Abstract
Osteoblasts and adipocytes share a common precursor in adult bone marrow and there is a degree of plasticity between the two cell lineages. This has important implications for the etiology of not only osteoporosis but also several other diseases involving an imbalance between osteoblasts and adipocytes. Understanding the process of differentiation of osteoblasts and adipocytes and their trans-differentiation is crucial in order to identify genes and other factors that may contribute to the pathophysiology of such diseases. Several transcriptional regulators have been shown to control osteoblast and adipocyte differentiation and function. Regulation of cell commitment occurs at the level of the progenitor cell through cross talk between complex signaling pathways and epigenetic mechanisms such as DNA methylation, chromatin remodeling, and microRNAs. Here we review the complex precursor cell microenvironment controlling osteoblastogenesis and adipogenesis during tissue development, maintenance, and pathology.
Keywords: Osteoblastogenesis, Adipogenesis, Progenitor, Transcription, Microenvironment
Introduction
Bone marrow stroma contains pluripotent mesenchymal progenitor cells that can give rise to many mesenchymal lineages, including chondroblasts, adipocytes, or osteoblasts. At birth, hematopoietic red marrow occupies the bone marrow space while adipocytes are barely present but their number and size increase with aging [1–3]. In the elderly and in osteoporosis, the increased volume of marrow adipose tissue correlates with a decrease in bone mass [4] and many conditions that can induce bone loss, such as estrogen insufficiency, disuse, hindlimb unloading, and microgravity exposure show increased bone marrow adiposity [5–7]. The differentiation of mesenchymal stem cells (MSCs) into adipocytes or osteoblasts is tightly regulated by mechanisms promoting cell fate into one lineage while repressing the other. Interestingly, osteoblastic cells derived from trabecular bone fragments have the potential to differentiate into multiple mesenchymal lineages including adipocytes in vitro [8]. Furthermore, adipocytic cells cultured from marrow show the ability to revert to a more proliferative phase and then differentiate in an osteogenic direction [9, 10]. This suggests that adipocytic and osteogenic cells share a common precursor in adult marrow and that there is a high degree of plasticity between the two cell lineages even at the most advanced stages of maturation. Regulation of adipogenesis and osteogenesis occurs at the level of the precursor cell in adult marrow, which has important implications for the etiology of not only osteoporosis but also several other diseases involving an imbalance between osteoblasts and adipocytes. Understanding the process of differentiation of osteoblasts and adipocytes and their trans-differentiation is crucial in order to identify genes and other factors that may contribute to the pathophysiology of such diseases.
Pathological conditions associated with osteoblast–adipocyte lineage plasticity
Several related genetic disorders have the common feature of superficial/dermal ossification with the formation of islands of heterotopic bone in skin and subcutaneous fat [11]. These diseases are associated with heterozygous inactivating mutations of guanine nucleotide-binding protein (α) stimulating activity polypeptide 1 (GNAS) and include progressive osseous heteroplasia (POH), Albright hereditary osteodystrophy/pseudopseudohypoparathyroidism (AHO/PPHP), pseudohypoparathyroidism (PHP), and osteoma cutis (OC). The tissue distribution of heterotopic ossification lesions in GNAS inactivation disorders such as POH suggests that the pathogenesis involves abnormal differentiation of MSCs and/or more committed precursor cells that are present in skin, subcutaneous fat, muscle, tendon, and ligament tissue. Considerable evidence supports the notion that tissues contain multipotential progenitor cells that can give rise to osteoblasts and adipocytes [12–18]. In addition, vascular endothelial cells were reported to transform into multipotent stem-like cells [19].
Another example of a disease involving fat and bone tissue interaction is multiple myeloma (MM). Within the bone marrow microenvironment, MM cells interact with bone marrow stromal cells, endothelial cells, osteoclasts, osteoblasts, adipocytes, immune cells, and the extracellular matrix [20]. MM cells suppress osteoblast differentiation and thereby inhibit bone formation. Several mechanisms appear to be involved, such as direct blocking of the activity of the osteoblast transcription factor Runt-related transcription factor 2 (Runx2) in mesenchymal and osteoprogenitor cells through direct cell-to-cell contact with the involvement of very late antigen a (VLA-4)/vascular cell adhesion molecule 1 (VCAM-1).
In addition to energy storage, adipose tissue plays an important physiological role as an endocrine organ by secreting adipokines, such as leptin and adiponectin. Therefore, dysregulation of osteoblastogenesis and adipogenesis may contribute to the pathophysiology of diseases such as obesity, atherogenesis, diabetes, and inflammation [21].
