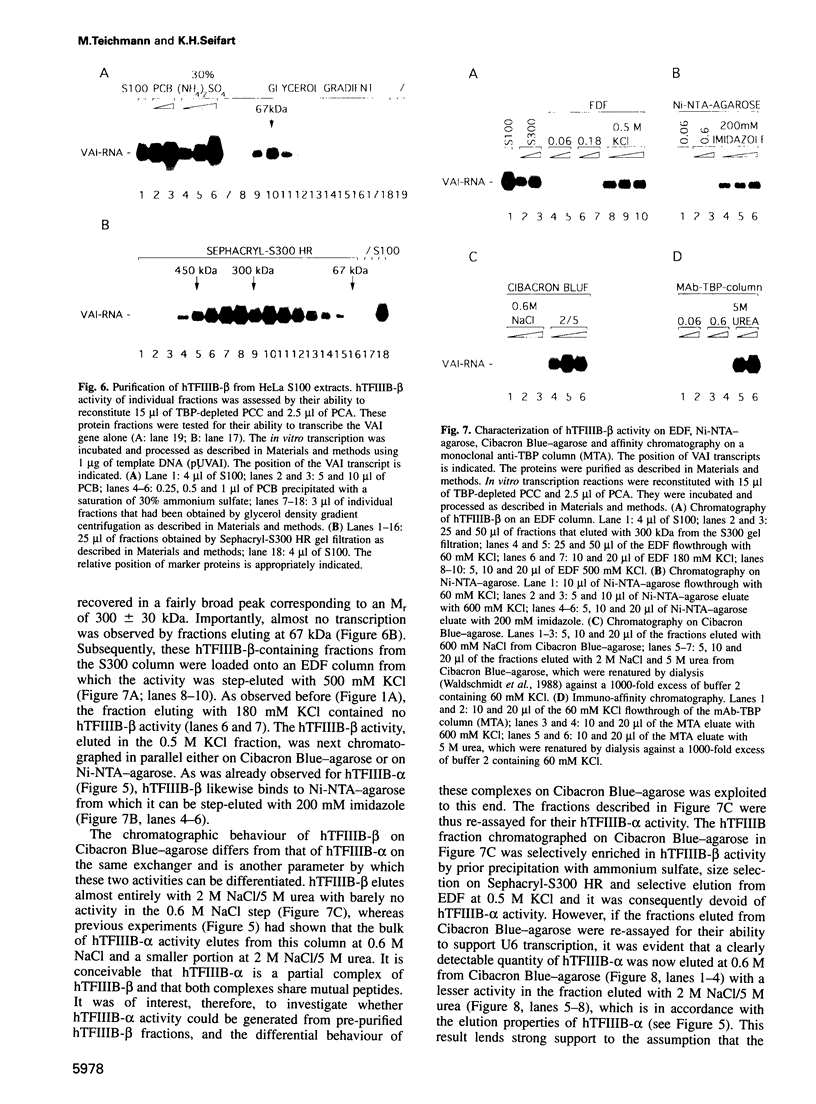
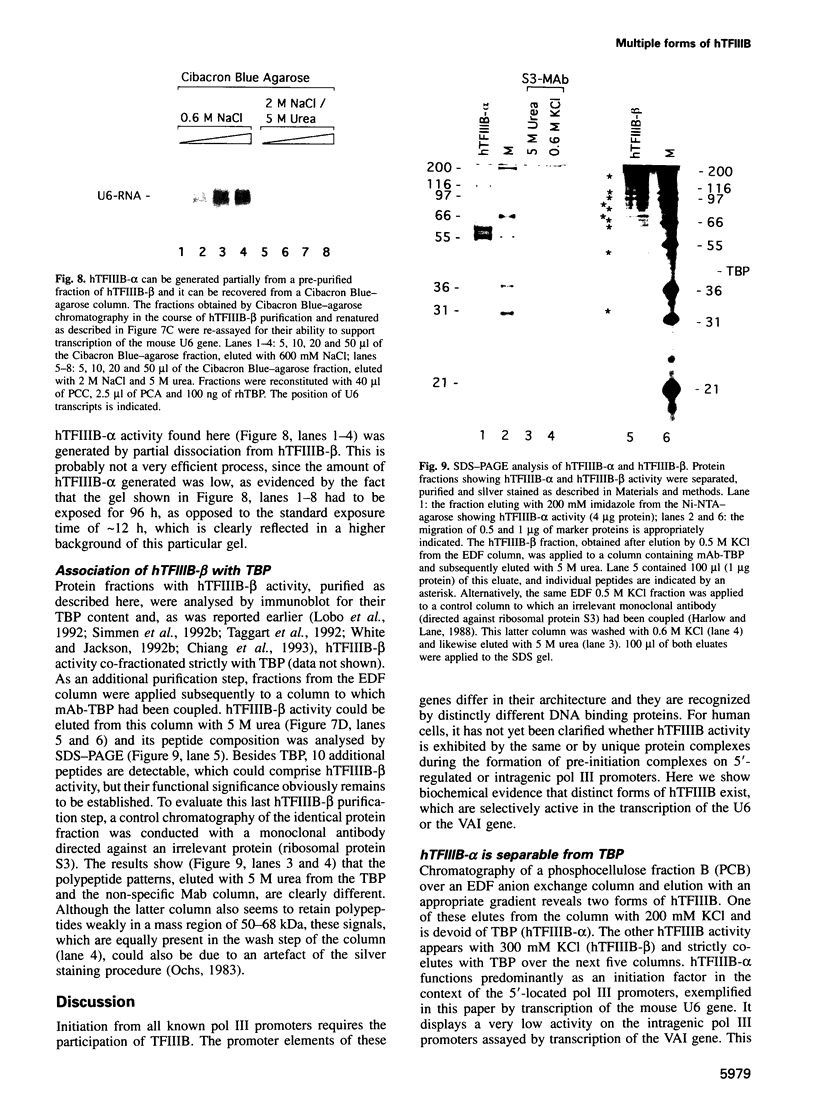
Human transcription factor hTFIIIB is necessary to initiate transcription correctly from all RNA polymerase III (pol III) genes which are governed by structurally different promoters, and it is unclear whether hTFIIIB complexes, required for intragenic or 5'-located pol III promoters, are composed of unique or different components. We show here that two different forms of hTFIIIB can be separated physically by ion exchange chromatography. hTFIIIB-alpha shows strong preference for transcription of the U6 over the VAI gene and does not contain TATA binding protein (TBP). After SDS-PAGE and renaturation of proteins, the transcriptional activity of hTFIIIB-alpha can be reconstituted by fractions corresponding to a mean M(r) of 25, 60 and 90 kDa. Upon gradient centrifugation or gel filtration, the activity of hTFIIIB-alpha is associated with an M(r) of 60 +/- 10 kDa, indicating that the components of the complex tend to dissociate. In contrast, hTFIIIB-beta is predominantly active on intragenic pol III promoters. It reveals an M(r) of 300 +/- 30 kDa upon gel filtration and, besides TBP, it contains several associated factors (TAFs). Two of these proteins reveal an M(r) of 60 kDa and 90 kDa, and it is conceivable that they are related to polypeptides of similar mass functionally identified in hTFIIIB-alpha. These protein are probably required for the recruitment of pol III to the initiation site at 5'-located and intragenic promoters.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buratowski S., Zhou H. A suppressor of TBP mutations encodes an RNA polymerase III transcription factor with homology to TFIIB. Cell. 1992 Oct 16;71(2):221–230. doi: 10.1016/0092-8674(92)90351-c. [DOI] [PubMed] [Google Scholar]
- Chiang C. M., Ge H., Wang Z., Hoffmann A., Roeder R. G. Unique TATA-binding protein-containing complexes and cofactors involved in transcription by RNA polymerases II and III. EMBO J. 1993 Jul;12(7):2749–2762. doi: 10.1002/j.1460-2075.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert T., Hahn S. A yeast TFIIB-related factor involved in RNA polymerase III transcription. Genes Dev. 1992 Oct;6(10):1940–1949. doi: 10.1101/gad.6.10.1940. [DOI] [PubMed] [Google Scholar]
- Comai L., Tanese N., Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992 Mar 6;68(5):965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Cormack B. P., Struhl K. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell. 1992 May 15;69(4):685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- Henry N. L., Sayre M. H., Kornberg R. D. Purification and characterization of yeast RNA polymerase II general initiation factor g. J Biol Chem. 1992 Nov 15;267(32):23388–23392. [PubMed] [Google Scholar]
- Henry R. W., Sadowski C. L., Kobayashi R., Hernandez N. A TBP-TAF complex required for transcription of human snRNA genes by RNA polymerase II and III. Nature. 1995 Apr 13;374(6523):653–656. doi: 10.1038/374653a0. [DOI] [PubMed] [Google Scholar]
