Abstract
Scope
The cathelicidin antimicrobial peptide (CAMP) gene is induced by 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), lithocholic acid, curcumin, nicotinamide and butyrate. Discovering additional small molecules that regulate its expression will identify new molecular mechanisms involved in CAMP regulation and increase understanding of how diet and nutrition can improve immune function.
Methods and results
We discovered that two stilbenoids, resveratrol and pterostilbene, induced CAMP promoter-luciferase expression. Synergistic activation was observed when either stilbenoid was combined with 1α,25(OH)2D3. Both stilbenoids increased CAMP mRNA and protein levels in the monocyte cell line U937 and synergy was observed in both U937 and the keratinocyte cell line, HaCaT. Inhibition of resveratrol targets sirtuin-1, cyclic AMP production and the c-Jun N-terminal, phophoinositide 3 and AMP-activated kinases did not block induction of CAMP by resveratrol or synergy with 1α,25(OH)2D3. Nevertheless, inhibition of the extracellular signal-regulated 1/2 and p38 mitogen-activated protein kinases, increased CAMP gene expression in combination with 1α,25(OH)2D3 suggesting that inhibition of these kinases by resveratrol may explain, in part, its synergy with vitamin D.
Conclusions
Our findings demonstrate for the first time that stilbenoid compounds may have the potential to boost the innate immune response by increasing CAMP gene expression particularly in combination with 1α,25(OH)2D3.
Keywords: cathelicidin antimicrobial peptide, innate immunity, resveratrol, stilbenoid, vitamin D receptor
1 - Introduction
Modulating the expression of endogenous antimicrobial peptides (AMPs) or proteins provides a viable approach for boosting the innate immune response as bacterial pathogens are less likely to develop resistance to AMPs [1]. Nutrients consumed in our food or through dietary supplements may provide a practical means to improve immune function by increasing the expression of AMPs [2]. The human cathelicidin antimicrobial peptide (CAMP) gene is an ideal candidate for increasing barrier defense as the peptide is effective at killing a wide range of bacteria and is expressed by both immune and epithelial cells [3].
The expression of the human CAMP gene is induced by 1α,25(OH)2D3, lithocholic acid, butyrate, and vitamin B3 [4–10]. The first two compounds induce expression by acting as ligands for the vitamin D receptor (VDR) which binds to the CAMP gene promoter [4, 5], butyrate treatment increases PU.1 and CREB1 recruitment to the CAMP promoter [8, 11] and vitamin B3 increases C/EBPε binding to the CAMP promoter [10]. Based on a mammalian two-hybrid study, it was proposed that polyunsaturated fatty acids (PUFAs) may act as low affinity ligands like lithocholic acid and thus regulate VDR-target gene expression [12]. In this same study, curcumin was identified as novel ligand for the VDR in colon cancer cell and shown to induce CYP24A1 gene expression. Recently, we demonstrated that curcumin modestly induced CAMP gene expression through a VDR-independent pathway in myeloid and colon cells, but PUFAs did not [13].
In addition to the VDR, it was shown that the primary bile salt chenodeoxycholic acid (CDCA) induced the expression of the human CAMP gene in a biliary carcinoma cell line through the farnesoid X receptor (FXR) [14]. It was proposed that CDCA increased binding of FXR to the CAMP promoter and activated gene expression, but the binding site for FXR was not identified [14]. With the possibility of additional VDR ligands and other steroid hormone receptors binding to the VDRE in the CAMP promoter, we hypothesized that additional small molecules may modulate CAMP gene expression. The discovery of additional small-molecule regulators of the CAMP gene would increase our knowledge of the biologically relevant pathways involved in regulating CAMP gene expression and could lead to better understanding of how diet and nutrition affect immune function and/or the development of therapeutically useful natural compounds to boost the innate immune response.
To identify new compounds that regulate CAMP gene expression, the NIH Clinical Collection of 446 molecules that are being used in human clinical trials was screened in U937 myeloid cells transfected with the human cathelicidin promoter sequence cloned into the two-step transcriptional activator (TSTA) luciferase reporter construct [15]. We discovered that both resveratrol and pterostilbene activated the CAMP promoter and endogenous CAMP gene expression was induced in both myeloid and keratinocyte cell lines by either stilbenoid. Furthermore, when pterostilbene or resveratrol was combined with 1α,25(OH)2D3 or its analogs there was a significant synergistic increase in CAMP gene expression above levels for cells treated with either active vitamin D or the stilbenoid alone.
2 - Materials and Methods
2.1 - Cell Culture
The myeloid leukemia cell line U937 and the keratinocyte cell line HaCaT were grown in RPMI 1640 or DMEM, respectively, supplemented with 10% FBS and antibiotics (100 units penicillin/streptomycin; Life Technologies, Carlsbad, CA). Cells were treated with various combinations of compounds at concentrations and times indicated in the figure legends. Resveratrol, 1,25 (OH)2D3 and sirtinol were purchased from Sigma-Aldrich Corporation (St. Louis, MO); pterostilbene was purchased from VWR (Radnor, PA). The AMP kinase (AMPK) inhibitor BML-275 and adenylate cyclase inhibitor 2',3'-dideosyadenosine (2',3'-DDA) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The kinase inhibitors for ERK1/2 (AZD6244), p38 MAP kinase (SB203580), c-Jun kinase (SP600125) and PI3 kinase (LY294022) were all purchased from Selleck Chemicals (Houston, TX).
