Abstract
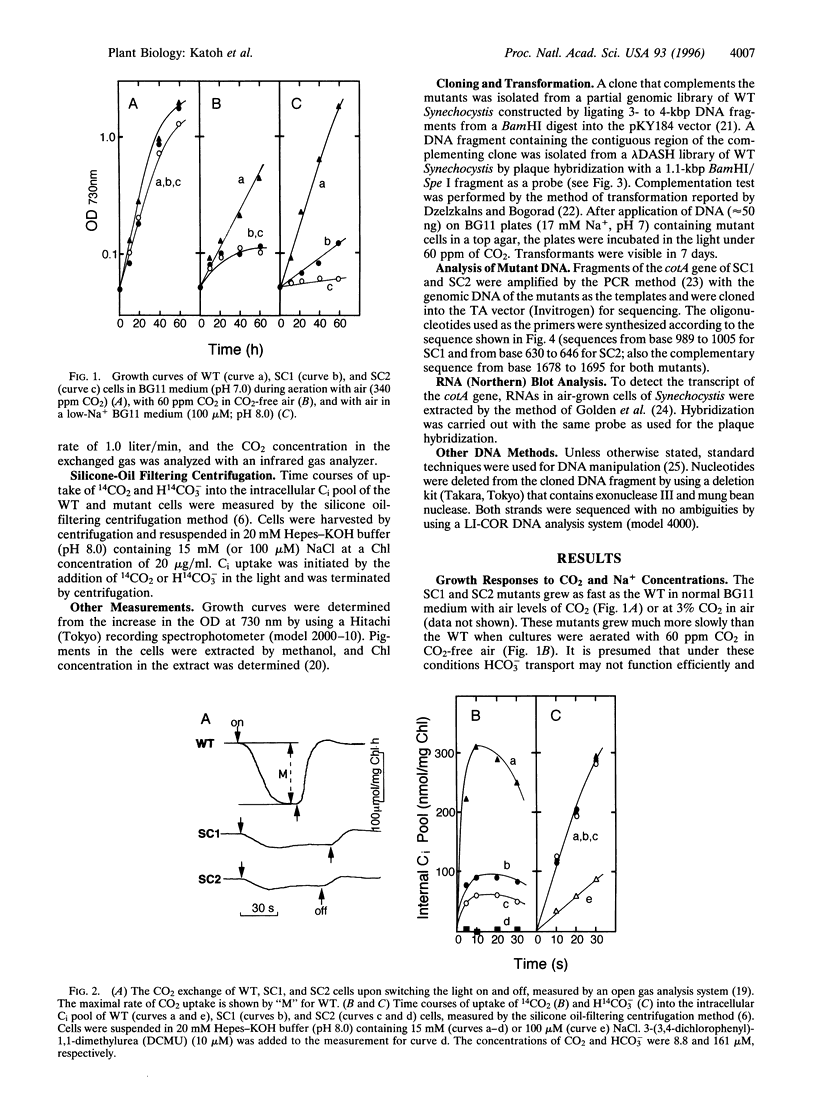
We have isolated mutants of Synechocystis PCC6803 that grew very slowly in a low-sodium medium, which is unfavorable for HCO3(-) transport, and examined two of these mutants (SC1 and SC2) for their ability to take up CO2 and HCO3(-) in the light. The CO2 transport activity of SC1 and SC2 was much lower than that of the wild type (WT), whereas there was no difference between the mutants and the WT in their activity of HCO3(-) transport. A clone containing a 3.9-kilobase-pair insert DNA that transforms both mutants to the WT phenotype was isolated from a genomic library of WT Synechocystis. Sequencing of the insert DNA in the region of mutations in SC1 and SC2 revealed an open reading frame (designated cotA), which showed significant amino-acid sequence homology to cemA encoding a protein found in the inner envelope membrane of chloroplasts. The cotA gene is present in a single copy and was not cotranscribed with any other gene(s). cotA encodes a protein of 247 amino acids containing four transmembrane domains. There was substitution of a single base in SC1 and two bases in SC2 in their cotA genes. A possible role of the cotA gene product in CO2 transport is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dzelzkalns V. A., Bogorad L. Molecular analysis of a mutant defective in photosynthetic oxygen evolution and isolation of a complementing clone by a novel screening procedure. EMBO J. 1988 Feb;7(2):333–338. doi: 10.1002/j.1460-2075.1988.tb02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Kandasamy R. A. Na-Independent HCO(3) Transport and Accumulation in the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1992 Feb;98(2):560–568. doi: 10.1104/pp.98.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Characterization of the na-requirement in cyanobacterial photosynthesis. Plant Physiol. 1988 Nov;88(3):757–763. doi: 10.1104/pp.88.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. High Affinity Transport of CO(2) in the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1991 Nov;97(3):943–953. doi: 10.1104/pp.97.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Selective and Reversible Inhibition of Active CO(2) Transport by Hydrogen Sulfide in a Cyanobacterium. Plant Physiol. 1989 Sep;91(1):387–394. doi: 10.1104/pp.91.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H., Kohchi T., Sano T., Shirai H., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. III. Gene organization of the large single copy region from rbcL to trnI(CAU). J Mol Biol. 1988 Sep 20;203(2):333–351. doi: 10.1016/0022-2836(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Identification and Characterization of the ictA/ndhL Gene Product Essential to Inorganic Carbon Transport of Synechocystis PCC6803. Plant Physiol. 1992 Aug;99(4):1604–1608. doi: 10.1104/pp.99.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Slaughter C. A., Südhof T. C. The structure of cytochrome b561, a secretory vesicle-specific electron transport protein. EMBO J. 1988 Sep;7(9):2697–2703. doi: 10.1002/j.1460-2075.1988.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Ethoxyzolamide Inhibition of CO(2) Uptake in the Cyanobacterium Synechococcus PCC7942 without Apparent Inhibition of Internal Carbonic Anhydrase Activity. Plant Physiol. 1989 Jan;89(1):37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Sekiguchi K., Nagano Y., Matsuno R. Chloroplast envelope protein encoded by chloroplast genome. FEBS Lett. 1993 Jan 18;316(1):93–98. doi: 10.1016/0014-5793(93)81743-j. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi C., Ito K. Multicopy suppression: an approach to understanding intracellular functioning of the protein export system. J Bacteriol. 1992 Mar;174(5):1454–1461. doi: 10.1128/jb.174.5.1454-1461.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey D. L., Gray J. C. An open reading frame encoding a putative haem-binding polypeptide is cotranscribed with the pea chloroplast gene for apocytochrome f. Plant Mol Biol. 1990 Aug;15(2):347–356. doi: 10.1007/BF00036920. [DOI] [PubMed] [Google Scholar]