Semaphorins are cell surface proteins sharing an extracellular Sema motif that binds to plexin family receptors, among others [1]. We have shown previously that Sema4D is expressed by platelets, serving as a contact-dependent amplifier of platelet activation by promoting activation of the tyrosine kinase, Syk, downstream of the collagen receptor, GP VI/FcRγ [2–4]. Deletion of Sema4D impairs thrombus growth in mice by reducing the number of fully-activated, stably-adherent platelets in the region closest to the vessel wall [5]. Sema4D(−/−) platelets have diminished responses to collagen, but normal responses to thrombin, TxA2 and ADP [2, 4]. Collagen-induced Syk activation and the subsequent activation of phospholipase Cγ2 are most robust when Sema4D is present and platelets are allowed to form stable contacts [4].
These observations have helped to define the role of platelet Sema4D. In the setting of vascular injury, Syk is activated by binding to a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) in FcRγ. However, while our previous studies show that Sema4D does not amplify G protein-dependent signaling, left unsettled was whether signal amplification is specific for GPVI or applies to other ITAM receptors as well. Here we have addressed that issue. Human platelets express two other ITAM-containing receptors, Clec-2 and FcR-IIA. Mouse platelets express Clec-2 [6]. Unlike GPVI/FcRγ and FcR-IIA, Clec-2 contains only half of an ITAM motif or “hemi-ITAM” (YXXL). Signaling occurs when two molecules of phosphorylated Clec-2 engage a single molecule of Syk [6, 7]. The known Clec-2 ligands are the snake venom toxin, rhodocytin [6] and podoplanin. Podoplanin/Clec-2 interactions play an essential role in separating the lymphatic and vascular systems during embryonic development [8–10].
Reagents and procedures
Rhodocytin was purified from Calloselasma rhodosoma venom [11]. Two separate batches differing somewhat in potency were used for these studies. Anti-phosphotyrosine (4G10P; Millipore, Billerica, MA), anti-Syk (N19; Santa Cruz Biotechnology, Santa Cruz, CA), anti- Clec-2 (17D9; Abcam, Cambridge, MA), and anti-phospho-Syk Y519/520 (Cell Signaling, Danvers, MA, USA). Lec3.2.8.1 CHO cells stably expressing hSema4D (1–657) containing a C-terminal His tag [12] were provided by Dr. Yvonne Jones (University of Oxford). Sema4D(−/−) mice [13] were backcrossed onto a C57 BL/6 background for >10 generations. Comparisons were made with mice obtained from heterozygous crosses. Platelet isolation. Blood was collected from the inferior vena cava of anesthetized mice. Platelets were isolated by centrifugation and resuspended in modified Tyrode’s buffer. Immunoprecipitation and immunoblotting. Platelets were lysed with buffer (1% NP-40, 50 mM Tris, 150 mM NaCl, 1 mM EDTA) containing protease (Sigma-Aldrich) and phosphatase inhibitors (Calbiochem, San Diego, CA). Immunoprecipitation and immunoblotting were performed as described [4].
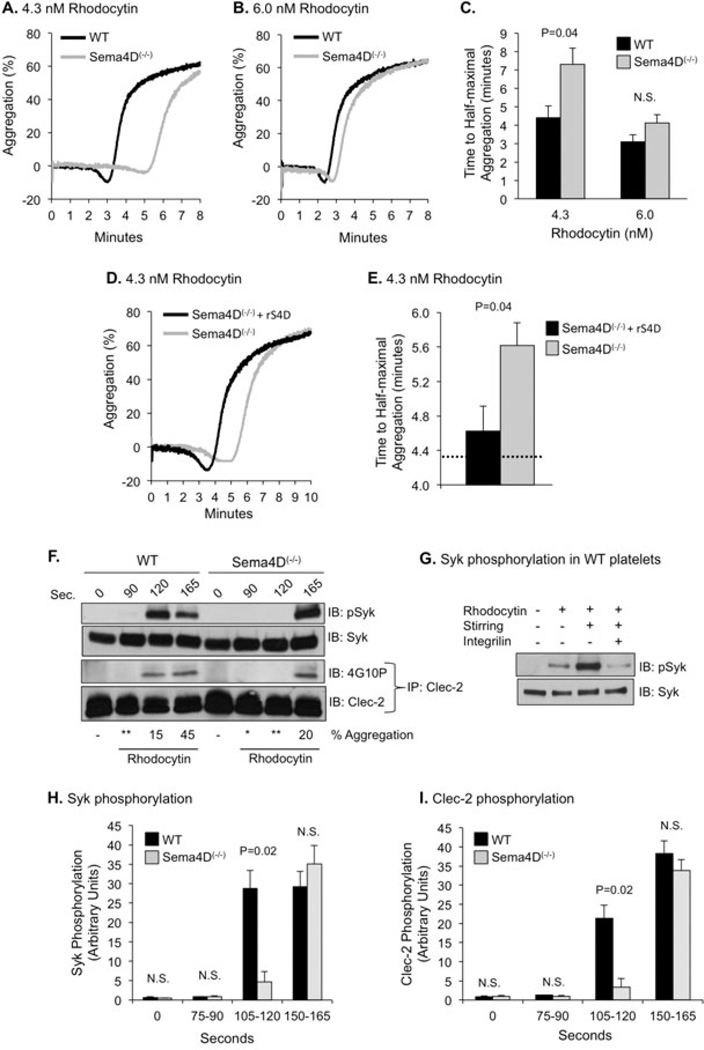
Loss of Sema4D expression produces a defect in rhodocytin-induced platelet aggregation that can be reversed with recombinant Sema4D
As others have noted, rhodocytin has a steep dose response curve [14]. Decreasing the concentration delays the onset of aggregation without markedly affecting the extent of aggregation (Figure 1A&B). Loss of Sema4D yielded a defect that could be overcome by raising the rhodocytin concentration (Figure 1B&C) or adding soluble Sema4D (Figure 1D&E). We next examined rhodocytin-induced Syk phosphorylation. In WT platelets, phosphorylation increased as the platelets began to aggregate (Figure 1F). This increase was blunted either by omitting stirring or by blocking aggregation with the αIIbβ3 antagonist, Integrilin, indicating that Clec-2-dependent Syk phosphorylation, like GPVI/FcRγ-dependent Syk phosphorylation, is contact-dependent (Figure 1G). Consistent with the aggregation studies, maximal Syk and Clec-2 phosphorylation were delayed in the absence of Sema4D (Figure 1F, H&I).