2.2 – Small Molecule Library Screen
A portion of the human CAMP promoter (nucleotides −693 to +14) [5] was cloned into the two-step transcriptional amplification vector that expresses firefly luciferase (FFL) and was kindly provided by Michael Carey, University of California at Los Angeles (Fig. 1) [15]. U937 (5 × 107) cells were transfected with 5 μg of the TSTA-CAMP-FFL and phTKRL that expresses Renilla luciferase (RL; Promega Corporation, Madison, WI) for normalization of FFL expression. Transfections were performed using the Neon System (Tip-100, 1400v, 30ms, 1 pulse) as described by the manufacturer (Life Technologies) and cells were incubated with RPMI1640 medium supplemented with 10% FBS and no antibiotics. At 8 h post transfection, the cells were evenly seeded into four 96-well plates with antibiotics and treated with control compounds (DMSO, ethanol or 1α,25(OH)2D3) or test compounds from the NIH Clinical Collection (NCC-003) (BioFocus DPI, Inc, Little Chesterford, UK) at a 10 μM concentration. At 24 h post-transfection, Dual-Glo Luciferase assays (Promega Corporation) were performed as instructed by the manufacturer and quantified using a SpectraMAXL luminometer (Molecular Devices, Sunnyvale, CA). Compounds that induced CAMP reporter activity were tested against the promoter-less TSTA vector to verify that induction was dependent on the presence of the CAMP promoter.
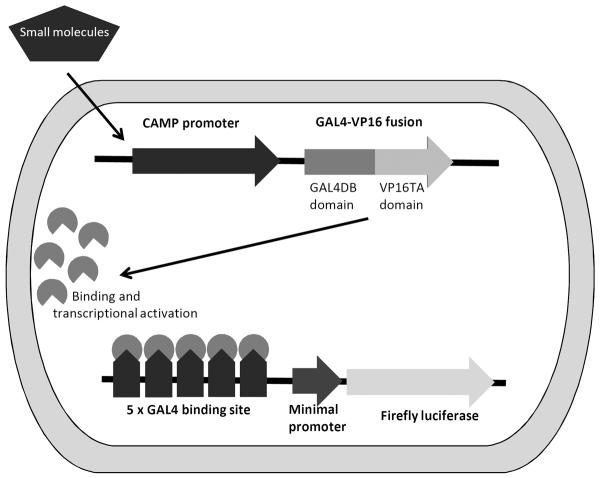
Figure 1. Schematic of TSTA-hCAMP-FFL reporter plasmid.
Small molecules that induce expression from the human CAMP promoter lead to the expression of the GAL4-VP16 fusion transcription activator protein. This transcriptional activator binds to the five GAL4 binding sites upstream of the minimal promoter driving expression of the firefly luciferase (FFL) gene. Activation of the CAMP promoter by the small molecule is indirectly measured by the amount of luciferase activity [15].
2.3 - RNA isolation and quantitative real-time PCR (QRT-PCR)
Total RNA from 2 × 106 U937 cells was prepared with Trizol as described by the manufacturer (Life Technologies). All cDNAs were synthesized from 2 μg of RNA using Superscript III reverse transcriptase as described by the manufacturer (Life Technologies). The cDNAs were analyzed by Q-PCR using Taqman probes specific for human CAMP, CYP24A1, β-actin and 18S rRNA as described previously [13]. Reactions were performed in triplicate for each sample, normalized to18S rRNA and the fold change was calculated using ΔΔCT values (treatment versus untreated) or the ratio of target gene/housekeeping gene (18S rRNA) was determined (ratio = 2-(Cttarget-Ct18S)). To determine statistical significance between two different means, a Student's T-test was performed (p < 0.05). To compare more than two means, ANOVA was performed followed by a Fisher's least significant difference procedure (p < 0.05).
2.4 - Flow Cytometery
U937 cells were treated with 10 nM 1α,25(OH)2D3 with or without 10 μM pterostilbene or resveratrol for 24 h. Cells were fixed, permeabilized, blocked and stained with primary and secondary or secondary antibody alone as described previously [13]. The primary antibody for hCAP-18 was rabbit anti-hCAP18, kindly provided by Niels Borregaard [16], and the secondary antibody was a Dylight 649 Fab' 2 donkey anti-rabbit (Jackson Immunoresearch, Pike West Grove, PA, USA). Fluorescence activated cell sorting (FACS) was performed on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and the results were analyzed by BD CellQuest™ Pro software (BD Biosciences).