- Hernandez N. TBP, a universal eukaryotic transcription factor? Genes Dev. 1993 Jul;7(7B):1291–1308. doi: 10.1101/gad.7.7b.1291. [DOI] [PubMed] [Google Scholar]
- Huet J., Conesa C., Manaud N., Chaussivert N., Sentenac A. Interactions between yeast TFIIIB components. Nucleic Acids Res. 1994 Aug 25;22(16):3433–3439. doi: 10.1093/nar/22.16.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A. The TATA-binding protein participates in TFIIIB assembly on tRNA genes. Nucleic Acids Res. 1992 Dec 25;20(24):6451–6454. doi: 10.1093/nar/20.24.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D., Wingender E., Seifart K. H. Transcription complexes for various class III genes differ in parameters of formation and stability towards salt. J Mol Biol. 1987 Jan 20;193(2):303–313. doi: 10.1016/0022-2836(87)90221-x. [DOI] [PubMed] [Google Scholar]
- Joazeiro C. A., Kassavetis G. A., Geiduschek E. P. Identical components of yeast transcription factor IIIB are required and sufficient for transcription of TATA box-containing and TATA-less genes. Mol Cell Biol. 1994 Apr;14(4):2798–2808. doi: 10.1128/mcb.14.4.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., Geiduschek E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990 Jan 26;60(2):235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Joazeiro C. A., Pisano M., Geiduschek E. P., Colbert T., Hahn S., Blanco J. A. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell. 1992 Dec 11;71(6):1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekamp M. S., Weil P. A. Partial purification and characterization of the Saccharomyces cerevisiae transcription factor TFIIIB. J Biol Chem. 1986 Feb 25;261(6):2819–2827. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobo S. M., Lister J., Sullivan M. L., Hernandez N. The cloned RNA polymerase II transcription factor IID selects RNA polymerase III to transcribe the human U6 gene in vitro. Genes Dev. 1991 Aug;5(8):1477–1489. doi: 10.1101/gad.5.8.1477. [DOI] [PubMed] [Google Scholar]
- Lobo S. M., Tanaka M., Sullivan M. L., Hernandez N. A TBP complex essential for transcription from TATA-less but not TATA-containing RNA polymerase III promoters is part of the TFIIIB fraction. Cell. 1992 Dec 11;71(6):1029–1040. doi: 10.1016/0092-8674(92)90397-u. [DOI] [PubMed] [Google Scholar]
- López-De-León A., Librizzi M., Puglia K., Willis I. M. PCF4 encodes an RNA polymerase III transcription factor with homology to TFIIB. Cell. 1992 Oct 16;71(2):211–220. doi: 10.1016/0092-8674(92)90350-l. [DOI] [PubMed] [Google Scholar]
- Margottin F., Dujardin G., Gérard M., Egly J. M., Huet J., Sentenac A. Participation of the TATA factor in transcription of the yeast U6 gene by RNA polymerase C. Science. 1991 Jan 25;251(4992):424–426. doi: 10.1126/science.1989075. [DOI] [PubMed] [Google Scholar]
