Abstract
Glutamate induced excitotoxic injury through over-activation of N-methyl-D-aspartate receptors (NMDARs) plays a critical role in the development of many neurodegenerative diseases. The present study was undertaken to evaluate the role of CGX-1007 (Conantokin G) as a neuroprotective agent against NMDA-induced excitotoxicity. Conantokin G, a cone snail peptide isolated from Conus geographus is reported to selectively inhibit NR2B containing NMDARs with high specificity and is shown to have potent anticonvulsant and antinociceptive effects. CGX-1007 significantly reduced the excitotoxic cell death induced by NMDA in organotypic hippocampal brain slice cultures in a concentration dependent manner. In contrast, ifenprodil, another NR2B specific antagonist failed to offer neuroprotection against NMDA-induced excitotoxicity. We further determined that the neuroprotection observed is likely due to action of CGX-1007 at multiple NMDA receptor subtypes. In a series of electrophysiology experiments, CGX-1007 inhibited NMDA-gated currents in human embryonic kidney (HEK) 293 cells expressing NMDA receptors containing either NR1a/NR2B or NR1a/NR2A subunit combinations. CGX-1007 produced a weak inhibition at NR1a/NR2C receptors, whereas it had no effect on NR1a/NR2D receptors. Further, the inhibition of NMDA receptors by CGX-1007 was voltage-dependent with greater inhibition seen at hyperpolarized membrane potentials. The voltage-dependence of CGX-1007 activity was also observed in recordings of NMDA-gated currents evoked in native receptors expressed in cortical neurons in culture. Based on our results, we conclude that CGX-1007 is a potent neuroprotective agent that acts as an antagonist at both NR2A and NR2B containing receptors.
Keywords: Neuroprotection, Organotypic, hippocampal brain slice, Conantokin G, HEK 293 cells
1. Introduction
Glutamate-induced excitotoxic cell death is an important mechanism of neuronal injury in neurodegenerative diseases, stroke and other brain insults (Choi and Rothman, 1990; Villmann and Becker, 2007) and is thought to be primarily due to the excessive activation of N-methyl D-aspartate (NMDA) receptors. NMDA receptors (NMDARs) are hetero-oligomers comprised of two NR1 subunits and one or more types of NR2 subunit (A-D) (Dingledine et al., 1999; Wenthold et al., 2003). While it is undisputed that changes in intracellular calcium due to over-stimulation of NMDARs and subsequent loss of mitochondrial membrane potential leads to excitotoxic cell death, the role of NMDAR subunit composition in mediating excitotoxicity is still not clear (Hardingham and Bading, 2010; Martel et al., 2009; Liu et al., 2007; von Engelhardt et al., 2007). Evidence suggests that changes in NMDAR subunits during development may influence the pharmacology of excitotoxicity (Sinor et al., 2000; Zhou and Baudry, 2006). NR2B-containing NMDA receptors mediate excitotoxicity in young neurons (14 days in vitro) while mostly NR2A-containing receptors mediate cell death in older cultures (21 days in vitro; von Engelhardt et al., 2007). CGX-1007, a widely reported NR2B antagonist, exhibits potent anticonvulsant and antinociceptive properties (Armstrong et al., 1998; Barton and White, 2004; Xiao et al., 2008). In addition, CGX-1007 is found to be neuroprotective against ischemic brain injury and staurosporine-induced apoptosis (Williams et al., 2000, 2002). However, the neuroprotective effect of CGX-1007 in excitotoxic cell death and the NMDA receptor subuntis involved has not been examined. Therefore, the current study was designed to determine if CGX-1007 is neuroprotective against NMDA-induced excitotoxicity in organotypic hippocampal slice cultures.
Results from the present study indicate that while CGX-1007 is quite effective in attenuating excitotoxic cell death in the in vitro organotypic slice culture preparation, ifenprodil, a NR2B selective antagonist (Williams, 2001), did not confer protection against NMDA-mediated cell death. This data suggests that CGX-1007 was not as selective for NR2B containing receptors as ifenprodil. Therefore, we examined the effect of CGX-1007 on NMDA-induced currents in HEK 293 cells expressing NMDARs with different combinations of NR2 subunits. In contrast to some reports (Donevan and McCabe, 2000, Klein et al., 2001; Teichert et al., 2007), but consistent with the neuroprotection data obtained here and other reports (Wittekindt et al., 2001; Barton et al., 2004; Ragnarsson et al., 2006), we found that CGX-1007 was quite effective in blocking NMDA-gated currents in receptors containing either NR2A or NR2B subunits. These results suggest that CGX-1007 may be a potent molecule for conferring neuroprotection against excitotoxic insults and that this effect is mediated by efficacy at multiple NMDA receptor subtypes.