3 - Results
3.1 – Chemical Library Screen
To screen chemical libraries for small molecule activators of CAMP gene expression, a two-step transcriptional activator (TSTA) reporter construct [15] containing 710 bp of the upstream promoter region (−696 to +14) of the CAMP gene was generated (Fig. 1). This strategy was utilized to augment the activity of the human CAMP promoter [5]. Rather than directly inducing the firefly luciferase gene (one-step activation), the CAMP promoter induces expression of a GAL4DBD-vp16 fusion protein, a very potent transcriptional activator, that binds to five GAL4 binding site repeats in the plasmid and thus driving expression of the firefly luciferase gene (two-step activation, Fig. 1). Using this reporter construct resulted in a 30–40-fold increase in absolute firefly relative light units (RLUs) as compared with the one-step construct (data not shown).
The expression of the CAMP gene is induced in the U937 myeloid leukemia cell line when it is treated with 1α,25(OH)2D3, LCA, butyrate or curcumin [5, 13, 17]; therefore, we selected this cell line for transfection with the TSTA-CAMP construct and the small molecule library screen. To verify that this system would detect activators of the CAMP gene, U937 cells were transfected with TSTA-CAMP and treated with ethanol or DMSO (both negative controls) or 100 nM 1α,25(OH)2D3 (positive control). Ethanol and DMSO did not activate the TSTA-CAMP construct, but 1α,25(OH)2D3 increased FFL activity by 3-4-fold. A Z-factor of 0.86 was calculated from three independent experiments indicating that the system would be robust enough to detect activators of the CAMP gene (data not shown).
The NIH Clinical Collection was screened and compounds that induced the TSTA-CAMP promoter construct 2-fold or greater compared to the DMSO control, without significantly decreasing RL activity, were retested in triplicate. Candidate compounds that consistently activated the TSTA-CAMP construct were tested in triplicate on U937 cells transfected with a promoter-less TSTA vector to exclude those compounds that non-specifically activated the backbone of the vector (data not shown). The NIH Clinical Collection compounds were also tested in combination with 10 nM 1α,25(OH)2D3 to identify small molecules that could cooperatively induce CAMP together with 1α,25(OH)2D3. Three compounds that passed all of the criteria for candidate activators, calcipitriene, resveratrol and pterostilbene, were used in subsequent experiments. Calcipitriene is a synthetic derivative or analog of 1α,25(OH)2D3 while resveratrol and pterostilbene belong to the stilbenoid class of compounds which are believed to have numerous health benefits. The identification of calcipitriene was not surprising because it, like 1α,25(OH)2D3, is a known VDR ligand and would be expected to induce CAMP gene expression. Activation by both VDR ligands demonstrated that the TSTA-FFL assay was robust enough to identify bona fide inducers of the CAMP gene.
3.2 – Induction of endogenous CAMP gene expression by candidate compounds
As a secondary screen, we tested the novel ability of resveratrol and pterostilbene to increase endogenous CAMP mRNA expression in cell culture. CAMP gene expression was consistently induced 2–4 fold in U937 cells treated with 10 μM resveratrol or pterostilbene as compared to controls (Fig. 2A). Furthermore, combining either pterostilbene or resveratrol (10 μM) with 1α,25(OH)2D3 (10 nM) induced CAMP levels about 3-fold higher than 1α,25(OH)2D3 alone (Fig. 2B & C).
Figure 2. Induction of endogenous CAMP gene expression by stilbenoid compounds.
(Panel A) U937 cells were treated with either vehicle (untreated) 10 μM pterostilbene (PTR) or resveratrol (RSV) for 18 h. Synergistic induction of CAMP gene expression by both stilbenoid compounds and 1,25(OH)2 D3. U937 cells were treated with 1,25(OH)2D3 and either without (w/out) or with (w/) 10 μM PTR (panel B) or RSV (panel C). Levels of CAMP gene expression were measured by qRT-PCR and normalized to 18S rRNA levels. Results are shown as fold change compared to cells without the stilbenoid (panel A) or 1,25(OH)2 D3 (panels B and C). Statistical significance was determined using a Student's t-test, *p=0.01; **p<0.0001, #p<0.05 and ##p<0.01.
To determine if resveratrol specifically modulated expression of the CAMP gene or vitamin D target genes in general, we examined the response of another VDR target gene, CYP24A1, and a non-VDR target gene, β-actin (Supporting Information Fig. 1). 1α,25(OH)2 D3 strongly induced CYP24A1 mRNA expression, but resveratrol did not (Supporting Information Fig. 1A). In addition, a combinatorial induction was not observed with resveratrol and 1α,25(OH)2 D3 (Supporting Information y Fig. 1A). The expression of β-actin was not induced by either 1α,25(OH)2 D3, resveratrol or a combination of both (Supporting Information Fig. 1B). Taken together, the data suggest that resveratrol primarily modulates CAMP gene expression and that it is not due to a non-specific transcriptional effect.
Human CAMP gene expression is induced by 1α,25(OH)2 D3 in keratinocytes [4, 6]. To determine if the stilbenoids would also induce CAMP in keratinocytes, HaCat cells were treated with resveratrol at 10 μM or 1α,25(OH)2D3 at 10 nM alone or a combination of both. There was no significant increase in CAMP expression in cells treated with resveratrol alone when compared to the untreated control (Fig. 3). Cells treated with 1α,25(OH)2D3 showed a small increase in CAMP expression; however, in combination with resveratrol there was an approximately three-fold increase over 1α,25(OH)2D3 alone (Fig. 3).
