Abstract
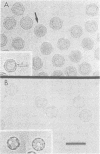
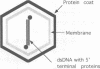
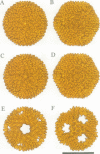
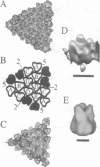
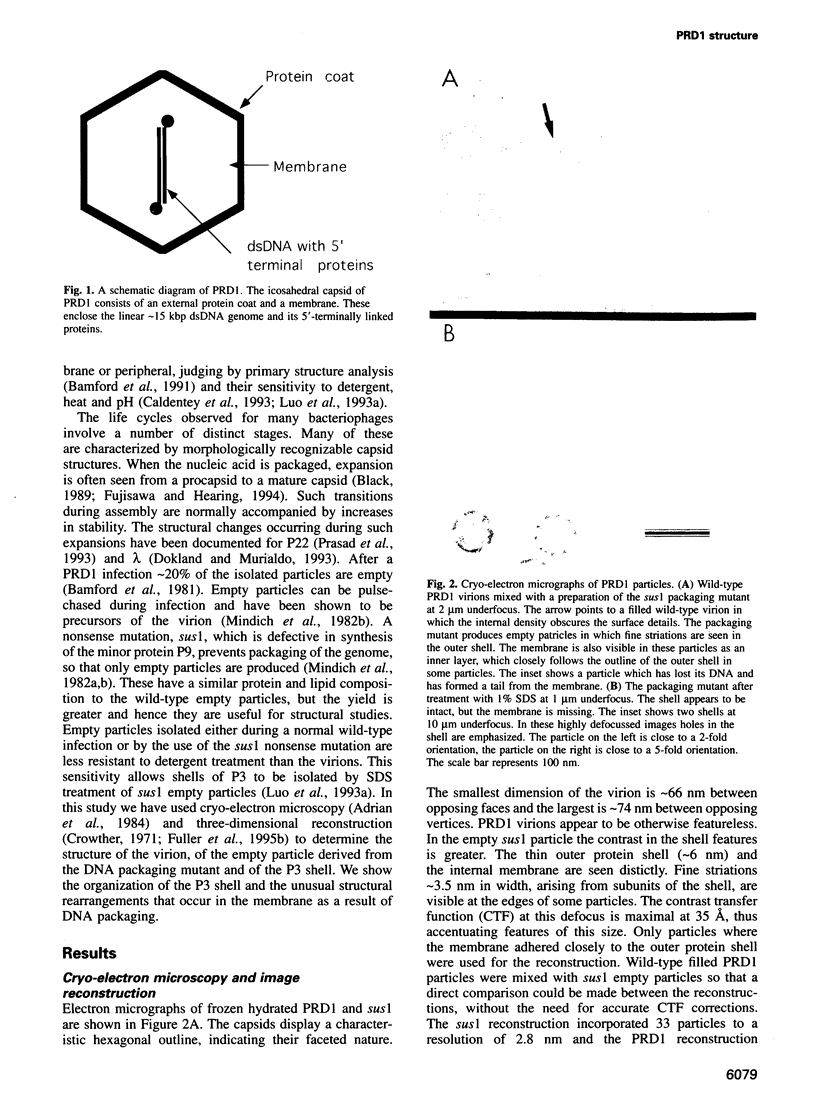
Bacteriophage PRD1 contains a linear dsDNA genome enclosed by a lipid membrane lying within a protein coat. Determination of the structure of the detergent-treated particle to 2 nm by cryo-electron microscopy and three-dimensional reconstruction has defined the position of the major coat protein P3. The coat contains 240 copies of trimeric P3 packed into positions of local 6-fold symmetry on a T = 25 lattice. The three-dimensional structures of the PRD1 virion and a DNA packaging mutant to a resolution of 2.8 nm have revealed specific interactions between the coat and the underlying membrane. The membrane is clearly visible as two leaflets separated by 2 nm and spanned by transmembrane density. The size of the coat does not change upon DNA packaging. Instead, the number of interactions seen between the protein shell and the membrane and the order of the membrane components increase. Thus the membrane of PRD1 plays a role in assembly which is akin to that played by the nucleocapsid in other membrane viruses.
