Abstract
We compared urinary levels of cytokines in patients with and without albuminuria, proteinuria, and kidney disease (GFR < 60 ml/min/1.73m2) after hematopoietic cell transplant (HCT). Plasma and urine were collected at baseline and weekly through day-100 and monthly through year-1, for measurement of IL-6, gp130, sIL6r, IL10, IL15, MCP1 and urine albumin to creatinine ratios (ACR). Cox-proportional hazards modeling examined associations between urinary cytokine levels and development of these renal endpoints. The association of ACR with the hazard of overall mortality was assessed using Cox regression.
Increasing urinary IL-6 and IL-15 were associated with an increased risk of developing proteinuria. Urinary MCP-1 during the first 100 days post-HCT was associated with kidney disease at 1 year. The degree of albuminuria at any time point in the first 100 days post-transplant was related to the subsequent risk of death (for ACR 30-299, HR=1.91; 95%CI:1.27-2.87; for ACR >300, HR=2.82; 95%CI:1.60-4.98).
After HCT, elevated urinary levels of proinflammatory cytokines are associated with development of albuminuria and proteinuria, suggesting early intrarenal inflammation as an important pathogenetic mechanism. Albuminuria and proteinuria within the first 100 days post-HCT are associated with decreased overall survival.
Keywords: albuminuria, proteinuria, chronic kidney disease, mortality, hematopoietic cell transplant
Introduction
Acute and chronic kidney disease (CKD) are common complications in long-term survivors of hematopoietic cell transplant (HCT). With the indications for and the number of transplants increasing and patient survival improving, so, too, will the burden of CKD on the health care system. The cumulative incidence of CKD varies from 23-60% in adult studies 1, 2, 3 to as high as 62% in children 4. Mortality rates among patients with CKD in this setting are significantly higher than in transplant recipients who retain normal renal function, even when controlled for co-morbidity 5. Additionally, the presence of proteinuria at transplant day 100 is a significant risk factor for mortality from all causes. Little is known about the pathophysiology of proteinuria or CKD in transplant survivors. Our recent work has identified both acute and chronic graft-vs.-host disease (GVHD) as risk factors for post-transplant kidney disease suggesting renal inflammation in its pathogenesis2. This finding challenges the prevailing view that renal injury in this setting results from either radiation nephropathy or toxic injury from drugs.
As a measure of renal inflammation, we analyzed urinary cytokines that are produced in the kidney, excreted in the urine, and their detection may offer insight into pathogenesis and progression of renal disease. Therefore, we sought to determine if increased urinary levels of cytokines in patients during their first 100 days after transplant were associated with the development of albuminuria and proteinuria. We also wanted to determine if elevations in urinary cytokine levels during this early time period were associated with development of chronic kidney disease and ultimately mortality.
Methods
Patient Selection
Patients over the age of 2 years undergoing their first HCT during the time period 2003-2012 were eligible to enroll in an ongoing prospective study if they met the following eligibility criteria: a) a baseline creatinine at screening within the limits of normal for age in children, <1.3 mg/dL in women, and < 1.5 mg/dL in men and/or an estimated GFR of >80 mL/min/1.73m2 by MDRD equation; b) not currently taking angiotensin receptor blockers or angiotensin converting enzyme inhibitors; c) no history of diabetes at time of enrollment into the study; and d) consented to a protocol approved by the Institutional Review Board of Seattle Children's Hospital (SCH). Urinary albumin to creatinine ratios were analyzed on 311 patients and 206 of the patients were randomly chosen for analysis of urinary cytokines.