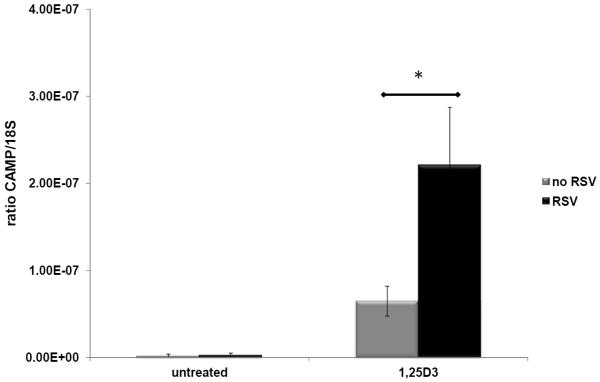
Figure 3. Induction of endogenous CAMP gene expression by resveratrol (RSV) in combination with vehicle (untreated) or 1,25(OH)2D3 (1,25D3) in human keratinocytes.
The human HaCaT cell line was treated with either ethanol vehicle, 10 μM RSV, 10 nM 1,25(OH)2D3 or a combination for 18 h. Levels of CAMP gene expression were measured by qRT-PCR and normalized to 18S rRNA levels. Results are shown as a ratio of CAMP/18S. Statistical significance was determined using a Student's t-test, *p=0.0007. Data are from two-independent experiments.
3.3 – CAMP Protein Expression
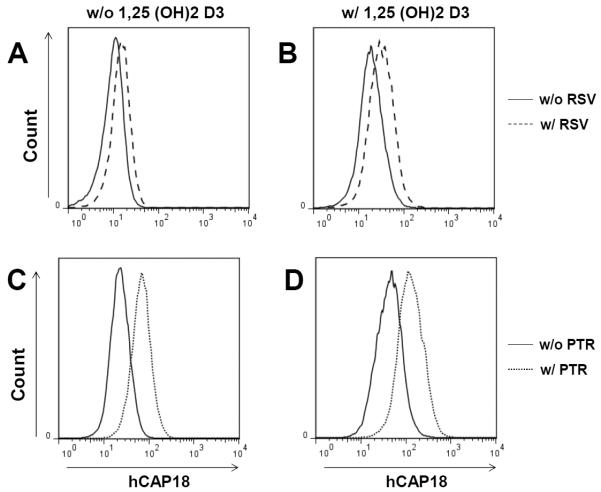
To determine if stilbenoids induced CAMP protein (hCAP18) levels, intracellular staining and FACS for hCAP18 was used to determine changes in protein expression (Fig. 4). As expected, U937 cells treated with 1α,25(OH)2D3 (1 nM) for 24 h (Fig. 4 B and D, solid curves) showed a significant shift to the right in the population's mean fluorescent intensity compared with untreated cells (Fig. 4 A and C) indicating induction of hCAP18. A modest shift was observed in cells treated with either resveratrol or pterostilbene (10 μM) without 1α,25(OH)2D3 indicating that both stilbenoids induced hCAP18 protein expression (Fig. 4 A and C, dashed or dotted curves versus solid curves). Cells incubated with either resveratrol or pterostilbene (10 μM) together with 1α,25(OH)2D3 (1 nM) showed increased hCAP18 protein expression with mean fluorescent intensities higher than those with either compound alone (Fig. 4 B and D, dashed or dotted curves versus solid curves). These results were consistent with the levels of induction of CAMP mRNA observed in U937 cells.
Figure 4. Induction of cathelicidin protein (hCAP18) expression in U937 cells by stilbenoid compounds.
U937 cells were treated with either 10 μM resveratrol (RSV, panel A) or 10 μM pterostilbene (PTR, panel C) alone or in combination with 1 nM 1,25(OH)2D3 (Panels B and D) for 24 h. Intracellular staining for hCAP18 and FACS was used to determine the expression level of hCAP18 in the cells. Results are representative of two independent experiments.
3.4 –Mechanism of Induction of CAMP by Stilbenoids
The molecular targets that mediate the effects of resveratrol are numerous and include siurtuins, cyclo- and lipooxygenases, reductases, protein kinases and transcription factors [18]. We tested several potential resveratrol targets to determine the molecular mechanism by which it increased CAMP gene expression.