2. Materials and Methods
2.1. Organotypic hippocampal brain slice cultures
Hippocampal brain slice cultures were grown using the interface culture method described by Stoppini et al., (1991) with slight modifications (Noraberg et al., 1999). Under pentobarbital anesthesia (40 mg/kg; i.p.), P10-P11 Sprague Dawley rat pups were decapitated, and their brains quickly removed and hippocampi were isolated under sterile conditions. 400 [.proportional]m thick transverse hippocampal sections were cut using a McIlwain tissue chopper (Vibratome, O'Fallon, MO). The sections were placed in ice-cold Gey's balanced salt solution (Sigma, St. Louis, MO) supplemented with 6.5 mg/ml glucose. Slices were separated, examined and trimmed under a microscope and undamaged sections with an intact cell layer were plated onto membrane inserts (Millicell CM, Millipore, Bedford, MA) in a 6 well plate (4 slices per insert/well), containing 1 ml of culture medium. The culture medium composed of 50% MEM with glutamax + Hepes, 25% horse serum and 25% Hank's balanced salt solution (all from Gibco/Invitrogen, Carlsbad, CA), supplemented with D-Glucose to a final concentration of 25 mM and pH 7.2. Hippocampal explants were maintained in an incubator at 5% CO2-95% air at 37°C and 95% humidity level. Media changes were done three times a week and the cultures were examined routinely for infections, neuronal survival and growth. All experiments were performed in 14 day in vitro cultures. One day prior to the experiment, culture medium was replaced with serum-free Neurobasal medium with 2% B27 supplement (Gibco/Invitrogen, Carlsbad, CA) and 25 mM D-glucose. Animal handling and experimental procedures were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Propidium iodide staining and live cell imaging
Excitotoxic neurodegeneration in hippocampal slice cultures is monitored by propidium iodide (PI) uptake (Vornov et al., 1991; Noraberg, 2004). PI is a stable fluorescent dye, nontoxic to neurons and binds to DNA of dead or dying cells to yield a bright red fluorescence. Before the start of the experiments, hippocampal explants were assessed for basal cell death by adding PI (2 μM; 45 min exposure; day 1). PI uptake was measured by fluorescent imaging, using an Axiovert TE 200 (Carl Zeiss, Inc., Germany). Only slices showing no or minimal cell death were selected for study and those slices were exposed to either NMDA (10 μM) alone, NMDA (10 μM) + CGX-1007, or NMDA (10 μM)+ ifenprodil for 4 hr. Each well of the six-well plate contained four hippocampal slices. For the experiment, there are two control wells (slices exposed to NMDA alone) and 4 test wells (NMDA + CGX-1007 or NMDA + ifenprodil), with the compounds to be tested used at different concentrations. After 4hrs of exposure to NMDA, NMDA + CGX-1007, or NMDA + ifenprodil, slices were transferred to fresh neurobasal medium containing PI. Slices were then imaged 24 hrs later to determine the extent of NMDA-induced excitotoxic cell death (day 2). Finally, to normalize the percent of cell death in each slice, glutamate (10 mM) was added to the slice cultures to kill all remaining cells and the slices were again imaged after a 24 hr period (day 3). Fluorescent intensities in images obtained on Day 2 were compared to that observed on Day 3 (see below). The number of slices used in the study ranged from12-25 slices per concentration. All drugs and compounds used in this assay are obtained from Sigma, St. Louis, MO., unless mentioned otherwise. CGX-1007 (CGX-1007) was from Cognetix, Inc., and a generous gift of Dr. Baldomero Olivera, University of Utah. The amino acid sequence of CGX-1007 was confirmed by mass spectrometry.
2.3. Analysis of cell death
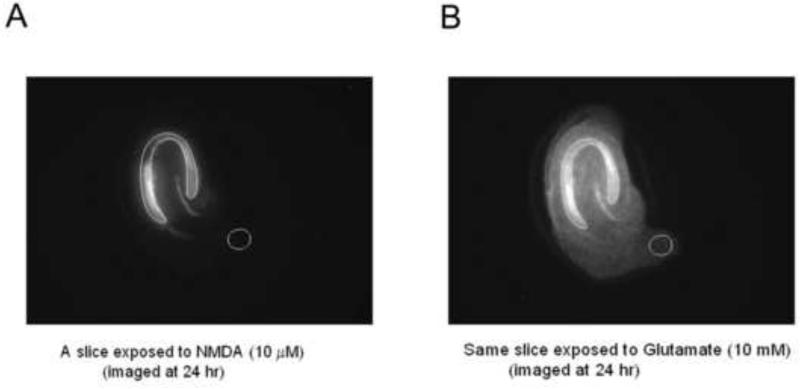
Axiovision software was used for image analysis and determination of cell death in hippocampal brain slices. A region of interest (ROI) comprising the CA1 and CA3 layers of hippocampal neurons were outlined for each individual slice in the images obtained following glutamate treatment (Figure 1) and brightness intensity within the ROI was determined. Defining the ROI in the image taken on Day 3, following glutamate treatment, reduced the risk of biasing the ROI in favor of a given treatment condition.
Figure 1.
Analysis of PI fluorescent intensity in the region of interest (ROI) was carried out using Axiovision software. A, B: CA1-CA3 pyramidal cell layer was selected and an outline was drawn in slices showing complete cell death following glutamate treatment. This ROI map was then applied to the image that was obtained following treatment with NMDA alone or NMDA with the test compounds. The reason for selecting the region of interest in the glutamate image first was due to the easy visualization of layers because of maximal cell death, as compared to the NMDA or NMDA+drug image, especially when neuroprotection was robust. The cell death ratio (PI uptake ratio) for each culture was calculated by determining the ratio of the PI intensity in the ROI in the image following treatment to the ROI in the same culture that was subsequently treated with glutamate. Data is thus expressed as % of total cell death.
The ROI map determined for each slice was then applied to the corresponding slice on the image taken on Day 2, 24 hours following the initial drug treatment. A cell death ratio was obtained by dividing the intensity of the image in the ROI in a slice under experimental conditions to the intensity of the same ROI in the very same slice following glutamate administration and was thus expressed as % of total fluorescent intensity. Background intensity values were subtracted from each image prior to calculation of the PI uptake ratio (Figure 1).