Technique of HCT
All patients undergoing HCT received a preparative conditioning regimen followed by infusion of donor hematopoietic cells. The day of hematopoietic cell infusion is termed “day zero,” by convention. Myeloablative regimens were typically cyclophosphamide-based (with either total body irradiation (TBI) or targeted busulfan) for allogeneic transplants; autologous graft recipients received a number of different regimens. Reduced intensity conditioning regimens consisted of fludarabine and TBI at 2-4 Gy 6. The kidneys are not shielded during TBI. Allogeneic graft recipients received prophylaxis against acute GVHD with immunosuppressive drugs, usually cyclosporine or tacrolimus plus methotrexate or mycophenolate mofetil 7. Prophylaxis for infections included acyclovir to prevent herpes simplex virus and varicella zoster infection, trimethoprim/ sulfamethoxazole to prevent Pneumocystic jivecii infection, oral fluconazole or itraconazole for prophylaxis of fungal infection, and pre-emptive ganciclovir for cytomegalovirus disease among viremic patients 8-13. Prophylactic ursodiol was given routinely14.
Specimen Collection and Analytical Methods
Urine samples were collected from patients at baseline, prior to any conditioning therapy, weekly through day 100, and monthly through the first year after transplant. Urine was also collected between the hours of 8-10 a.m., immediately placed on ice, separated into 2 mL aliquots and frozen at −80 degrees until time of analysis.
Blood was collected in a citrated tube between the hours of 8-10 a.m. at baseline, (prior to the conditioning regimen), and then weekly through day 100 post-HCT. Blood was centrifuged at 4 degrees Celsius for 15 minutes and plasma was aspirated and frozen (−80°C) in 2 mL aliquots until analysis.
Aliquots of urine and plasma were rapidly thawed and the concentrations of the following factors were determined using the Luminex microbead system: IL-6, gp130, sIL6r, IL10, IL15, and MCP1. The intra-assay and inter-assay coefficient of variation is ≤10% for these analytes. We chose these cytokines based on preliminary data from patients and hematopoietic cell donors suggesting that single nucleotide polymorphisms in genes coding for these cytokines were associated with the development of CKD (data not shown). Serum levels of C-reactive protein (CRP) were measured in those patients with overt proteinuria. Urinary albumin was determined using an immunoturbidimetric assay using a Cobas c 11 analyzer in aliquots of untreated urine samples. The inter-assay coefficient of variation (CV) of the assay is 0.7-2.2% and intra-assay CV is 1.0-1.6%. A quantitative determination of urine creatinine was measured on Roche/Hitachi modular automated clinical chemistry analyzers. This enzymatic method is based on the conversion of creatinine with the aid of creatininase, creatinase, and sarcosine oxidase to glycine, formaldehyde and hydrogen peroxide. Catalyzed by peroxidase the liberated hydrogen peroxide reacts with 4-aminophenazone and HTIBa to form a quinone imine chromogen. The color intensity of the quinone imine chromogen formed is directly proportional to the creatinine concentration in the reaction mixture. The inter-assay CV is 2.1-3.7% and the intra-assay CV is 0.8-1.0%.
Clinical Parameters
Clinical data included baseline patient characteristics: subject age, gender, race/ethnicity, indication for HCT, preparative regimen, total body irradiation, and use of busulfan or cyclophosphamide as part of the conditioning regimen. Primary indications for transplant were categorized as acute leukemia, chronic leukemia, myelodysplastic syndrome, and all other groups. The patient's blood pressure, medications, and temperature were recorded in the morning on the day samples were collected. Hypertension was defined as an elevated blood pressure ≥140/90 or use of antihypertensive medications. In children and adolescents we defined hypertension as a systolic or diastolic blood pressure >95th percentile based on gender, age, and height published in 200415. Severity of acute graft-versus-host disease (aGVHD) was categorized as grades 0-1 and grades 2-4. Presence of diabetes was recorded from the medical chart or use of insulin.