- Meissner W., Holland R., Waldschmidt R., Seifart K. H. Transcription factor IIA stimulates the expression of classical polIII-genes. Nucleic Acids Res. 1993 Feb 25;21(4):1013–1018. doi: 10.1093/nar/21.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers R. E., Sharp P. A. TATA-binding protein and associated factors in polymerase II and polymerase III transcription. Mol Cell Biol. 1993 Dec;13(12):7953–7960. doi: 10.1128/mcb.13.12.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murphy S., Yoon J. B., Gerster T., Roeder R. G. Oct-1 and Oct-2 potentiate functional interactions of a transcription factor with the proximal sequence element of small nuclear RNA genes. Mol Cell Biol. 1992 Jul;12(7):3247–3261. doi: 10.1128/mcb.12.7.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983 Dec;135(2):470–474. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Poon D., Weil P. A. Immunopurification of yeast TATA-binding protein and associated factors. Presence of transcription factor IIIB transcriptional activity. J Biol Chem. 1993 Jul 25;268(21):15325–15328. [PubMed] [Google Scholar]
- Rigby P. W. Three in one and one in three: it all depends on TBP. Cell. 1993 Jan 15;72(1):7–10. doi: 10.1016/0092-8674(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Kornberg R. D. Purification and characterization of polyadenylate-binding protein. Methods Enzymol. 1990;181:332–352. doi: 10.1016/0076-6879(90)81134-g. [DOI] [PubMed] [Google Scholar]
- Sadowski C. L., Henry R. W., Lobo S. M., Hernandez N. Targeting TBP to a non-TATA box cis-regulatory element: a TBP-containing complex activates transcription from snRNA promoters through the PSE. Genes Dev. 1993 Aug;7(8):1535–1548. doi: 10.1101/gad.7.8.1535. [DOI] [PubMed] [Google Scholar]
- Schneider H. R., Waldschmidt R., Jahn D., Seifart K. H. Purification of human transcription factor IIIC and its binding to the gene for ribosomal 5S RNA. Nucleic Acids Res. 1989 Jul 11;17(13):5003–5016. doi: 10.1093/nar/17.13.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M. C., Reeder R. H., Hahn S. Variants of the TATA-binding protein can distinguish subsets of RNA polymerase I, II, and III promoters. Cell. 1992 May 15;69(4):697–702. doi: 10.1016/0092-8674(92)90233-3. [DOI] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Simmen K. A., Bernués J., Lewis J. D., Mattaj I. W. Cofractionation of the TATA-binding protein with the RNA polymerase III transcription factor TFIIIB. Nucleic Acids Res. 1992 Nov 25;20(22):5889–5898. doi: 10.1093/nar/20.22.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen K. A., Bernués J., Parry H. D., Stunnenberg H. G., Berkenstam A., Cavallini B., Egly J. M., Mattaj I. W. TFIID is required for in vitro transcription of the human U6 gene by RNA polymerase III. EMBO J. 1991 Jul;10(7):1853–1862. doi: 10.1002/j.1460-2075.1991.tb07711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen K. A., Waldschmidt R., Bernués J., Parry H. D., Seifart K. H., Mattaj I. W. Proximal sequence element factor binding and species specificity in vertebrate U6 snRNA promoters. J Mol Biol. 1992 Feb 20;223(4):873–884. doi: 10.1016/0022-2836(92)90249-j. [DOI] [PubMed] [Google Scholar]