Figure 1. Platelet responses to rhodocytin in the absence of Sema4D.
(A&B) Platelets from Sema4D(+/+) (WT) and Sema4D(−/−) mice were stimulated with rhodocytin at time zero. (C) Mean ± SEM, N≥5. N.S. = not significant. (D&E) Sema4D(−/−) platelets were preincubated with 80 µg/ml recombinant Sema4D (rS4D) for 10 min at room temperature and stimulated with 4.3 nM rhodocytin at time zero. Mean ± SEM, N=6. The dashed line indicates the time to half-maximal aggregation for WT platelets rhodocytin for comparison (from Figure 1C). (F) Platelets were stimulated with 8.9 nM rhodocytin with stirring. The extent of aggregation is indicated at the bottom of each lane. * refers to platelets that have changed shape but have no measurable aggregation; ** platelets that have completed shape change and just begun to aggregate. Proteins were precipitated with anti-Clec-2 and immunoblotted with phosphotyrosine antibody, 4G10P. Lysates were also probed for Syk phosphorylated on Y519/520. (G) WT platelets were incubated with 10 µM Integrilin for 10 min followed by 8.9 nM rhodocytin for 150 sec with or without stirring as indicated. Lysates were probed for phospho-Syk. N=3. A representative immunoblot is shown. (H) Platelets were activated with 8.9 nM rhodocytin under stirred conditions. Syk phosphorylation was detected with anti-pSyk. Mean ± SEM, N=3. (I) Platelets were activated under stirred conditions with 8.9 nM rhodocytin. Phosphorylation of Clec-2 was detected by immunoprecipitating with anti-Clec-2 and blotting with 4G10P (N=3).
Thus, our studies show that Sema4D supports maximal Syk phosphorylation downstream of Clec-2 in a contact-dependent manner, just as it does for GPVI/FcRγ [4]. Notably, however, there are differences as well as similarities between Clec-2 and GPVI/FcRγ. As already noted, GPVI/FcRγ forms a 1:1 complex with Syk, while Clec-2 has a modified ITAM and forms a 2:1 complex. GPVI/FcRγ is phosphorylated by Src family members [15], while Clec-2 is phosphorylated by Syk in a positive feedback loop following rhodocytin-induced receptor clustering [14, 16]. Although we observed previously that GPVI/FcRγ phosphorylation occurs normally in Sema4D(−/−) platelets [4], here we found that loss of Sema4D impairs Clec-2 phosphorylation as well, presumably because of the involvement of Syk.
In summary, these results indicate for the first time that optimal Syk activation downstream of Clec-2 , like optimal activation downstream of GPVI, is dependent on contacts between platelets and on Sema4D. The observed reduction in Clec-2 signaling in the absence of Sema4D reflects a decrease in both Clec-2 phosphorylation and Syk activation. Collectively, these results suggest that the contribution of Sema4D in platelets applies to ITAM-containing receptors as a class, and is not limited to GPVI/FcRγ.
Acknowledgements
We thank Mark Kahn for critically reading the manuscript. These studies were supported by NIH P50-HL81012 (L.F.B.) and the American Heart Association 10POST3420003 (K.M.W.).
Footnotes
Author Contributions
K. M. Wannemacher designed the study, performed experiments, analyzed the data, and wrote the paper. H. Jiang performed experiments. P. R. Hess. performed experiments. Y. Shin and K. Suzuki-Inoue provided reagents/analytical tools. L. F. Brass designed the study, analyzed data and wrote the paper.
Conflict-of-interest disclosures
The authors declare no competing financial interests.
References
- 1.Siebold C, Jones EY. Structural insights into semaphorins and their receptors. Seminars in cell & developmental biology. 2012 doi: 10.1016/j.semcdb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wannemacher KM, Wang L, Zhu L, Brass LF. The role of semaphorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22:461–465. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wannemacher KM, Zhu L, Jiang H, Fong KP, Stalker TJ, Lee D, Tran AN, Neeves KB, Maloney S, Kumanogoh A, Kikutani H, Hammer DA, Diamond SL, Brass LF. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood. 2010;116:5707–5715. doi: 10.1182/blood-2010-04-279943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 7.Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, O'Callaghan CA, Futterer K, Watson SP. CLEC-2 activates Syk through dimerization. Blood. 2010;115:2947–2955. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 11.Shin Y, Morita T. Rhodocytin, a functional novel platelet agonist belonging to the heterodimeric C-type lectin family, induces platelet aggregation independently of glycoprotein Ib. Biochem Biophys Res Commun. 1998;245:741–745. doi: 10.1006/bbrc.1998.8516. [DOI] [PubMed] [Google Scholar]
- 12.Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, Yoshida K, Kikutani H. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 14.Severin S, Pollitt AY, Navarro-Nunez L, Nash CA, Mourao-Sa D, Eble JA, Senis YA, Watson SP. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hemimmunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286:4107–4116. doi: 10.1074/jbc.M110.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J Thromb Haemost. 2009;7:1192–1199. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]