Cardiovascular endpoints were also recorded from the medical charts if listed or extracted from questionnaires related to history of myocardial infarction, angina, and/or congestive heart failure. Chronic kidney disease (CKD) was defined as a GFR <60 mL/min/1.73 m2 at 1 year post-transplant. The GFR was estimated using the MDRD equation in adults and the Schwartz formula in children and adolescents. The degree of albuminuria was expressed as a urinary albumin-to-creatinine ratio (ACR). Normal ACR was <30 mg/g creatinine; albuminuria was 30-299 mg/g creatinine; and proteinuria was defined as ≥ 300 mg/g creatinine. The adjustment variables included in each model were chosen a priori based on previous studies and knowledge of risk factors related to the clinical events.
As the cause of death in patients post-HCT is often multifactorial, a root cause of death was determined for each patient by (SH and GM) after review of the death certificates when available and review of the medical charts, notes and correspondence for each patient. Both SH and GM were blinded to urinary ACR and cytokine study results.
Statistical Methods
The association between ACR and the risk of overall mortality was examined using Cox regression, adjusting for severity of disease at time of transplant (categorized as low vs. intermediate vs. high), type of donor (categorized as matched sibling vs. non-sibling relative or mismatched sibling vs. unrelated vs. autologous), patient age at transplant, and baseline ACR, defined as the ACR value closest to but before day of HCT. In keeping with our previous work, ACR was restricted to day 100 and the association of this value with subsequent death was examined; day-100 ACR was defined as the ACR value closest to day 100 in the window of day 80 to day 120 (if no value was available in this time, then day-100 ACR was considered to be missing). Day-100 ACR was treated as both a continuous and categorical variable. When continuous, ACR was modeled as both a linear and non-linear variable (using a cubic spline with knots at the 5th, 25th, 50th, 75th, and 95th percentiles); when categorical, day-100 ACR was grouped as < 30, 30-299, or ≥ 300 mg/g creatinine. In addition, ACR values at any time following HCT were considered and treated as a time-dependent covariate, both categorical and continuous as described above.
The association between cytokine levels and ACR was assessed by calculating the average value of all cytokine levels up to the time of the day-100 ACR for each patient. The means of these average values across patients were then compared among the three day-100 ACR groups using linear regression. In addition, a test for trend between the average cytokine values and ACR was performed, with the 3 ACR groups modeled as a continuous linear variable with integral values 1, 2, 3 for ACR groups < 30, 30-299, and ≥ 300 mg/g creatinine, respectively. We also modeled proteinuria as a time-to-event variable, with persistent proteinuria defined as the day on which the second of two consecutive ACR measurements exceeded 300 mg/g creatinine. Average cytokine levels up to a particular point in time were then modeled as time-dependent covariates to assess the association of cytokine level with the hazard of persistent proteinuria. The average cytokine levels were modeled as both linear and non-linear continuous variables as described above.
The association between cytokine levels and CKD at 1 year among patients who survived to one year was examined using linear regression, where the average cytokine levels through one year were compared among those with and those without CKD, as determined by their GFR at this time. These analyses were adjusted for the presence of diabetes and hypertension.
The cytokines included in this study were: IL-10, IL-15, IL-6, sIL6r, gp130 and MCP1.
To assess whether urinary levels of selected cytokines were primarily a reflection of plasma levels, correlation coefficients and R-values were calculated between plasma and urine levels of the cytokines listed above in those patients with an ACR ≥ 300 mg/g creatinine at day 100 post-HCT.
Results
Patient demographic and clinical information are presented in Table 1. A total of 311 patients had at least one ACR value; the median age of the 311 patients was 48 years. The majority (64%) of patients was male, and 76% of patients identified themselves as Caucasian. The most common diagnosis was AML, and nearly half of the patients were transplanted from an unrelated donor. The majority (75%) of patients received peripheral blood cells as their source of hematopoietic cells. For analysis of the relation of day 100 ACR to mortality, the entire cohort (N=311) was examined. Of these 311, 206 patients were randomly chosen for analyses involving urinary cytokines. Table 2 provides a summary of median values with 5th and 95th percentiles for ACR and the cytokines examined. The median number of urinary samples per patient was 6 with a range of 1-21.
Table 1.