Full text
PDF
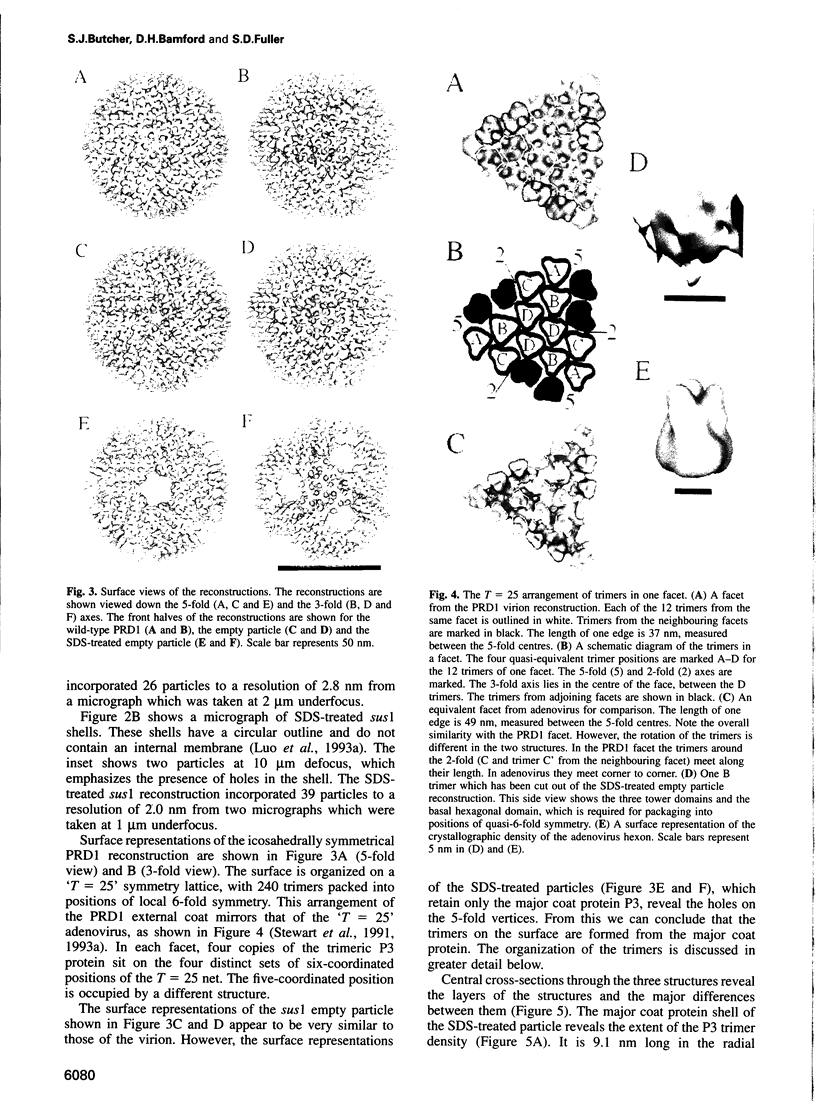


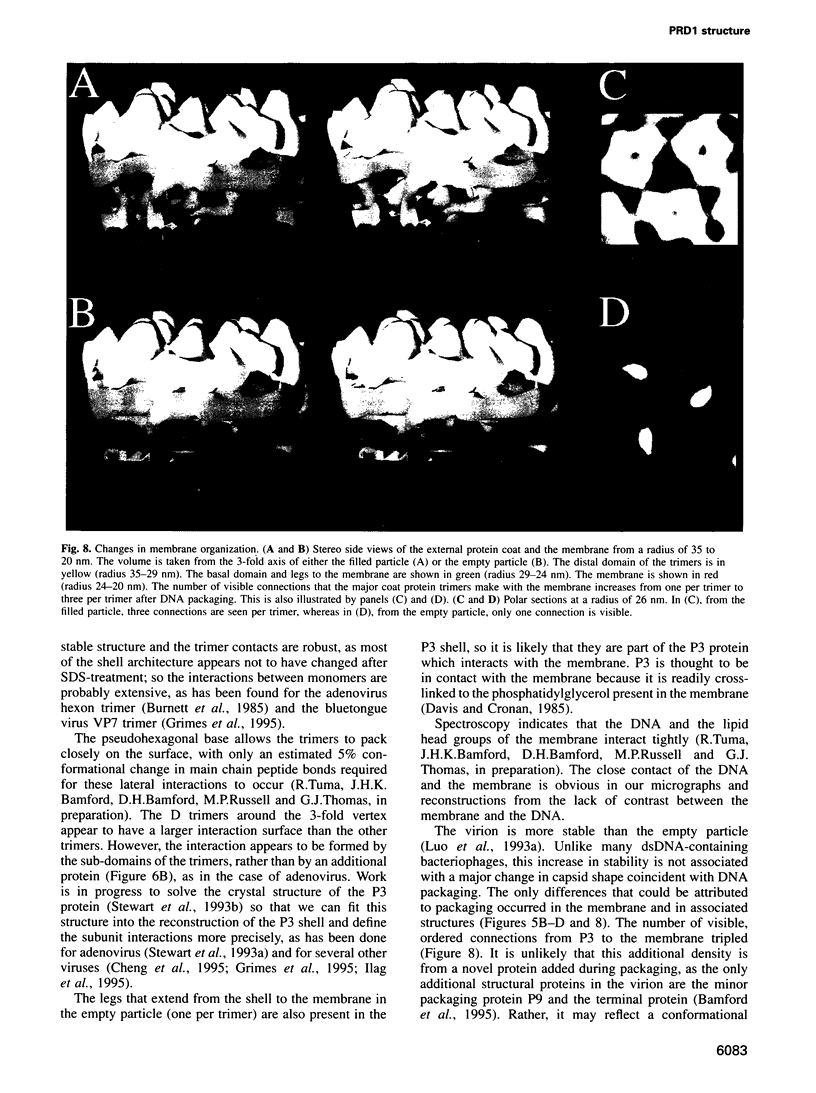



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Bamford J. K., Towse S. A., Thomas G. J., Jr Structural study of the lipid-containing bacteriophage PRD1 and its capsid and DNA components by laser Raman spectroscopy. Biochemistry. 1990 Jun 26;29(25):5982–5987. doi: 10.1021/bi00477a015. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Caldentey J., Bamford J. K. Bacteriophage PRD1: a broad host range DSDNA tectivirus with an internal membrane. Adv Virus Res. 1995;45:281–319. doi: 10.1016/s0065-3527(08)60064-0. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Rouhiainen L., Takkinen K., Söderlund H. Comparison of the lipid-containing bacteriophages PRD1, PR3, PR4, PR5 and L17. J Gen Virol. 1981 Dec;57(Pt 2):365–373. doi: 10.1099/0022-1317-57-2-365. [DOI] [PubMed] [Google Scholar]
- Bamford D., McGraw T., MacKenzie G., Mindich L. Identification of a protein bound to the termini of bacteriophage PRD1 DNA. J Virol. 1983 Aug;47(2):311–316. doi: 10.1128/jvi.47.2.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D., Mindich L. Structure of the lipid-containing bacteriophage PRD1: disruption of wild-type and nonsense mutant phage particles with guanidine hydrochloride. J Virol. 1982 Dec;44(3):1031–1038. doi: 10.1128/jvi.44.3.1031-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford J. K., Bamford D. H. Capsomer proteins of bacteriophage PRD1, a bacterial virus with a membrane. Virology. 1990 Aug;177(2):445–451. doi: 10.1016/0042-6822(90)90508-o. [DOI] [PubMed] [Google Scholar]
- Bamford J. K., Hänninen A. L., Pakula T. M., Ojala P. M., Kalkkinen N., Frilander M., Bamford D. H. Genome organization of membrane-containing bacteriophage PRD1. Virology. 1991 Aug;183(2):658–676. doi: 10.1016/0042-6822(91)90995-n. [DOI] [PubMed] [Google Scholar]
- Bamford J. K., Luo C., Juuti J. T., Olkkonen V. M., Bamford D. H. Topology of the major capsid protein P3 of bacteriophage PRD1: analysis using monoclonal antibodies and C-terminally truncated proteins. Virology. 1993 Dec;197(2):652–658. doi: 10.1006/viro.1993.1640. [DOI] [PubMed] [Google Scholar]
- Black L. W. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Adsorption of bacteriophages specific for Pseudomonas aeruginosa R factors RP1 and R1822. Biochem Biophys Res Commun. 1974 Apr 8;57(3):893–900. doi: 10.1016/0006-291x(74)90630-5. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Rutherford E. L. Basic characterization of a lipid-containing bacteriophage specific for plasmids of the P, N, and W compatibility groups. Can J Microbiol. 1975 Feb;21(2):152–163. doi: 10.1139/m75-023. [DOI] [PubMed] [Google Scholar]
- Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994 Sep 1;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Burnett R. M., Grütter M. G., White J. L. The structure of the adenovirus capsid. I. An envelope model of hexon at 6 A resolution. J Mol Biol. 1985 Sep 5;185(1):105–123. doi: 10.1016/0022-2836(85)90186-x. [DOI] [PubMed] [Google Scholar]
- Caldentey J., Luo C., Bamford D. H. Dissociation of the lipid-containing bacteriophage PRD1: effects of heat, pH, and sodium dodecyl sulfate. Virology. 1993 Jun;194(2):557–563. doi: 10.1006/viro.1993.1294. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Kuhn R. J., Olson N. H., Rossmann M. G., Choi H. K., Smith T. J., Baker T. S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995 Feb 24;80(4):621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R. H., Reddy V. S., Olson N. H., Fisher A. J., Baker T. S., Johnson J. E. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure. 1994 Apr 15;2(4):271–282. doi: 10.1016/s0969-2126(00)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Davis T. N., Cronan J. E., Jr An alkyl imidate labeling study of the organization of phospholipids and proteins in the lipid-containing bacteriophage PR4. J Biol Chem. 1985 Jan 10;260(1):663–671. [PubMed] [Google Scholar]
- Davis T. N., Muller E. D., Cronan J. E., Jr The virion of the lipid-containing bacteriophage PR4. Virology. 1982 Jul 30;120(2):287–306. doi: 10.1016/0042-6822(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Dokland T., Murialdo H. Structural transitions during maturation of bacteriophage lambda capsids. J Mol Biol. 1993 Oct 20;233(4):682–694. doi: 10.1006/jmbi.1993.1545. [DOI] [PubMed] [Google Scholar]
- Dreiseikelmann B. Translocation of DNA across bacterial membranes. Microbiol Rev. 1994 Sep;58(3):293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- Fuller S. D., Berriman J. A., Butcher S. J., Gowen B. E. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell. 1995 Jun 2;81(5):715–725. doi: 10.1016/0092-8674(95)90533-2. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Furcinitti P. S., van Oostrum J., Burnett R. M. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989 Dec 1;8(12):3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Grimes J., Basak A. K., Roy P., Stuart D. The crystal structure of bluetongue virus VP7. Nature. 1995 Jan 12;373(6510):167–170. doi: 10.1038/373167a0. [DOI] [PubMed] [Google Scholar]