2.4. Transfection of HEK 293 Cells
The cDNA clones of rat NMDA receptors (NR1a, NR1b, NR2A, NR2B, NR2C and NR2D) contained in mammalian expression vectors pRC/CMV, pRK5 or pRK7 were a generous gift of Dr. David Lynch (University of Pennsylvania). Human Embryonic Kidney (HEK) 293 cells were obtained from ATCC (Manassas, VA, USA) and grown on 75 cm2 flask in Eagle's minimum essential medium supplemented with 10 % fetal bovine serum (Gibco/Invitrogen, Carlsbad, CA) and 100 U/ml penicillin-streptomycin (Sigma, St. Louis, MO) and maintained at 37°C in a 5% CO2 environment. Cells maintained in culture were split twice weekly, after reaching 90% confluency. Twenty-four hours prior to the transfection, cells were split and plated at 30-50% confluency on poly-L-lysine coated glass coverslips in a 35 mm dish. HEK cells were transiently transfected with cDNA encoding NMDA receptor subunits and Green fluorescent protein (GFP) using Calcium Phosphate precipitation (Chen and Okayama, 1987; Kendrick et al., 1998). Prior to transfection, DNA sequences of the NMDA receptor subunits were confirmed by the sequencing core facility, School of Medicine, University of Utah. The cDNA for NR1/NR2/GFP were mixed in 1:1:0.5 ratio. Cells were grown in the presence of 1 mM APV (Sigma, MO, USA), to prevent NMDA receptor-mediated excitotoxic cell death after transfection (Krupp et al., 1998; Tovar and Westbrook, 1999). After 12-16 hours transfection mixture was removed, cells were rinsed and replaced with fresh medium. Whole cell recordings were made within 24-48 hours after transfection.
2.5. Primary neuronal cultures
Primary cultures of cortical neurons were prepared from embryonic (E-16) mice of CF-1 strain (Charles River, Wilmington, MA), as described previously (Alex et al., 2006, Otto et al., 2002). The cells were plated at a low density of 100,000 cells/ml on poly-L-lysine (Sigma)-coated coverslips placed in 35-mm dishes. Cultures were maintained in a humidified incubator at 37°C and 7% CO2 for 3 wk. The culture medium was replaced with fresh DMEM every 2–3 days. Experiments were done on neurons maintained in culture for 17-19 days.
2.6. Electrophysiological recordings and solutions
Whole-cell voltage clamp recordings were performed on transfected HEK 293 cells and primary cortical neurons. Patch pipettes (2-4 M ) were pulled using a micropipette electrode puller (Sutter Instruments, CA) and were filled with internal recording solution containing 153 mM CsCl, 10 mM EGTA-CsOH, 10 mM HEPES, and 4 mM MgCl2 (290 mOsm; pH 7.3). The cells plated on glass coverslips were placed in a recording chamber perfused continuously with Mg2+-free extracellular solution containing, 142 mM NaCl, 1.5 mM KCl, 1 mM CaCl2, 20 mM Sucrose, 10 mM Glucose and 10 mM HEPES (310 mOsm; pH 7.3). Working concentrations of CGX-1007 were prepared from a stock of 0.1 mM by diluting in extracellular solution.
Recordings of evoked NMDA currents were made using either an EPC-7 or Axopatch 200B amplifier in voltage-clamp mode. NMDA (100 μM) and glycine (50 μM) was used to facilitate the maximal activation of NMDA receptors and evoke NMDA-gated currents in transfected HEK 293 cells. NMDA (10 μM) and glycine (10 μM) were used to evoke NMDA-gated currents in cortical neurons. TTX (500 nM), a sodium channel blocker, was included in the external solution to block synaptic transmission in cortical neurons. The cells were perfused with NMDA alone (control) and then NMDA + CGX-1007 solutions through a gravity-fed three-barelled microperfusion system, positioned within 100 μm of the cell. Either NMDA or NMDA + CGX-1007 was applied for 0.5 sec and the currents evoked were filtered at 2-10 kHz and recorded in a personal computer using pClamp 9.0 (Axon Instruments, CA). Response to NMDA obtained in the presence of CGX-1007 was compared to that of NMDA alone in each cell.
2.7. Data Analysis
All data are presented as Mean +/− SEM. A p value of < 0.05 was considered significant. Statistical significance was determined using Student's t- test or ANOVA. The concentration-response curves for CGX-1007 in transfected cells were best fit using four parameter logistic equation and IC50 calculated using Prism (Graphpad Software). The data for recombinant NMDARs were collected from 2-4 separate transfections of HEK 293 cells having an n of at least 3 from each transfection.