- Taggart A. K., Fisher T. S., Pugh B. F. The TATA-binding protein and associated factors are components of pol III transcription factor TFIIIB. Cell. 1992 Dec 11;71(6):1015–1028. doi: 10.1016/0092-8674(92)90396-t. [DOI] [PubMed] [Google Scholar]
- Timmers H. T., Meyers R. E., Sharp P. A. Composition of transcription factor B-TFIID. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8140–8144. doi: 10.1073/pnas.89.17.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers H. T., Sharp P. A. The mammalian TFIID protein is present in two functionally distinct complexes. Genes Dev. 1991 Nov;5(11):1946–1956. doi: 10.1101/gad.5.11.1946. [DOI] [PubMed] [Google Scholar]
- Waldschmidt R., Jahn D., Seifart K. H. Purification of transcription factor IIIB from HeLa cells. J Biol Chem. 1988 Sep 15;263(26):13350–13356. [PubMed] [Google Scholar]
- Waldschmidt R., Jahn D., Teichmann M., Jahn M., Meissner W., Seifart K. H. Physical and immunological characterization of human transcription factor IIIA. Eur J Biochem. 1990 Nov 26;194(1):167–176. doi: 10.1111/j.1432-1033.1990.tb19441.x. [DOI] [PubMed] [Google Scholar]
- Waldschmidt R., Seifart K. H. TFIIA is required for in vitro transcription of mammalian U6 genes by RNA polymerase III. J Biol Chem. 1992 Aug 15;267(23):16359–16364. [PubMed] [Google Scholar]
- Waldschmidt R., Wanandi I., Seifart K. H. Identification of transcription factors required for the expression of mammalian U6 genes in vitro. EMBO J. 1991 Sep;10(9):2595–2603. doi: 10.1002/j.1460-2075.1991.tb07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Jackson S. P. Mechanism of TATA-binding protein recruitment to a TATA-less class III promoter. Cell. 1992 Dec 11;71(6):1041–1053. doi: 10.1016/0092-8674(92)90398-v. [DOI] [PubMed] [Google Scholar]
- White R. J., Jackson S. P., Rigby P. W. A role for the TATA-box-binding protein component of the transcription factor IID complex as a general RNA polymerase III transcription factor. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1949–1953. doi: 10.1073/pnas.89.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis I. M. RNA polymerase III. Genes, factors and transcriptional specificity. Eur J Biochem. 1993 Feb 15;212(1):1–11. doi: 10.1111/j.1432-1033.1993.tb17626.x. [DOI] [PubMed] [Google Scholar]
- Yoon J. B., Murphy S., Bai L., Wang Z., Roeder R. G. Proximal sequence element-binding transcription factor (PTF) is a multisubunit complex required for transcription of both RNA polymerase II- and RNA polymerase III-dependent small nuclear RNA genes. Mol Cell Biol. 1995 Apr;15(4):2019–2027. doi: 10.1128/mcb.15.4.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S. K., Boulanger P. A., Berk A. J. Resolution of human transcription factor TFIIIC into two functional components. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3585–3589. doi: 10.1073/pnas.84.11.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]