Activation of Sirt1
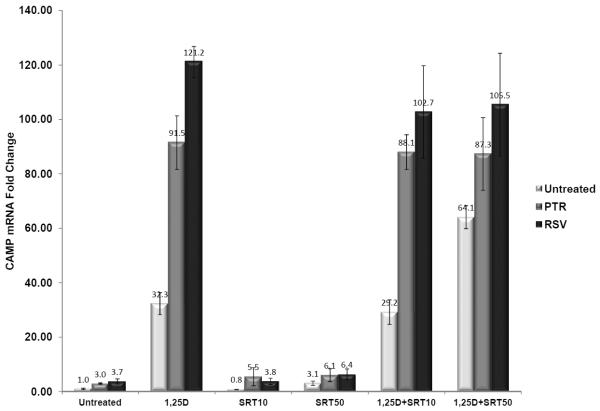
The metabolic effects of resveratrol are tied to its ability to indirectly activate Sirt1 in vivo [19–22]. To determine if activation of Sirt1 was involved in the induction of CAMP gene expression, we treated cells with the Sirt1 inhibitor sirtinol [23]. Pterostilbene and resveratrol induced CAMP gene expression to similar levels in both untreated and sirtinol-treated U937 cells and sirtinol did not interfere with the synergy of 1α,25(OH)2D3 when combined with either stilbenoid (Fig. 5). Furthermore, NAM, another Sirt1 inhibitor, had no effect on CAMP gene expression in U937 cells (data not shown). Taken together, the data do not support a role for Sirt1 activation in the induction of the CAMP gene by either stilbenoid.
Figure 5. Inhibition of SIRT1 does not block stilbenoid-mediated CAMP induction.
Effects of sirtinol on the synergistic induction of CAMP by 1,25(OH)2D3 and either stilbenoid compound. U937 cells were treated with 10 μM of either pterostilbene (PTR) or resveratrol (RSV), and with or without 1nM 1,25(OH)2D3 (1,25D) or Sirtinol (10 or 50 μM, [SRT10 or SRT50], respectively) for 18 h. Sirtinol did not inhibit CAMP mRNA induction by either PTR or RSV. CAMP gene expression was determined by qRT-PCR and normalized to 18S rRNA levels. Changes in gene expression are represented as fold-change compared to the untreated control (first bar graph). Data presented are from one experiment, but representative of results from three individual experiments.
Activation of cAMP signaling
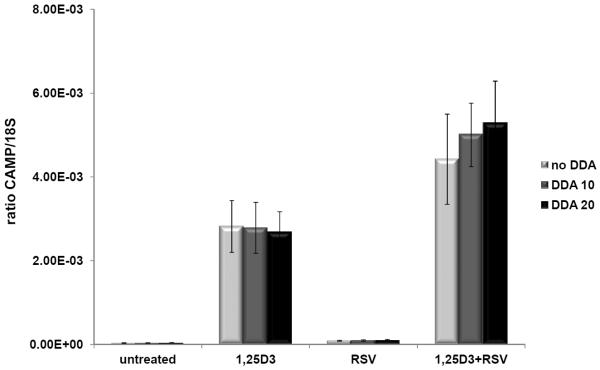
Resveratrol increases cAMP levels by inhibiting cAMP-degrading phosphodiesterases (PDEs) ultimately leading to the activation of the CamKKβ-AMPK pathway [24]. This pathway activates both PGC-1α and Sirt1 and may explain the metabolic effects of resveratrol [24]. cAMP signaling is very complex and numerous other transcription factors are activated including the cAMP responsive element binding protein 1 (CREB1) [25]. cAMP signaling induces CAMP gene expression in mucosal epithelial cells via activation of the CREB1 and activator protein-1 (AP-1) transcription factors [11]. To determine if an increase of cAMP levels mediated the induction of CAMP by resveratrol, we pretreated U937 cells with the adenyl cyclase inhibitor 2',5'-dideoxyadenosine (2',3'-DDA) to block the production of cAMP, but CAMP induction by resveratrol was not blocked (Fig. 6). Furthermore, cells treated with the PDE inhibitor rolipram, which mimics resveratrol by increasing cAMP levels, did not increase cathelicidin expression (data not shown) nor did stimulating cAMP production with forskolin (data not shown). Taken together these data do not support a role for increased cAMP levels in mediating the induction of CAMP expression in U937 monocytic cells by resveratrol or pterostilbene.
Figure 6. 2',3'-dideoxyadenosine, a cAMP pathway inhibitor, did not affect resveratrol (RSV)-enhanced hCAMP expression.
U937 cells were treated with combinations of vehicle (untreated), 1,25(OH)2D3 (1,25D3) or RSV in the presence of 2',3'-dideoxyadenosine (DDA) at 10 or 20 mM (DDA 10 or DDA 20, respectively) for 18 h. Levels of CAMP gene expression were measured by QRT-PCR and normalized to 18S rRNA levels. Results are shown as a ratio of CAMP/18S. The data represent two-independent experiments combined.