3. Results
3.1. CGX-1007 prevents NMDA-induced excitotoxic cell death in hippocampal slice cultures
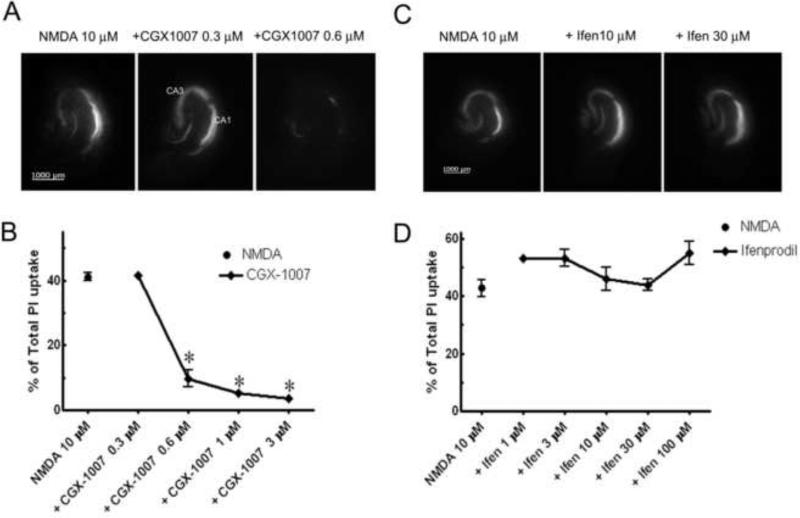
Excessive glutamate release during seizures and other acute brain insults can result in considerable cell loss in the brain. Therefore, identification of potential neuroprotective agents has important clinical implications. Using in vitro organotypic hippocampal slice cultures, we modeled excitotoxic neuronal injury by incubating slices in NMDA and examined the ability of CGX-1007 in preventing NMDA-induced cell death. In hippocampal slice cultures, exposure to NMDA (10 μM, 4 hr insult) induced substantial cell death as determined by significant PI uptake after 24 hr (Figure 2). When CGX-1007 (0.3-3 μM) was co-applied with NMDA for four hours, concentration-dependent neuroprotection was observed, with an EC50 of 0.44 ± 0.02 μM (Figure 2a, b). In contrast, ifenprodil, a NR2B selective antagonist of NMDA receptors (Kew et al., 1998; Williams, 2001) failed to offer any neuroprotection against NMDA-induced excitotoxicity in hippocampal slice cultures at any concentration tested (1.0-100.0 μM) (Figure 2c, d). The difference between the neuroprotection results observed with CGX-1007 and ifenprodil suggest that the mechanism of action of CGX-1007 is not exclusively through inhibition of NR2B-containing NMDA receptors.
Figure 2.
NMDA (10 μM) induced excitotoxic cell death in CA1 and CA3 neuronal cell layers of hippocampus maintained in culture. A, B: CGX-1007 offered significant neuroprotection against NMDA-induced excitotoxicity at concentrations greater than 0.3 μM (p < 0.05, One Way ANOVA). C, D: Ifenprodil (Ifen; 1-100 μM) did not have any effect against NMDA-induced excitotoxic cell death (p > 0.05, One Way ANOVA).
3.2. CGX-1007 inhibits both NR1a/NR2A and NR1a/NR2B containing NMDA receptors
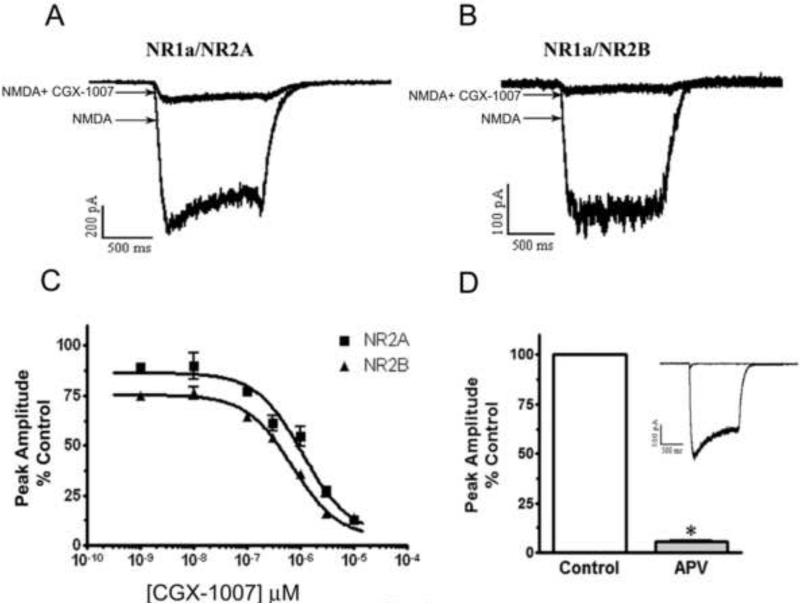
Previous studies of CGX-1007-induced inhibition of recombinant NMDA receptors had revealed both subunit selective and non-selective actions. However, the neuroprotection data we have obtained suggests that CGX-1007 may not be selective for NR2B containing receptors. The present set of experiments was designed to evaluate the subunit selectivity of CGX-1007 on NMDA receptors expressed in HEK 293 cells. HEK cells were transiently transfected with different combinations of NR1 and NR2 receptor subunits and recordings were made within 48 hrs post-transfection. Whole cell recordings of NMDA-gated currents evoked by NMDA (100 μM) and glycine (50 μM) were compared with that in the presence of CGX-1007. CGX-1007 (0.001-10 μM) inhibited NMDA-gated currents in cells expressing either NR2A or NR2B-containg NMDA receptors (Figure 3a, b, c). These NMDA-gated currents were sensitive to APV and were completely blocked by 100 μM of APV (Figure 3d).
Figure 3.
In HEK 293 cells expressing NR1a/NR2A and NR1a/NR2B receptors, CGX-1007 inhibits NMDA-gated currents in a concentration dependent manner. A, B: NMDA-gated currents evoked by NMDA (100 μM) and glycine (50 μM), as compared to NMDA response in the presence of CGX-1007 (3 μM). C: Plots of the concentration-response of CGX-1007 (0.001-10 μM) on NMDA-gated currents from cells transfected with NR1a/NR2A and NR1a/NR2B receptors. CGX-1007 inhibited the currents from both NR1a/NR2A and NR1a/NR2B containing receptors (p < 0.05, two-way ANOVA). D: NMDA-gated currents obtained from the cells expressing NR1a/NR2A and NR1a/NR2B receptors were completely inhibited by APV (100 μM).