Transcriptional regulators of osteoblast–adipocyte differentiation
Genetic approaches in which genes are silenced or overexpressed reveal factors controlling the plasticity between the osteoblast–adipocyte cell lineages. This provides insight into the critical pathways that determine the fate of the bone marrow MSCs. Several transcriptional regulators have been shown to control the osteoblast and adipocyte lineages. Runx2 is expressed in osteoblasts at all stages of development and its targeted disruption abolishes the osteoblast lineage [22–24]. Also, inactivation of Osterix (Osx) by gene targeting results in a block in osteoblast development downstream of Runx2 [25]. Activating transcription factor 4 (ATF4) [26] and activator protein 1 (AP-1) [17] further promote the transition to functional osteoblasts. Conversely, peroxisome proliferator-activated receptor gamma (PPARγ) is crucial for adipocyte differentiation and function [27], and members of the CCAAT/enhancer binding proteins (C/EBP) family of transcription factors have been implicated in controlling aspects of adipocyte biology [28]. The balance between osteogenic and adipogenic transcription factors in multipotent mesenchymal precursor cells regulates their quiescence and determines lineage commitment. Furthermore, osteogenic and adipogenic transcription factors can affect each other; PPARγ is a suppressor of Runx2 expression and transcriptional activity thus inhibiting osteoblast differentiation [29, 30].
Regulation of the commitment between the two cell lineages occurs through cross talk between complex signaling pathways including those derived from parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP), bone morphogenetic proteins (BMPs) and retinoic acid (RA), transforming growth factor β (TGFβ), wingless-type MMTV integration site (Wnt) proteins, hedgehogs (Hhs), delta/jagged proteins, fibroblastic growth factors (FGFs), insulin, and insulin-like growth factors (IGFs), and leptin (reviewed in [31, 32]). Additional factors that were identified to play an important role in osteoblast and adipocyte differentiation include ∆FBJ murine osteosarcoma viral oncogene homolog B (∆FosB), a naturally occurring truncated form of FosB [17], Msh homeobox 2 (Msx2) [33], transcriptional coactivator with PDZ-binding motif (TAZ) [34], inhibitor of DNA binding 4 (Id4) [35], Sirtuin 1 (Sirt1) [36], and early B cell factor 1 (Ebf1) [37]. However, their specificity as determinants of age-related changes in bone homeostasis is unclear. In contrast, in the case of the retinoblastoma protein (pRB; [38]), V-maf musculoaponeurotic fibrosarcoma oncogene homolog (Maf) [39] and vascular endothelial growth factor A (VEGF; [40]), also reported to control the balance between osteoblast and adipocyte cell fates in vivo, expression levels are reduced in mesenchymal cells during aging. Loss of the tumor suppressor gene retinoblastoma protein (Rb) favors adipogenesis over osteogenesis resulting in reduced levels of calcified bone and increased levels of brown fat, and Rb-deficient preosteoblasts show multipotency at the expense of commitment to the osteogenic state. The basic leucine-zipper transcription factor Maf was demonstrated to be central to osteoblast lineage commitment through binding to Runx2 and directly regulating the osteocalcin-coding gene bone gamma-carboxyglutamic acid-containing protein 1 (Bglap1). Furthermore, Maf disturbs adipocyte differentiation by suppressing the interaction of Cebpδ and the cAMP response element-binding protein (CREB) gene (Crebbp). VEGF stimulates osteoblastogenesis at the expense of adipogenesis by regulating the expression levels of Runx2 and PPARγ by mechanisms that appear to be affected by a functional interaction with the nuclear envelope protein lamin A. Defective processing of lamin A by the enzyme Zmpste24 has also been linked to changes in osteoblastogenesis and adipogenesis in the bone marrow [41]. Furthermore, cell shape and cytoskeletal tension were reported to regulate stem cell lineage commitment to either osteoblastogenesis or adipogenesis by modulating endogenous Ras homolog gene family, member A (RhoA) activity [42] or stress response pathways [43].
Epigenetic mechanisms involved in osteogenic–adipogenic differentiation
Recently, Scheideler et al. [44] found that gene repression is most prevalent prior to commitment in both osteoblast and adipocyte cell lineages, and computational analysis suggested that gene repression before commitment of MSCs is mediated by microRNAs (miRNAs). Thus, miRNAs were proposed to be involved in regulating differentiation and cell fate decisions [45]. So far, only a few key miRNAs controlling the balance between osteogenesis and adipogenesis have been identified, such as miR-22 [46], miR-31 [47], miR-106a [47], miR-148a [47], miR-424 [47], miR-637 [48], miR-705 [49], miR-3077-5p [49], and several members of the miR-17 family [50]. Different families of miRNAs affect osteoblast–adipocyte differentiation by targeting distinct downstream targets, including histone deacetylase 6 (HDAC6), Osx and BMP2 for miR-22, miR-637, and the miR-17 family members miR-17-5p and miR-106a, respectively, as well as Runx2, core binding factor β (Cbfβ) and BMPs for miR-31, miR-106a, miR-148a and miR-424.