Modulation of Erk1/2, p38 MAPK, JNK, PI3K and AMPK pathways
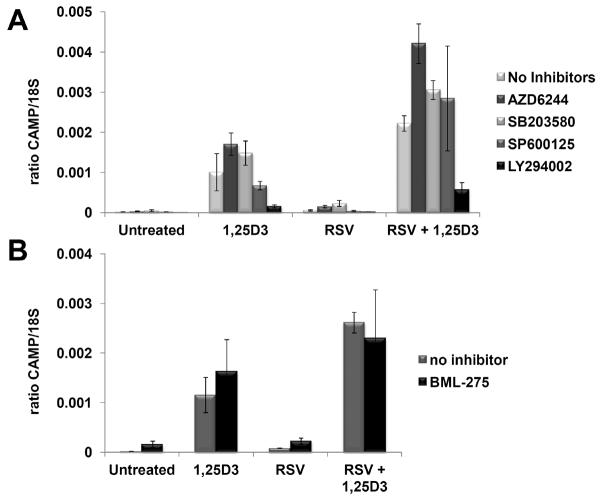
Resveratrol modulates the MAPK, PI3K/AkT and AMPK signaling pathways [18]. To determine if one or more of these pathways is involved in the action of resveratrol on the induction of CAMP gene expression, we treated U937 cells with inhibitors of these kinases and determined the effect they had on CAMP induction with or without 1α,25(OH)2D3. Numerous studies in different cell culture systems have demonstrated that resveratrol inhibits MAPK activity [26–28]. In U937 cells treated with the MAPK inhibitors AZD6244 (ERK1/2), SB203580 (p38 MAPK) and SP600125 (JNK), none of the inhibitors alone or in combination with resveratrol induced CAMP gene expression (Fig. 7A, Untreated) nor did they enhance or impair induction of the CAMP gene in a statistically significant manner (Fig. 7A, RSV). In combination with 1α,25(OH)2D3, ERK1/2 and p38 MAPK inhibitors increased CAMP expression about 50–70% higher than 1α,25(OH)2D3 alone (Fig. 7A, 1,25D3). However, neither was as effective as resveratrol which increased CAMP expression >200% above 1α,25(OH)2D3 alone (Fig. 7A, 1,25D3) and inhibition of JNK did not affect CAMP induction by 1α,25(OH)2D3. Inhibition of ERK1/2, p38 MAPK or JNK did not block the synergy observed with the combination of resveratrol and 1,25(OH)2D3 and, in fact, CAMP levels were increased above those seen with the combination alone (Fig. 7A, RSV + 1,25D3). These increases were likely due to the effect of these inhibitors on the induction by 1α,25(OH)2D3.
Figure 7. Inhibition of the MAPK, PI3K and AMPK pathways does not block the effect of resveratrol (RSV) on CAMP gene expression.
(Panel A) U937 cells were treated with combinations of vehicle (untreated), 1,25(OH)2D3 (1,25D3) or RSV in the presence or absence of inhibitors for ERK1/2 (AZD6244), p38 MAP kinase (SB203580), c-Jun kinase (SP600125) and PI3 kinase (LY294022) for 18 h. (Panel B) U937 were treated as described in panel A, but an inhibitor for AMP kinase, BML-275 was used. Levels of CAMP gene expression were measured by qRT-PCR and normalized to 18S rRNA levels. The data are from two individual experiments combined. Results are shown as a ratio of CAMP/18S.
Resveratrol inhibits PI3K activity [29] and so we tested the effect of PI3K inhibition on induction of the CAMP gene by 1α,25(OH)2D3. Induction of CAMP by 1α,25(OH)2D3 alone or in combination with resveratrol was inhibited by the PI3K inhibitor LY294002, but the synergy of 1α,25(OH)2D3 and resveratrol was still maintained (Fig. 7A, 1,25D3 vs RSV+1,25D3). The inhibition of VDR target genes by PI3K inhibition was described previously and suggests that the overall reduction in CAMP expression is due to the effect of LY294002 on the vitamin D receptor [30, 31].
Resveratrol activates the AMPK pathway [32–34]; therefore, we tested the effect of AMPK inhibition on induction of the CAMP gene by 1α,25(OH)2D3. The AMPK inhibitor BML-275 had no statistically significant effect on the ability of 1α,25(OH)2D3 to induce CAMP expression nor was the synergy with resveratrol affected by BML-275 (Fig. 7B).
Taken together, the data suggests that the inhibition of ERK1/2 and p38 MAPK by resveratrol may contribute to the enhanced expression of the CAMP gene observed with the combination of resveratrol and 1α,25(OH)2D3, but that modulation of JNK, PI3K and AMPK activities by resveratrol do not play a role in the synergy observed between 1α,25(OH)2D3 and resveratrol.
3.5 – Combinatorial induction of CAMP gene expression by stilbenoids and 1α,25(OH)2D3 analogs
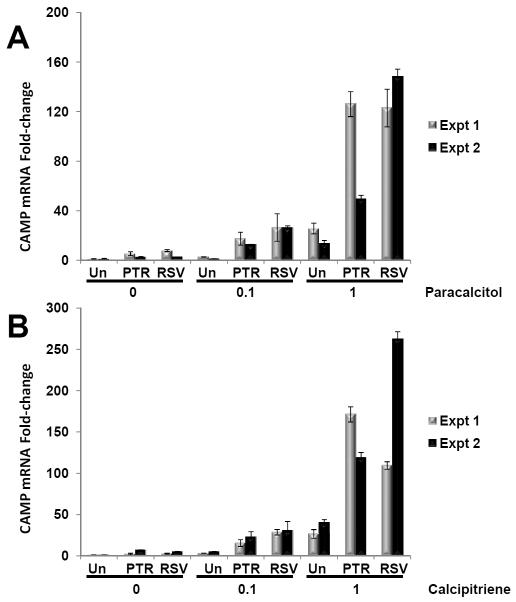
Synthetic analogs of 1α,25(OH)2D3 are used clinically because they have a similar or higher affinity for the VDR, but display significantly less activity in regulating calcium metabolism and causing hypercalcemia as does 1α,25(OH)2D3 [35]. We tested whether a combination of stilbenoid with calcipitriene (Dovonex, Leo Pharma, Inc., Parsippany, NJ), an analog used topically to treat plaque psoriasis, and paricalcitol (Zemplar, Abbott Laboratories, Abbott Park, IL), another analog used to prevent or treat secondary hyperparathyroidism associated with chronic renal failure, would induce CAMP expression in U937 cells (Fig. 8). Both resveratrol and pterostilbene induced CAMP mRNA levels three-to-20-fold higher than paracalcitol alone (Fig. 8A) and four-to-eight-fold higher than calcipitriene alone (Fig. 8B). These data demonstrate that both stilbenoids synergistically activate CAMP gene expression with vitamin D analogs.