The CGX-1007-induced inhibition of peak amplitude and area of the NMDA-gated currents were of the same magnitude in NR2A and NR2B containing receptors. At 3 μM of CGX-1007, 73 ± 7.8% inhibition of NMDA currents was seen in cells expressing NR2A-containing receptors, whereas, an inhibition of 83 ± 1.2% was seen at NR2B containing receptors. At the maximum concentration of CGX-1007 tested (10 μM), currents evoked in cells transfected with either NR1a/NR2A or NR1a/NR2B receptors were inhibited equally. The IC50 values for NR1a/NR2A and NR1a/NR2B receptors were 1.1 ± 0.15 μM and 0.6 ± 0.08 μM, respectively (Figure 3c). The concentration-response curve of CGX-1007 on NR2A and NR2B containing receptors showed only a 1.8 fold difference in their efficacy, which was similar to that observed by Wittekindt et al., (2001) in Xenopus oocytes. The present results confirm the action of CGX-1007 on both NR2A and NR2B-containing receptors, as suggested by studies on native NMDA receptors (Barton et al., 2004; Alex et al., 2006), although, the affinity of CGX-1007 at NR2B containing receptors was significantly higher than NR2A containing NMDARs.
3.3. CGX-1007 weakly inhibits NR2C- and has no effect on NR2D-containing receptors
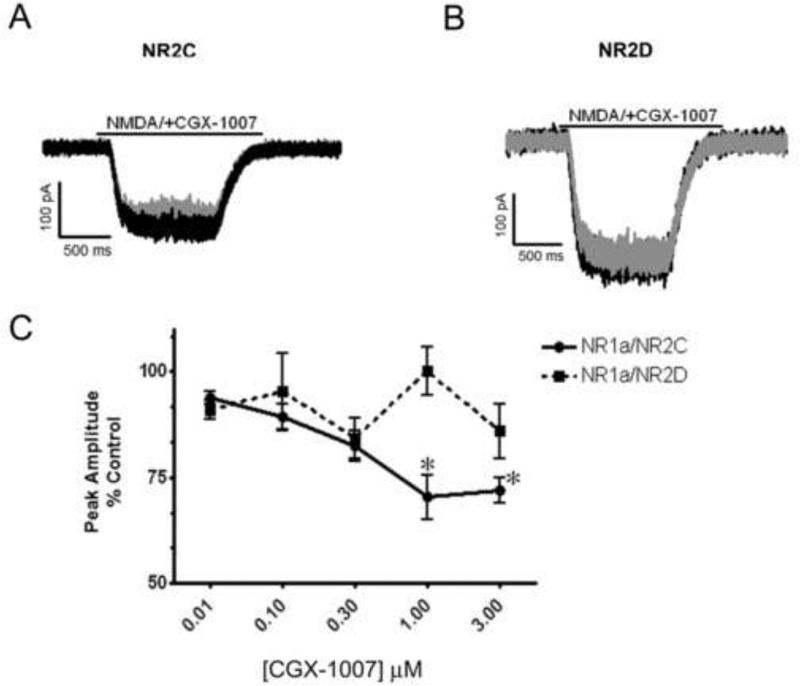
Since CGX-1007 inhibits both NR2A and NR2B-containing receptors, we further tested its effects on NMDA receptors composed of NR1a/NR2C and NR1a/NR2D subunits. CGX-1007 (0.01-3 μM) inhibited NMDA-gated currents in cells expressing NR1a/NR2C containing receptors. At the highest concentration tested (3 μM), a 28 ± 3% inhibition was observed (Figure 4a, c), revealing a weak potency of CGX-1007 at NR2C containing receptors. However, consistent with earlier reports CGX-1007 (Donevan and McCabe, 2000; Klein et al., 2001) had no significant effect on NR1a/NR2D-containing receptors at any of the concentrations tested in this study (Figure 4b, c).
Figure 4.
Effect of CGX-1007 on NR1a/NR2C and NR1a/NR2D containing receptors. A, B: Traces of NMDA-gated currents from NR1a/NR2C and NR1a/NR2D expressing cells. CGX-1007-induced a weak inhibition of the NMDA (100 μM) mediated currents in cells expressing NR2C subunit, while no significant inhibition was seen at NR1a/NR2D containing receptors. Grey traces represent inhibition of NMDA currents in the presence of CGX-1007 (3 μM), while black traces represent the control (NMDA alone) response. C: The concentration-response of CGX-1007 on NR2C and NR2D containing receptors. At the maximum concentration tested (3 μM), CGX-1007-induced a 30% inhibition on NR1a/NR2C receptors, where as it failed to produce any significant inhibition at NR1a/NR2D receptors. *, p < 0.05 as compared to control response (paired t-test).