Accumulating evidence suggests that epigenetic mechanisms involving post-translational and covalent modifications of histones in chromatin may be a central mechanism controlling gene transcription. Histone modifications can occur during specification into a cell lineage and in response to changes in the extracellular environment. For example, a change in cell fate from adipogenesis to osteoblastogenesis of bone marrow MSCs was shown to occur as a result of transactivation of agonist-bound PPARγ being repressed by a non-canonical cascade. Wnt5a was reported to activate Nemo-like kinase (NLK), which in turn phosphorylates a histone methyltransferase, SET domain bifurcated 1 (SETDB1), leading to the formation of a co-repressor complex that inactivates PPARγ function through histone H3-K9 methylation [51]. This indicated a novel molecular mechanism where a signal from a cell membrane receptor leads to altered histone modification and changes in gene regulation and cell-lineage decisions.
Co-factors promoting adipocyte differentiation while suppressing osteoblast differentiation were identified as zinc finger protein 467 (Zfp467), which may function by recruiting a HDAC associated co-repressor complex to suppress target gene transcription [52], and HDAC3, which mediates numerous developmental signaling pathways [53]. Furthermore, histone demethylases KDM4B and KDM6B were shown to epigenetically regulate commitment of MSCs to the osteoblast–adipocyte lineage [54]. Depletion of KDM4B or KDM6B significantly reduced osteogenic differentiation and increased adipogenic differentiation by controlling DLX (by removing H3K9me3) and homeobox (HOX) expression (by removing H3K27me3), respectively.
Controlling osteoblast–adipocyte lineage plasticity and consequent therapeutic implications
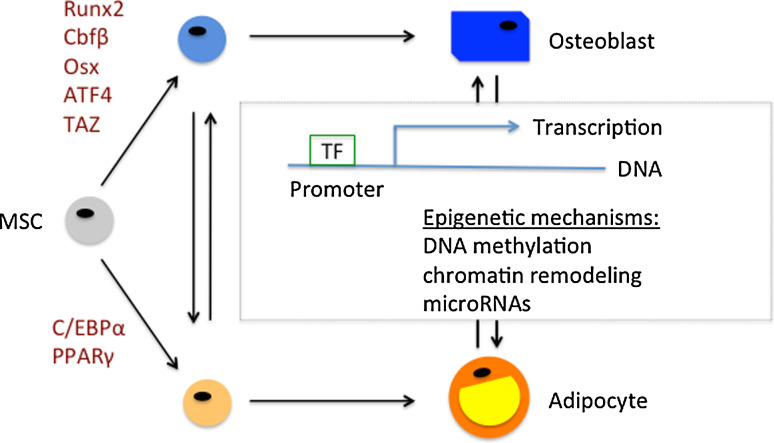
The data reviewed above suggest that the relationship between osteoblastogenesis and adipogenesis is complex [55] [56] (Fig. 1). Osteoblastogenesis is dominant over adipogenesis in the bone marrow of young animals and the cell-differentiation balance between the two cell fates is usually reversed in bone marrow of older or osteoporotic animals [57]. The differentiation of MSCs towards a specific lineage is dependent on activation of specific transcription factors (see Fig. 1) by local, hormonal and mechanical factors [58], including VEGF, BMPs, TGFβ, Wnts, FGFs, IGFs, 1,25-dihydroxyvitamin D3, PTH, glucocorticoids, insulin, and leptin. Recently, elegant genetic pulse-chase experiments indicated that osteoblastic cells are short-lived and nonreplicative, requiring replenishment from bone marrow-derived precursor cells [59]. Thus, it will be crucial to fully understand the precursor cell microenvironment during tissue development, maintenance and pathology in order to identify targets for pharmacological intervention in diseases related to osteoblast–adipocyte lineage plasticity.
Fig. 1.
