Abstract
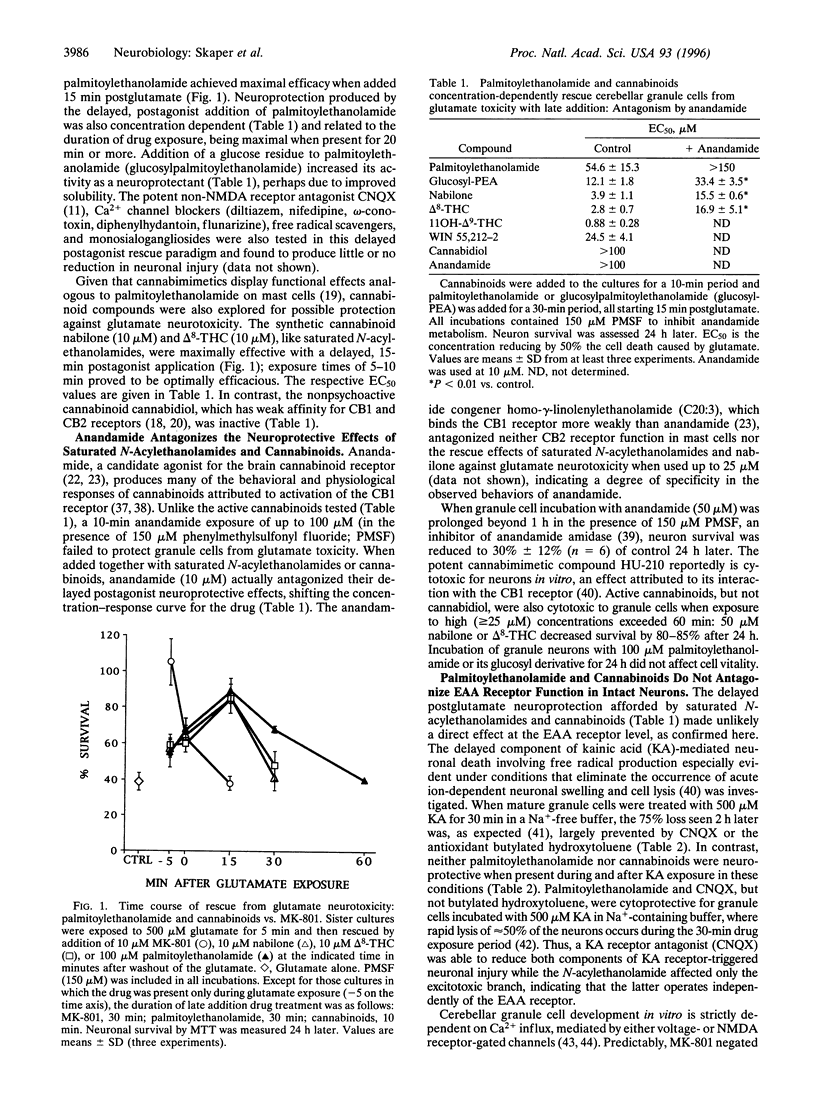
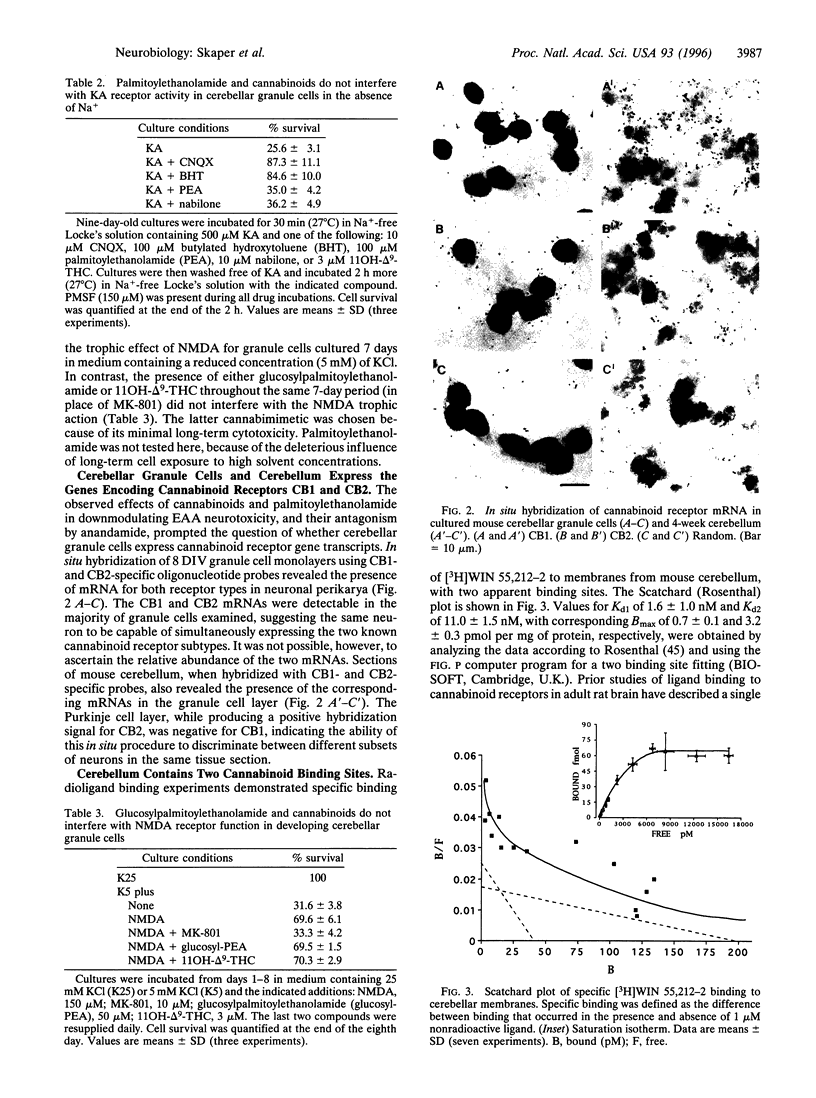
The amino acid L-glutamate is a neurotransmitter that mediates fast neuronal excitation in a majority of synapses in the central nervous system. Glutamate stimulates both N-methyl-D-aspartate (NMDA) and non-NMDA receptors. While activation of NMDA receptors has been implicated in a variety of neurophysiologic processes, excessive NMDA receptor stimulation (excitotoxicity) is thought to be primarily responsible for neuronal injury in a wide variety of acute neurological disorders including hypoxia-ischemia, seizures, and trauma. Very little is known about endogenous molecules and mechanisms capable of modulating excitotoxic neuronal death. Saturated N-acylethanolamides like palmitoylethanolamide accumulate in ischemic tissues and are synthesized by neurons upon excitatory amino acid receptor activation. Here we report that palmitoylethanolamide, but not the cognate N-acylamide anandamide (the ethanolamide of arachidonic acid), protects cultured mouse cerebellar granule cells against glutamate toxicity in a delayed postagonist paradigm. Palmitoylethanolamide reduced this injury in a concentration-dependent manner and was maximally effective when added 15-min postglutamate. Cannabinoids, which like palmitoylethanolamide are functionally active at the peripheral cannabinoid receptor CB2 on mast cells, also prevented neuron loss in this delayed postglutamate model. Furthermore, the neuroprotective effects of palmitoylethanolamide, as well as that of the active cannabinoids, were efficiently antagonized by the candidate central cannabinoid receptor (CB1) agonist anandamide. Analogous pharmacological behaviors have been observed for palmitoylethanolamide (ALI-Amides) in downmodulating mast cell activation. Cerebellar granule cells expressed mRNA for CB1 and CB2 by in situ hybridization, while two cannabinoid binding sites were detected in cerebellar membranes. The results suggest that (i) non-CB1 cannabinoid receptors control, upon agonist binding, the downstream consequences of an excitotoxic stimulus; (ii) palmitoylethanolamide, unlike anandamide, behaves as an endogenous agonist for CB2-like receptors on granule cells; and (iii) activation of such receptors may serve to downmodulate deleterious cellular processes following pathological events or noxious stimuli in both the nervous and immune systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Leon A., Levi-Montalcini R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions. 1993;39(Spec No):C145–C147. doi: 10.1007/BF01972748. [DOI] [PubMed] [Google Scholar]
- Aronica E., Dell'Albani P., Condorelli D. F., Nicoletti F., Hack N., Balázs R. Mechanisms underlying developmental changes in the expression of metabotropic glutamate receptors in cultured cerebellar granule cells: homologous desensitization and interactive effects involving N-methyl-D-aspartate receptors. Mol Pharmacol. 1993 Nov;44(5):981–989. [PubMed] [Google Scholar]