Figure 8. Synergistic induction of CAMP gene expression by stilbenoids and vitamin D analogs used in the clinic.
U937 cells were treated with vehicle (0) or vitamin D analog (0.1 or 1 nM paracalcitol or calcipitriene) in absence (Un) or presence of pterostilbene (PTR) or resveratrol (RSV) for 18 h. Levels of CAMP gene expression were measured by QRT-PCR and normalized to 18S rRNA levels. Changes in gene expression are represented as fold-change compared to the untreated control (no analog or stilbenoid). Data from two individual experiments are shown.
4 – Discussion
Screening of the NIH Clinical Collection of 446 compounds led to the novel discovery of two stilbenoids that induce the human CAMP gene. Although the induction of CAMP by resveratrol and pterostilbene was modest, they synergistically induced CAMP gene expression when combined with 1α,25(OH)2D3. This synergy was observed in both monocyte and keratinocyte cell lines. The only other bona fide inducer identified in the collection was calcipitriene, a 1α,25(OH)2 D3 analog.
Resveratrol has numerous well-documented health benefits; however, its mechanisms of action remain unclear because direct molecular targets of resveratrol are numerous and difficult to identify [18]. We tested the potential role for several molecular targets in mediating the effects of resveratrol on vitamin D induction of CAMP gene expression. This included activation of Sirt1 and cAMP production as well as the inhibition of MAPK, PI3K and AMPK activities. These pathways do not appear to be involved in the synergy that we observe, but the inhibition of ERK1/2 and p38 MAPK enhanced 1α,25(OH)2D3 induction of CAMP suggesting that the effect of resveratrol on CAMP expression may be due, in part, to the inhibition of these kinases. Expression of the VDR target gene CYP24A1 was not enhanced by resveratrol alone or in combination with 1α,25(OH)2D3 suggesting that the effect on CAMP expression was not due to an enhancement of vitamin D-signaling in general. The differential recruitment of transcriptional factors or cofactors to the CAMP gene promoter remains to be determined.
Resveratrol has been shown to induce endoplasmic reticulum stress and we have observed increased XBP-1 splicing in our cells treated with resveratrol (data not shown) [36, 37]. Furthermore, Park and colleagues showed that endoplasmic reticulum stress induced with either thapsigargin (Tg) or tunicamycin increased expression of the CAMP gene in HaCaT and normal human keratinocytes [38]. Nevertheless, they demonstrated that the induction of endoplasmic reticulum stress in the presence of 1α,25(OH)2D3 did not show a synergistic effect, but instead suppressed vitamin D-induced CAMP expression [38]. These findings would indicate that endoplasmic reticulum stress induced by resveratrol does not contribute to the synergy that we observed in this study.
Although, the mechanism by which resveratrol induces CAMP gene expression remains unclear, the discovery that resveratrol in combination with vitamin D enhances CAMP gene expression is intriguing and consistent with previous findings that a number of natural small molecules regulate CAMP expression [2]. The potential of combining vitamin D with stilbenoids to improve immunity remains to be determined. Bioavailability of stilbenoids upon their oral consumption is a problem as they are metabolized into glucuronated and sulfonated byproducts by the intestine and liver [39]. Nevertheless, topical applications to improve barrier defense in wounds or infections could be envisioned as active forms of vitamin D are used to treat psoriasis and resveratrol is used in cosmetics [40–42]. Interestingly, topical resveratrol inhibits herpes simplex virus replication in vitro and in vivo in mice [43, 44]. Future work is required to determine if vitamin D alone or in combination with resveratrol will be useful for boosting the innate immune response or barrier defense against infection.
Supplementary Material
Acknowledgements
The authors thank Michael Carey (University of California at Los Angeles) for the two-step transcriptional amplification vector that expresses firefly luciferase (FFL), Niels Borregaard (University of Copenhagen, Denmark) for the hCAP18 antibody and Siva Kolluri (Oregon State University) for expert advice on screening the small molecule library. This research was supported by a grant from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (5R01AI65604) to AFG.