3.4. Voltage-dependent block of NMDA-gated currents by CGX-1007
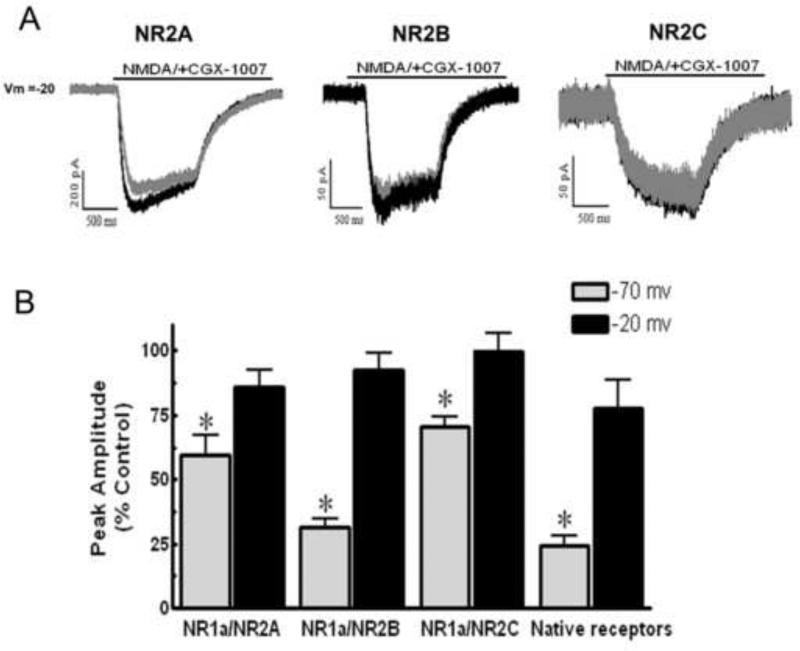
To investigate whether membrane potential affected the CGX-1007-induced inhibition of NMDA receptors, we compared NMDA-gated currents in both HEK cells and cortical neurons at different holding potentials. Our results in HEK cells indicate that CGX-1007 blocks NMDA-gated currents in a voltage-dependent fashion. At a holding potential of −20 mV, CGX-1007-induced inhibition of NMDA-gated currents was significantly less than that seen at −70 mV in all the receptor combinations studied. Currents evoked via activation of NR1a/NR2B containing receptors exhibited significantly less inhibition by CGX-1007 at −20 mV, as compared to that at −70 mV (p = 0.0001, Student's t- test). A decreased efficacy of CGX-1007 at −20 mV was also seen on HEK cells expressing NR1a/NR2A subunits, when compared to that seen at −70 mV (p < 0.05, Student's t- test). Inhibition of NMDA currents in cells expressing NR1a/NR2C receptors was completely eliminated at −20 mV (99.89 ± 6.7% of control), as compared to that observed at −70 mV (70.38 ± 4.1% of control; p = 0.007, Student's t- test). NR1a/NR2D transfected cells were not tested, as CGX-1007 (1 μM) did not show any significant effect on NMDA-gated currents at −70 mV. Similar results with native NMDA receptors were obtained in cortical neurons maintained in dissociated cell culture. CGX-1007-induced inhibition was significantly attenuated at depolarized membrane potentials (Figure 5b), similar to that seen with transfected HEK cells.
Figure 5.
A: NMDA-gated currents from HEK 293 cells expressing either NR1a/NR2A, NR1a/NR2B, or NR1a/NR2C containing receptors, recorded at a holding potential of -20 mV. Grey traces represent NMDA-gated currents in the presence of CGX-1007 (1 μM) and black traces represent the control response. B: CGX-1007-induced inhibition of NMDA currents was attenuated when the holding potential was -20 mV instead of -70 mV. CGX-1007 loses efficacy at native receptors when cultured cortical neurons are held at -20 mV (*, p > 0.05; Student's t-test).
4. Discussion
Excessive glutamate release as a result of various neurological insults induces substantial cell loss in the brain. Therefore, compounds with neuroprotective effects may be an excellent choice for acute treatment of status epilepticus and other CNS insults. CGX-1007, a 17 amino acid peptide toxin isolated from marine cone snail, has been shown to have anticonvulsant activity in several acute animal seizure models (Armstrong et al., 1998; Barton and White, 2004). The present study demonstrates for the first time that CGX-1007 confers significant neuroprotection against NMDA-induced excitotoxicity in organotypic hippocampal brain slice cultures. The subunit selectivity of CGX-1007 in mediating neuroprotection was evaluated using a heterologous expression system of HEK 293 cells, expressing different NMDA receptor subunit combinations. The data presented here indicates that CGX-1007 inhibits currents evoked in NMDA receptors containing NR2A, NR2B or NR2C subunits, with an order of efficacy of NR1a/NR2B> NR1a/NR2A >>NR1a/NR2C. The activity of CGX-1007 at a number of NMDA receptor types provides an explanation for its neuroprotective actions, since ifenprodil, a highly selective NR2B-containing receptor antagonist failed to prevent cell death following NMDA exposure in the hippocampal slice culture. The binding sites on the NMDA receptor for CGX-1007 and ifenprodil are quite different. While CGX-1007 is competitive antagonist thought to bind at the glutamate binding site of the receptor (Doneven and McCabe, 2000), ifenprodil is a non-competitive antagonist that may also have an indirect effect on NMDARs through an increase in proton inhibition (Williams, 2001) and allosteric modulation of the polyamine binding (Han et al., 2008).