- Balázs R., Jørgensen O. S., Hack N. N-methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988 Nov;27(2):437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Koh J. Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988 Jan;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Methods for antagonizing glutamate neurotoxicity. Cerebrovasc Brain Metab Rev. 1990 Summer;2(2):105–147. [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Cosi C., Spoerri P. E., Comelli M. C., Guidolin D., Skaper S. D. Glucocorticoids depress activity-dependent expression of BDNF mRNA in hippocampal neurones. Neuroreport. 1993 May;4(5):527–530. doi: 10.1097/00001756-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Corwin R. L., Robinson J. K., Felder C. C., Devane W. A., Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993 Dec;46(4):967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Csernansky C. A., Canzoniero L. M., Sensi S. L., Yu S. P., Choi D. W. Delayed application of aurintricarboxylic acid reduces glutamate-induced cortical neuronal injury. J Neurosci Res. 1994 May 1;38(1):101–108. doi: 10.1002/jnr.490380113. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Chin S. A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993 Sep 1;46(5):791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Dysarz F. A., 3rd, Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605–613. [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J. C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994 Dec 15;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990 Jul;13(7):286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- Epps D. E., Natarajan V., Schmid P. C., Schmid H. O. Accumulation of N-acylethanolamine glycerophospholipids in infarcted myocardium. Biochim Biophys Acta. 1980 Jun 23;618(3):420–430. doi: 10.1016/0005-2760(80)90260-x. [DOI] [PubMed] [Google Scholar]
- Facci L., Dal Toso R., Romanello S., Buriani A., Skaper S. D., Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Kingsbury A., Balázs R., Jørgensen O. S. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987 Jul;7(7):2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H. S., Lauritzen L., Strand A. M., Moesgaard B., Frandsen A. Glutamate stimulates the formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cortical neurons in culture. Biochim Biophys Acta. 1995 Oct 5;1258(3):303–308. doi: 10.1016/0005-2760(95)00134-x. [DOI] [PubMed] [Google Scholar]
- Hartley D. M., Choi D. W. Delayed rescue of N-methyl-D-aspartate receptor-mediated neuronal injury in cortical culture. J Pharmacol Exp Ther. 1989 Aug;250(2):752–758. [PubMed] [Google Scholar]
- Hillard C. J., Edgemond W. S., Campbell W. B. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995 Feb;64(2):677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Hollister L. E. Health aspects of cannabis. Pharmacol Rev. 1986 Mar;38(1):1–20. [PubMed] [Google Scholar]
- Howlett A. C. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Kato K., Puttfarcken P. S., Lyons W. E., Coyle J. T. Developmental time course and ionic dependence of kainate-mediated toxicity in rat cerebellar granule cell cultures. J Pharmacol Exp Ther. 1991 Jan;256(1):402–411. [PubMed] [Google Scholar]