- Ilag L. L., Olson N. H., Dokland T., Music C. L., Cheng R. H., Bowen Z., McKenna R., Rossmann M. G., Baker T. S., Incardona N. L. DNA packaging intermediates of bacteriophage phi X174. Structure. 1995 Apr 15;3(4):353–363. doi: 10.1016/s0969-2126(01)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilainen M. M., Grahn A. M., Bamford J. K., Bamford D. H. Binding of an Escherichia coli double-stranded DNA virus PRD1 to a receptor coded by an IncP-type plasmid. J Bacteriol. 1993 May;175(10):3089–3095. doi: 10.1128/jb.175.10.3089-3095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M., Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994 May 6;77(3):321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Luo C., Butcher S., Bamford D. H. Isolation of a phospholipid-free protein shell of bacteriophage PRD1, an Escherichia coli virus with an internal membrane. Virology. 1993 Jun;194(2):564–569. doi: 10.1006/viro.1993.1295. [DOI] [PubMed] [Google Scholar]
- Luo C., Hantula J., Tichelaar W., Bamford D. H. Bacteriophage PRD1 proteins: cross-linking and scanning transmission electron microscopy analysis. Virology. 1993 Jun;194(2):570–575. doi: 10.1006/viro.1993.1296. [DOI] [PubMed] [Google Scholar]
- Metcalf P., Cyrklaff M., Adrian M. The three-dimensional structure of reovirus obtained by cryo-electron microscopy. EMBO J. 1991 Nov;10(11):3129–3136. doi: 10.1002/j.1460-2075.1991.tb04874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Bamford D., Goldthwaite C., Laverty M., Mackenzie G. Isolation of nonsense mutants of lipid-containing bacteriophage PRD1. J Virol. 1982 Dec;44(3):1013–1020. doi: 10.1128/jvi.44.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Bamford D., McGraw T., Mackenzie G. Assembly of bacteriophage PRD1: particle formation with wild-type and mutant viruses. J Virol. 1982 Dec;44(3):1021–1030. doi: 10.1128/jvi.44.3.1021-1030.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Cohen J., Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976 Apr;126(1):177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung H., Vanden Boom T., Cronan J. E., Jr The major capsid protein of the lipid-containing bacteriophage PR4 is the precursor of two other capsid proteins. Virology. 1994 Jan;198(1):17–24. doi: 10.1006/viro.1994.1003. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Bamford D. H. Quantitation of the adsorption and penetration stages of bacteriophage phi 6 infection. Virology. 1989 Jul;171(1):229–238. doi: 10.1016/0042-6822(89)90530-8. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Siak J. S., Gray R. H. Characteristics of PRD1, a plasmid-dependent broad host range DNA bacteriophage. J Virol. 1974 Sep;14(3):689–699. doi: 10.1128/jvi.14.3.689-699.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula T. M., Savilahti H., Bamford D. H. Comparison of the amino acid sequence of the lytic enzyme from broad-host-range bacteriophage PRD1 with sequences of other cell-wall-peptidoglycan lytic enzymes. Eur J Biochem. 1989 Mar 1;180(1):149–152. doi: 10.1111/j.1432-1033.1989.tb14625.x. [DOI] [PubMed] [Google Scholar]