Abbreviations
- (1α,25 (OH)2 D3)
1α,25 hydroxyvitamin D3
- (CAMP)
cathelicidin antimicrobial peptide
- (TSTA)
Two Step Transcriptional Activator
- (VDR)
Vitamin D Receptor
Footnotes
The authors have declared no conflict of interest.
5. References
- [1].Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- [2].Campbell Y, Fantacone ML, Gombart AF. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur J Nutr. 2012;51:899–907. doi: 10.1007/s00394-012-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- [4].Wang TT, Nestel FP, Bourdeau V, Nagai Y, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- [5].Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- [6].Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schauber J, Oda Y, Buchau AS, Yun QC, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- [8].Termen S, Tollin M, Rodriguez E, Sveinsdottir SH, et al. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Mol Immunol. 2008;45:3947–3955. doi: 10.1016/j.molimm.2008.06.020. [DOI] [PubMed] [Google Scholar]
- [9].Peric M, Koglin S, Dombrowski Y, Gross K, et al. VDR and MEK-ERK dependent induction of the antimicrobial peptide cathelicidin in keratinocytes by lithocholic acid. Mol Immunol. 2009;46:3183–3187. doi: 10.1016/j.molimm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- [10].Kyme P, Thoennissen NH, Tseng CW, Thoennissen GB, et al. C/EBPepsilon mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J Clin Invest. 2012;122:3316–3329. doi: 10.1172/JCI62070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chakraborty K, Maity PC, Sil AK, Takeda Y, Das S. cAMP stringently regulates human cathelicidin antimicrobial peptide expression in the mucosal epithelial cells by activating cAMP-response element-binding protein, AP-1, and inducible cAMP early repressor. J Biol Chem. 2009;284:21810–21827. doi: 10.1074/jbc.M109.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bartik L, Whitfield GK, Kaczmarska M, Lowmiller CL, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21:1153–1161. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem. 2012;24:754–759. doi: 10.1016/j.jnutbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].D'Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–1443. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- [15].Iyer M, Wu L, Carey M, Wang Y, et al. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci U S A. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- [17].Gombart AF, O'Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)2D3 in various tissues. J Steroid Biochem Mol Biol. 2007;103:552–557. doi: 10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- [18].Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- [19].Kaeberlein M, McDonagh T, Heltweg B, Hixon J, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- [20].Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- [21].Beher D, Wu J, Cumine S, Kim KW, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- [22].Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- [24].Park SJ, Ahmad F, Philp A, Baar K, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoeffler JP, Meyer TE, Yun Y, Jameson JL, Habener JF. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- [26].El-Mowafy AM, White RE. Resveratrol inhibits MAPK activity and nuclear translocation in coronary artery smooth muscle: reversal of endothelin-1 stimulatory effects. FEBS Lett. 1999;451:63–67. doi: 10.1016/s0014-5793(99)00541-4. [DOI] [PubMed] [Google Scholar]
- [27].Yu R, Hebbar V, Kim DW, Mandlekar S, et al. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol. 2001;60:217–224. doi: 10.1124/mol.60.1.217. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hmama Z, Nandan D, Sly L, Knutson KL, et al. 1alpha,25-dihydroxyvitamin D(3)-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J Exp Med. 1999;190:1583–1594. doi: 10.1084/jem.190.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dwivedi PP, Gao XH, Tan JC, Evdokiou A, et al. A role for the phosphatidylinositol 3-kinase--protein kinase C zeta--Sp1 pathway in the 1,25-dihydroxyvitamin D3 induction of the 25-hydroxyvitamin D3 24-hydroxylase gene in human kidney cells. Cell Signal. 2010;22:543–552. doi: 10.1016/j.cellsig.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [32].Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- [33].Baur JA, Pearson KJ, Price NL, Jamieson HA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Park CE, Kim MJ, Lee JH, Min BI, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- [35].Kenny EE, Pe'er I, Karban A, Ozelius L, et al. A genome-wide scan of Ashkenazi Jewish Crohn's disease suggests novel susceptibility loci. PLoS Genet. 2012;8:e1002559. doi: 10.1371/journal.pgen.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Park JW, Woo KJ, Lee JT, Lim JH, et al. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- [37].Wang FM, Galson DL, Roodman GD, Ouyang H. Resveratrol triggers the proapoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp Hematol. 2011;39:999–1006. doi: 10.1016/j.exphem.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park K, Elias PM, Oda Y, Mackenzie D, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- [40].Guilhou JJ. The therapeutic effects of vitamin D3 and its analogues in psoriasis. Expert Opin Investig Drugs. 1998;7:77–84. doi: 10.1517/13543784.7.1.77. [DOI] [PubMed] [Google Scholar]
- [41].Bernard P, Berthon JY. Resveratrol: an original mechanism on tyrosinase inhibition. Int J Cosmet Sci. 2000;22:219–226. doi: 10.1046/j.1467-2494.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- [42].Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008;7:2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- [43].Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, et al. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999;43:145–155. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- [44].Docherty JJ, Smith JS, Fu MM, Stoner T, Booth T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 2004;61:19–26. doi: 10.1016/j.antiviral.2003.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.