- Koh J. Y., Goldberg M. P., Hartley D. M., Choi D. W. Non-NMDA receptor-mediated neurotoxicity in cortical culture. J Neurosci. 1990 Feb;10(2):693–705. doi: 10.1523/JNEUROSCI.10-02-00693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Kater S. B. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989 Jul;12(7):265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Lipton S. A. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993 Dec;16(12):527–532. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Rosenberg P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994 Mar 3;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lynn A. B., Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994 Mar;268(3):1612–1623. [PubMed] [Google Scholar]
- Manthorpe M., Fagnani R., Skaper S. D., Varon S. An automated colorimetric microassay for neuronotrophic factors. Brain Res. 1986 Mar;390(2):191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Mercanti D., Galli C., Liguori M., Ciotti M. T., Gullà P., Calissano P. Identification of the Serum Complex Which Induces Cerebellar Granule Cell In Vitro Differentiation and Resistance to Excitatory Amino Acids. Eur J Neurosci. 1992;4(8):733–744. doi: 10.1111/j.1460-9568.1992.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nadler V., Mechoulam R., Sokolovsky M. The non-psychotropic cannabinoid (+)-(3S,4S)-7-hydroxy-delta 6- tetrahydrocannabinol 1,1-dimethylheptyl (HU-211) attenuates N-methyl-D-aspartate receptor-mediated neurotoxicity in primary cultures of rat forebrain. Neurosci Lett. 1993 Nov 12;162(1-2):43–45. doi: 10.1016/0304-3940(93)90555-y. [DOI] [PubMed] [Google Scholar]
- Natarajan V., Schmid P. C., Schmid H. H. N-acylethanolamine phospholipid metabolism in normal and ischemic rat brain. Biochim Biophys Acta. 1986 Aug 14;878(1):32–41. doi: 10.1016/0005-2760(86)90341-3. [DOI] [PubMed] [Google Scholar]
- Ohsawa F., Widmer H. R., Knusel B., Denton T. L., Hefti F. Response of embryonic rat hippocampal neurons in culture to neurotrophin-3, brain-derived neurotrophic factor and basic fibroblast growth factor. Neuroscience. 1993 Nov;57(1):67–77. doi: 10.1016/0306-4522(93)90112-s. [DOI] [PubMed] [Google Scholar]
- Pacheco M. A., Ward S. J., Childers S. R. Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Res. 1993 Feb 12;603(1):102–110. doi: 10.1016/0006-8993(93)91304-b. [DOI] [PubMed] [Google Scholar]
- Patel J., Zinkand W. C., Thompson C., Keith R., Salama A. Role of glycine in the N-methyl-D-aspartate-mediated neuronal cytotoxicity. J Neurochem. 1990 Mar;54(3):849–854. doi: 10.1111/j.1471-4159.1990.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Puttfarcken P. S., Getz R. L., Coyle J. T. Kainic acid-induced lipid peroxidation: protection with butylated hydroxytoluene and U78517F in primary cultures of cerebellar granule cells. Brain Res. 1993 Oct 8;624(1-2):223–232. doi: 10.1016/0006-8993(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Schmid H. H., Schmid P. C., Natarajan V. N-acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29(1):1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Compton D. R., Welch S. P., Razdan R. K., Mechoulam R., Martin B. R. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994 Jul;270(1):219–227. [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]