- Pakula T. M., Savilahti H., Bamford D. H. The organization of the right-end early region of bacteriophage PRD1 genome. Gene. 1989 Dec 21;85(1):53–58. doi: 10.1016/0378-1119(89)90463-0. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E., Barth P. T., Figurski D. H., Guiney D. G., Haas D., Helinski D. R., Schwab H., Stanisich V. A., Thomas C. M. Complete nucleotide sequence of Birmingham IncP alpha plasmids. Compilation and comparative analysis. J Mol Biol. 1994 Jun 24;239(5):623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- Paredes A. M., Brown D. T., Rothnagel R., Chiu W., Schoepp R. J., Johnston R. E., Prasad B. V. Three-dimensional structure of a membrane-containing virus. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9095–9099. doi: 10.1073/pnas.90.19.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Prevelige P. E., Marietta E., Chen R. O., Thomas D., King J., Chiu W. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J Mol Biol. 1993 May 5;231(1):65–74. doi: 10.1006/jmbi.1993.1257. [DOI] [PubMed] [Google Scholar]
- Rey F. A., Heinz F. X., Mandl C., Kunz C., Harrison S. C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995 May 25;375(6529):291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Bamford D. H. Linear DNA replication: inverted terminal repeats of five closely related Escherichia coli bacteriophages. Gene. 1986;49(2):199–205. doi: 10.1016/0378-1119(86)90280-5. [DOI] [PubMed] [Google Scholar]
- Stewart P. L., Burnett R. M., Cyrklaff M., Fuller S. D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991 Oct 4;67(1):145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- Stewart P. L., Fuller S. D., Burnett R. M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993 Jul;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. L., Ghosh S., Bamford D. H., Burnett R. M. Crystallization of the major coat protein of PRD1, a bacteriophage with an internal membrane. J Mol Biol. 1993 Mar 5;230(1):349–352. doi: 10.1006/jmbi.1993.1148. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Walin L., Tuma R., Thomas G. J., Jr, Bamford D. H. Purification of viruses and macromolecular assemblies for structural investigations using a novel ion exchange method. Virology. 1994 May 15;201(1):1–7. doi: 10.1006/viro.1994.1259. [DOI] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]