Patient demographic data and clinical characteristics
| Patient Characteristic | Frequency (%) | ||
|---|---|---|---|
| Age at transplantation | <20 | 32 (10) | |
| 20-39 | 63 (20) | ||
| 40-59 | 151 (49) | ||
| >60 | 65 (21) | ||
| Median age = 48 | |||
| Gender: | Female | 112 (36) | |
| Male | 199 (64) | ||
| Race: | African American | 10 (3) | |
| Caucasian | 236 (76) | ||
| Hispanic | 15 (5) | ||
| Other | 37 (12) | ||
| Not available | 13 (4) | ||
| Diagnosis | ANL* | 103 (33) | |
| MDS | 55 (18) | ||
| CML | 29 (9) | ||
| NHL | 31 (10) | ||
| ALL | 26 (8) | ||
| MM | 19 (6) | ||
| CLL | 12 (4) | ||
| AA | 11 (4) | ||
| Other | 25 (8) | ||
| Donor type | Allogeneic | 107 (34) | |
| Autologous | 53 (17) | ||
| Unrelated donor | 151 (49) | ||
| Conditioning regimen | Reduced intensity regimens (200cGy) | 56 (18) | |
| Myeloablative: CY/TBI 12-13.5 cGy | 60 (19) | ||
| Myeloablative: BU, CY only | 91 (29) | ||
| Other myeloablative regimens | 104 (33) | ||
| Baseline serum creatinine (mg/dL) | Median (5th to 95th percentile) | 0.9 (0.5-1.3) | |
| Baseline ACR (mg/g) | Median: 11.10 | ||
| 5th percentile 1.80 | |||
| 95th percentile 165.70 | |||
| Patient CMV serostatus | Positive | 171 (55) | |
| Negative | 139 (45) | ||
| Missing | 1 | ||
| Source of Stem Cells+ | BM | 56 (18) | |
| PBSC | 234 (75) | ||
| Cord | 21 (7) | ||
Acute non-lymphocytic leukemia
bone marrow, peripheral blood stem cell, cord blood
Table 2.
Summary of median values of ACR and analyte levels
| Analyte | Median # samples/patient (range) | Median Value pg/mL (range) | 5th; 95th Percentiles |
|---|---|---|---|
| ACR mg/g creatinine | 8 (1-21) | 38.1 (1.6-3000) | 3.2; 667 |
| IL6 pg/mL | 6 (1-21) | 7.9 (2.2-1603) | 3.6; 96.1 |
| gp130 pg/mL | 6 (1-21) | 7811 (30-109,580) | 412; 24,887 |
| sIL6r pg/mL | 6 (1-21) | 456 (30-15,390) | 67.4; 2370 |
| IL-15 pg/mL | 10 (1-21) | 3.6 (1-179.5) | 1; 15.1 |
| MCP-1 pg/mL | 6 (1-21) | 661 (15-91,600) | 105; 3810 |
| IL-10 pg/mL | 6 (1-21) | 2 (1-14.5) | 1; 4.4 |
Association between ACR and overall mortality
We previously showed that proteinuria at day 100 was associated with an increased risk of mortality unrelated to relapse of a malignant disorder (termed non-relapse mortality), relative to patients with a normal day-100 ACR (0-29 mg/g creatinine)(16). Since that report of 142 patients, we have accrued an additional 169 patients into our prospective study for a total of 311 patients with urinary ACR data. In the analysis of the entire study cohort to date, among 120 patients with normal ACR values at day 100 post-transplant, there were 29 (24%) subsequent deaths from all causes. In contrast, among 97 patients with albuminuria at day 100, there were 44 (45%) subsequent deaths. And among 24 patients with proteinuria (ACR ≥ 300 mg/g creatinine) at day 100, there were 15 (63%) subsequent deaths. Seventy patients did not have an ACR value in the window of 80-120 days following HCT (and therefore were regarded as missing a day-100 ACR value); 27 (39%) of these patients died by last contact. Treating mortality as a time-to-event endpoint, the hazard of death was 74% higher among patients with albuminuria at day 100 compared to patients with a normal day-100 ACR value (HR=1.74, 95% CI 1.08-2.81, p=.02). Patients with proteinuria at day 100 were at even greater risk of death compared to patients with normal ACR at this time (HR=3.62, 95% CI 1.91-6.87, p<.0001). These results were qualitatively the same after adjusting for baseline ACR.