Osteoblast–adipocyte lineage plasticity is a complex, multifactorial process that shapes tissue development, maintenance, and pathology. Mesenchymal stem cells (MSCs) differentiate into mature osteoblasts and adipocytes via intermediate precursor cell stages that are subjected to transdifferentiation. Local, hormonal and mechanical factors in MSCs result in the activation of transcription factors (TF) and epigenetic mechanisms, which jointly control the balance between osteoblastogenesis and adipogenesis
References
- 1.Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17:34–37. [PubMed] [Google Scholar]
- 2.Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87:4109–4119. [PubMed] [Google Scholar]
- 3.Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–767. doi: 10.1016/S0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 4.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res. 1974;17:57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- 6.Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res. 2002;17:668–677. doi: 10.1359/jbmr.2002.17.4.668. [DOI] [PubMed] [Google Scholar]
- 7.Wronski TJ, Morey-Holton E, Jee WS. Skeletal alterations in rats during space flight. Adv Space Res. 1981;1:135–140. doi: 10.1016/0273-1177(81)90254-4. [DOI] [PubMed] [Google Scholar]
- 8.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99(Pt 1):131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 10.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 11.Pignolo RJ, Xu M, Russell E, Richardson A, Kaplan J, Billings PC, Kaplan FS, Shore EM. Heterozygous inactivation of GNAS in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011;26:2647–2655. doi: 10.1002/jbmr.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy P, Marie PJ. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1(+) cells. J Cell Biochem. 2001;81:23–38. doi: 10.1002/1097-4644(20010401)81:1<23::AID-JCB1021>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Davis LA, Zur Nieden NI. Mesodermal fate decisions of a stem cell: the Wnt switch. Cell Mol Life Sci. 2008;65:2658–2674. doi: 10.1007/s00018-008-8042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 15.Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- 16.Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/S8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- 17.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 18.Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 19.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 23.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 24.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell. 2004;117:387–398. doi: 10.1016/S0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 27.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.M106424200. [DOI] [PubMed] [Google Scholar]
- 29.Khan E, Abu-Amer Y. Activation of peroxisome proliferator-activated receptor-gamma inhibits differentiation of preosteoblasts. J Lab Clin Med. 2003;142:29–34. doi: 10.1016/S0022-2143(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 30.Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, Kim SY, Shin CS. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 31.Ahdjoudj S, Fromigue O, Marie PJ. Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss. Histol Histopathol. 2004;19:151–157. doi: 10.14670/HH-19.151. [DOI] [PubMed] [Google Scholar]
- 32.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, Yatani H, Cao X, Komori T, Yamaguchi A, Yoneda T. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 34.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 35.Tokuzawa Y, Yagi K, Yamashita Y, Nakachi Y, Nikaido I, Bono H, Ninomiya Y, Kanesaki-Yatsuka Y, Akita M, Motegi H, Wakana S, Noda T, Sablitzky F, Arai S, Kurokawa R, Fukuda T, Katagiri T, Schonbach C, Suda T, Mizuno Y, Okazaki Y. Id4, a new candidate gene for senile osteoporosis, acts as a molecular switch promoting osteoblast differentiation. PLoS Genet. 2010;6:e1001019. doi: 10.1371/journal.pgen.1001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- 37.Hesslein DG, Fretz JA, Xi Y, Nelson T, Zhou S, Lorenzo JA, Schatz DG, Horowitz MC. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone. 2009;44:537–546. doi: 10.1016/j.bone.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, Kodama T, Yamaguchi A, Owen MJ, Takahashi S, Takayanagi H. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120:3455–3465. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivas D, Li W, Akter R, Henderson JE, Duque G. Accelerated features of age-related bone loss in zmpste24 metalloproteinase-deficient mice. J Gerontol A Biol Sci Med Sci. 2009;64:1015–1024. doi: 10.1093/gerona/glp089. [DOI] [PubMed] [Google Scholar]
- 42.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117–1127. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheideler M, Elabd C, Zaragosi LE, Chiellini C, Hackl H, Sanchez-Cabo F, Yadav S, Duszka K, Friedl G, Papak C, Prokesch A, Windhager R, Ailhaud G, Dani C, Amri EZ, Trajanoski Z. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 2008;9:340. doi: 10.1186/1471-2164-9-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010;7:36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Huang S, Wang S, Bian C, Yang Z, Zhou H, Zeng Y, Li H, Han Q, Zhao RC. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 2012;21:2531–2540. doi: 10.1089/scd.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J, Yang T, Han J, Yan K, Qiu X, Zhou Y, Fan Q, Ma B. MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. J Cell Biochem. 2011;112:1844–1856. doi: 10.1002/jcb.23106. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC, Bian XW, Zhou J, Lin MC, Lu G, Poon WS, Kung HF. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell. 2011;22:3955–3961. doi: 10.1091/mbc.E11-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao L, Yang X, Su X, Hu C, Zhu X, Yang N, Chen X, Shi S, Jin Y. Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 2013;4:e600. doi: 10.1038/cddis.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Li T, Wang S, Wei J, Fan J, Li J, Han Q, Liao L, Shao C, Zhao RC. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013;10:313–324. doi: 10.1016/j.scr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 52.Quach JM, Walker EC, Allan E, Solano M, Yokoyama A, Kato S, Sims NA, Gillespie MT, Martin TJ. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J Biol Chem. 2011;286:4186–4198. doi: 10.1074/jbc.M110.178251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5:e11492. doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11:50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 56.Lowe CE, O’Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011;124:2681–2686. doi: 10.1242/jcs.079699. [DOI] [PubMed] [Google Scholar]
- 57.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 58.Prockop DJ. Marrow stromal cells as stem cells for no hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 59.Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]