4.1. CGX-1007 provides neuroprotection against NMDA-induced excitotoxic cell death
Organotypic slice cultures provide an excellent model for studying the pharmacology of excitotoxic cell death as they preserve the cellular organization of the hippocampus. Cell death can also be monitored and quantified directly in the live tissue by using a standardized PI uptake method (Noraberg et al., 1999) and additionally, it is possible to normalize the data for individual slices by re-imaging after treating with a saturating concentration of glutamate at the end of the experiment. The present data shows for the first time, that CGX-1007, a presumed antagonist of NR2B-containing receptors, prevented excitotoxic cell death induced by NMDA in hippocampal slice cultures. In contrast, ifenprodil, another NR2B selective antagonist, failed to exert any neuroprotective effects in this assay. Similar observations were made in cultured cortical neurons where ifenprodil (10 μM) lost the ability to protect neurons against NMDA-mediated toxicity with increasing days in culture (von Engelhardt et al., 2007). Likewise, while ifenprodil is unable to block NMDA-mediated EPSCs at excitatory synapses in thalamic brain slices prepared from adult animals, CGX-1007 retains the ability to block EPSCs at the same synapse in the same preparation (Smeal et al., 2008). In addition, CGX-1007 has also been found to inhibit NMDA-mediated EPSCs at the CA3-CA1 synapse in hippocampal brain slices, at a developmental time point when other NR2B selective antagonists lose efficacy (Barton et al., 2004). Finally, while there is a reduced potency of CGX-1007 at synaptic NMDA receptors that correlates with the developmental switch from NR2B to NR2A-containing NMDA receptors (Alex et al., 2006), it continues to inhibit synaptic NMDA receptors, while ifenprodil completely loses efficacy at synaptic NMDA-receptors known to make this switch (Zhou and Baudry, 2006; Martel et al., 2009; Stocca and Vicini, 1998). It has been suggested that synaptic NMDARs are neuroprotective, whereas extrasynaptic NMDARs preferentially initiate cell death pathways (Hardingham et al., 2002; Hardingham and Bading, 2010). However, it is not clear whether synaptic NMDARs can contribute to excitotoxic cell death during chronic activation. Moreover, majority of the mature synapses express tri-heteromeric NMDARs containing both NR2A and NR2B subunits (Luo et al., 1997), which has been shown to have low sensitivity to NR2B-selective antagonists (Hatton and Paoletti, 2005). Therefore, the neuroprotection data obtained here, in light of the studies mentioned above, suggested that CGX-1007 would be efficacious at a number of different types of NMDA receptors.
4.2. CGX-1007-induced inhibition of recombinant NMDA receptors
Additional evidence for the broader subunit selectivity of CGX-1007 comes from the inhibition of NMDA-gated currents from receptors expressed heterologously in HEK 293 cells. Our results indicate that CGX-1007 completely inhibits NMDA-gated currents from cells expressing either NR1a/NR2A or NR1a/NR2B receptors in a concentration-dependent manner. In addition, our results indicate that CGX-1007 also blocks NR2C containing receptors, albeit to a lesser extent. Inhibition of NMDA currents in NR1a/NR2A and NR1a/NR2C expressing HEK cells indicate that CGX-1007 may not be a NR2B selective antagonist as previously reported (Donevan and McCabe, 2000; Klein et al., 2001). Indeed, the data presented here is consistent with studies in Xenopus oocyte expression system that showed CGX-1007-induced inhibition at NR1a/NR2A and NR1a/NR2B containing receptors (Wittekindt et al., 2001; Ragnarsson et al., 2006).
4.3. Voltage dependence in the action of CGX-1007
We report here that CGX-1007-induced inhibition of NMDA-gated currents from HEK cells expressing recombinant NMDA receptors is voltage-dependent. Holding the cells at a depolarized membrane potential attenuated the CGX-1007-induced inhibition of NMDA currents in all the receptor combinations studied (Fig. 5). Indeed, in HEK 293 cells expressing either NR1a/NR2A, NR1a/NR2B, or NR1a/NR2C containing NMDA receptors, antagonism of the currents by CGX-1007 at -20 mV was significantly attenuated. While the effect of CGX-1007 was only evaluated on diheteromeric NMDA receptor combinations expressed in HEK 293 cells, voltage dependence of the action of CGX-1007 on triheteromeric (NR1a/NR2A/NR2B) NMDARs is not known. Reports suggest that the majority of mature synapses in adult rat cortex are triheteromeric NR1/NR2A/NR2B receptors (Luo et al., 1997; Stocca and Vicini, 1998; Tovar and Westbrook, 1999; Thomas et al., 2006). To verify whether changes in membrane potentials have an effect on CGX-1007-induced inhibition of native NMDA receptors, we performed experiments on cortical neurons in culture expressing native receptors at a time point in this culture system when NR2A and NR2B are both known to be expressed (Alex et al., 2006). Results indicate that the inhibition of NMDA-gated currents in natively expressed receptors by CGX-1007 is indeed sensitive to changes in the membrane potential.
In conclusion, this report provides the first direct evidence for the neuroprotective effects of CGX-1007 against excitotoxic neuronal injury. The high potency of CGX-1007 (EC50 0.44 ± 0.02 μM) in reducing NMDA-induced cell death is most likely due to its high affinity at multiple subtypes of NMDA receptors. The data presented herein provides evidence for CGX-1007-induced inhibition of NMDA receptors containing NR2B, NR2A and NR2C subunits, though the affinity at NR2B containing receptors is higher. Finally, our data supports the hypothesis that CGX-1007 is not as selective for the NR2B receptor subunit, as previously reported, which may account for its broad-spectrum anticonvulsant and neuroprotective effects.