These results are consistent with our previous work (16). In addition, however, we extended our previous results by taking a more general approach to examining the association between ACR and all-cause mortality. Instead of categorizing ACR as 0-29, 30-300, and ≥300, we modeled day-100 ACR as a continuous non-linear function as described in Statistical Methods. Figure 1 shows the association of day-100 ACR and mortality using this approach. As seen from this figure, the risk of mortality increases steadily and somewhat linearly as day-100 ACR increases. Under the assumption of linearity, the risk of mortality is increased by 10% for each increase in ACR of 100 mg/g creatinine (HR=1.10, 95% CI 1.05-1.15, p<.0001). There was also no evidence to suggest that the association between day-100 ACR, modeled as a linear variable, and mortality was different in allogeneic vs. autologous patients (interaction test, p=.38). In other words, while autologous patients have a lower risk of mortality than allogeneic patients, the association between day-100 ACR and the risk of mortality in autologous patients appears to be similar to that among allogeneic patients.
Figure 1.
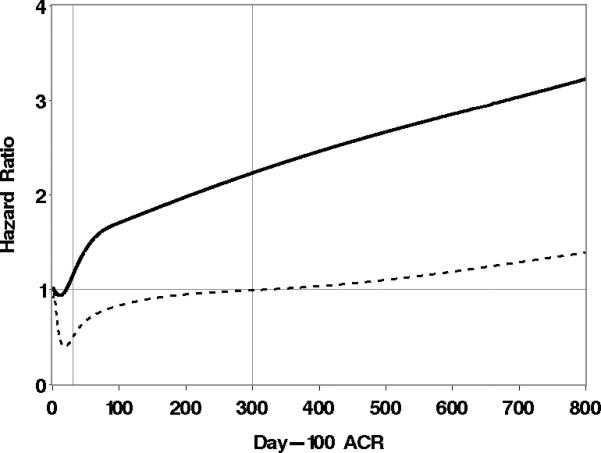
Association of day-100 ACR with the risk of overall mortality. Day-100 ACR is modeled as a cubic spline, and each point on the solid curve represents the hazard of death for the associated ACR value relative to the hazard of death at an ACR level of 2.6, which is the 5th percentile of day-100 ACR values. The dotted curve represents point-wise lower 95% confidence limits. The 5th, 25th, 50th, 75th, and 95th percentiles (the knots for the cubic spline) are 2.6, 10, 30.4, 95.6, and 715.7, respectively.
We also examined the association of ACR at any time post-HCT with the risk of mortality, not just ACR at day 100, with ACR now modeled as a time-dependent covariate. Doing so yielded the same qualitative conclusions as those obtained by examining the association between day-100 ACR and mortality. In particular, patients with an ACR in the range defined as albuminuria had an increased risk of death relative to patients with an ACR in the normal range (HR=1.91, 95% CI 1.27-2.87, p=.002), while patients with an ACR in the range defined as proteinuria had an even higher relative risk of death (HR=2.82, 95% CI 1.60-4.98, p=.0004). Modeling ACR as a continuous linear variable, there is a 5% increase in the hazard of death (HR=1.05; 95%CI 1.02-1.08) for each increase in ACR of 100 mg/g creatinine. Shown in Figure 2 is the association between ACR and mortality with ACR modeled as a continuous non-linear variable as described in Statistical Methods; this relationship is similar to that seen with day-100 ACR. As with day-100 ACR, there was no evidence to suggest that the association between ACR any time after HCT modeled as a linear variable and mortality was different in allogeneic vs. autologous patients (interaction test, p=.79).