Acknowledgements
The authors wish to thank Dr. David Lynch, University of Pennsylvania, for kindly providing the NMDA receptor subunit clones and helpful advices. We also thank Dr. Baldomero M. Olivera, Department of Biology, University of Utah for facilities and support and Ms. Amanda L. Pollock for technical assistance. This work was supported by GM-048677-11 to B. M. Olivera and K.S. Wilcox and NO1-NS-4-2359.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest
References
- Alex AB, Baucum AJ, Wilcox KS. Effect of Conantokin G on NMDA receptor-mediated spontaneous EPSCs in cultured cortical neurons. J Neurophysiol. 2006;96(3):1084–92. doi: 10.1152/jn.01325.2005. [DOI] [PubMed] [Google Scholar]
- Armstrong H, Zhou L, Layer R, Nielson J, McCabe RT, White HS. Anticonvulsant profile of Conantokin-G: a novel, broad spectrum NMDA antagonist. Epilpesia. 1998;36:39. [Google Scholar]
- Barton ME, White HS, Wilcox KS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on NMDA receptor-mediated EPSCs. Epilepsy Res. 2004;59(1):13–24. doi: 10.1016/j.eplepsyres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Barton ME, White HS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on kindling acquisition and expression. Epilepsy Res. 2004;59(1):1–12. doi: 10.1016/j.eplepsyres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7(8):2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–82. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Donevan SD, McCabe RT. Conantokin G is an NR2B-selective competitive antagonist of N- methyl-D-aspartate receptors. Mol Pharmacol. 2000;58(3):614–23. doi: 10.1124/mol.58.3.614. [DOI] [PubMed] [Google Scholar]
- Han X, Tomitori H, Mizuno S, Higashi K, Full C, Fukiwake T, Terui Y, Leewanich P, Nishimura K, Toida T, Williams K, Kashiwagi K, Igarashi K. Binding of spermine and ifenprodil to a purified, soluble regulatory domain of the N-methyl-D-aspartate receptor. J Neurochem. 2008;107(6):1566–77. doi: 10.1111/j.1471-4159.2008.05729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46(2):261–74. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Kendrick SJ, Dichter MA, Wilcox KS. Characterization of desensitization in recombinant N- methyl-D-aspartate receptors: comparison with native receptors in cultured hippocampal neurons. Brain Res Mol Brain Res. 1998;57(1):10–20. doi: 10.1016/s0169-328x(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18(6):1935–43. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Prorok M, Galdzicki Z, Castellino FJ. The amino acid residue at sequence position 5 in the conantokin peptides partially governs subunit-selective antagonism of recombinant N-methyl-D-aspartate receptors. J Biol Chem. 2001;276(29):26860–7. doi: 10.1074/jbc.M102428200. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron. 1998;20(2):317–27. doi: 10.1016/s0896-6273(00)80459-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27(11):2846–57. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J WY, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol. 1997;51(1):79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158(1):334–43. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Kristensen BW, Zimmer J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res Brain Res Protoc. 1999;3(3):278–90. doi: 10.1016/s1385-299x(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Noraberg J. Organotypic brain slice cultures: an efficient and reliable method for neurotoxicological screening and mechanistic studies. Altern Lab Anim. 2004;32(4):329–37. doi: 10.1177/026119290403200403. [DOI] [PubMed] [Google Scholar]
- Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol. 2002;61(4):921–7. doi: 10.1124/mol.61.4.921. [DOI] [PubMed] [Google Scholar]
- Ragnarsson L, Yasuda T, Lewis RJ, Dodd PR, Adams DJ. NMDA receptor subunit-dependent modulation by conantokin-G and Ala7-conantokin-G. J Neurochem. 2006;6(1):283–91. doi: 10.1111/j.1471-4159.2005.03574.x. [DOI] [PubMed] [Google Scholar]
- Sinor JD, Du S, Venneti S, Blitzblau RC, Leszkiewicz DN, Rosenberg PA, Aizenman E. NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro. J Neurosci. 2000;20(23):8831–7. doi: 10.1523/JNEUROSCI.20-23-08831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal RM, Keefe KA, Wilcox KS. Differences in excitatory transmission between thalamic and cortical afferents to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci. 2008;28(10):2041–52. doi: 10.1111/j.1460-9568.2008.06505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507( Pt 1):13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM. Novel conantokins from Conus parius venom are specific antagonists of N-methyl-D-aspartate receptors. J Biol Chem. 2007;282(51):36905–13. doi: 10.1074/jbc.M706611200. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95(3):1727–34. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19(10):4180–8. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13(6):594–615. doi: 10.1177/1073858406296259. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53(1):10–7. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Coyle JT. Direct observation of the agonist-specific regional vulnerability to glutamate, NMDA, and kainate neurotoxicity in organotypic hippocampal cultures. Exp Neurol. 1991;114(1):11–22. doi: 10.1016/0014-4886(91)90079-r. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–58. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Phillips JB, Lin Y, McCabe RT, Tortella FC. Neuroprotective efficacy and therapeutic window of the high-affinity N-methyl-D-aspartate antagonist conantokin-G: in vitro (primary cerebellar neurons) and in vivo (rat model of transient focal brain ischemia) studies. J Pharmacol Exp Ther. 2000;294(1):378–86. [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2(3):285–98. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Lu XM, Ling G, Tortella FC. Selective NR2B NMDA receptor antagonists are protective against staurosporine-induced apoptosis. Eur J Pharmacol. 2002;452(1):135–6. doi: 10.1016/s0014-2999(02)02327-0. [DOI] [PubMed] [Google Scholar]
- Wittekindt B, Malany S, Schemm R, Otvos L, Maccecchini ML, Laube B, Betz H. Point mutations identify the glutamate binding pocket of the N-methyl-D-aspartate receptor as major site of conantokin-G inhibition. Neuropharmacology. 2001;41(6):753–61. doi: 10.1016/s0028-3908(01)00112-5. [DOI] [PubMed] [Google Scholar]
- Xiao C, Huang Y, Dong M, Hu J, Hou S, Castellino FJ, Prorok M, Dai Q. NR2B-selective conantokin peptide inhibitors of the NMDA receptor display enhanced antinociceptive properties compared to non-selective conantokins. Neuropeptides. 2008;42(5-6):601–9. doi: 10.1016/j.npep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26(11):2956–63. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]