Figure 2.

Association of ACR beyond day of HCT with overall mortality. ACR is modeled as a cubic spline, and each point on the solid curve represents the hazard of death for the associated ACR value relative to the hazard of death at an ACR level of 3.2, which is the 5th percentile of all ACR values beyond day of HCT. The dotted curve represents point-wise lower 95% confidence limits. The 5th, 25th, 50th, 75th, and 95th percentiles (the knots for the cubic spline) are 3.2, 12.7, 38.1, 117.3, and 667, respectively.
The root cause of death in patients is shown in Table 3. As ACR increases, the percentage of deaths with GVHD as the underlying cause increases (17.9% to 29.4%).
Table 3.
Root causes of death in patients by ACR values.
| ACR group | Total deaths | Relapse | GVHD | Other* |
|---|---|---|---|---|
| ACR < 30 | 39 | 24 (61.5%) | 7 (17.9%) | 5 (12.8%) |
| ACR 30-299 | 50 | 33 (66%) | 11 (22%) | 6 (12%) |
| ACR >/= 300 | 17 | 8 (47.1%) | 5 (29.4%) | 3 (17.6%) |
Other causes of death include, multiorgan failure, pulmonary embolus, cardiac, CNS bleed and infection. ACR data at day 100 was not available on 4 patients.
Association between urinary cytokine levels and ACR
The mean cytokine levels to day 100 among the 3 ACR groups are summarized in Table 4. Statistically significant differences were found in the mean levels of IL-6, IL-15, IL-10, and MCP-1 between patients with an ACR < 30 mg/g creatinine and those with an ACR ≥ 300 mg/g creatinine. Looking instead at log-transformed cytokine levels, the same conclusion was found for IL-6 and IL-15, but the differences for MCP-1 and IL-10 were no longer statistically significant between the two extreme ACR groups. In addition, the trend test yielded statistically significant associations for both IL-6 and IL-15, each increasing as ACR group increases, with both the log-transformed data and the actual data. There was no suggestion that these associations were different among allogeneic HCT recipients as compared to autologous recipients.
Table 4.
Summary statistics for average urinary cytokine levels in pg/mL up to day 100 after transplant and their association with ACR at day 100.
| Cytokine | N | ACR Group | Mean Level | Median Level | p-value1 | trend p-value1 | p-value2 | trend p-value2 |
|---|---|---|---|---|---|---|---|---|
| IL6 | 88 | 0-29 | 18.90 | 10.56 | · | 0.0005 | · | <0.0001 |
| 48 | 30-299 | 35.97 | 17.92 | 0.14 | 0.02 | |||
| 23 | ≥300 | 73.68 | 27.44 | .0004 | .0002 | |||
| gp130 | 88 | 0-29 | 10614.66 | 9680.83 | 0.43 | 0.68 | ||
| 48 | 30-299 | 11203.42 | 10628.43 | 0.56 | 0.45 | |||
| 23 | ≥300 | 11507.27 | 9261.42 | 0.49 | 0.89 | |||
| sIL6r | 88 | 0-29 | 763.29 | 615.11 | 0.48 | 0.36 | ||
| 48 | 30-299 | 796.15 | 650.98 | 0.73 | 0.86 | |||
| 23 | ≥300 | 849.53 | 741.61 | 0.49 | 0.30 | |||
| IL10 | 88 | 0-29 | 2.00 | 2.00 | 0.02 | 0.07 | ||
| 48 | 30-299 | 2.12 | 2.00 | 0.39 | 0.47 | |||
| 23 | ≥300 | 2.41 | 2.00 | 0.02 | 0.06 | |||
| IL15 | 86 | 0-29 | 5.80 | 3.60 | 0.0008 | 0.002 | ||
| 50 | 30-299 | 8.63 | 5.62 | 0.04 | 0.09 | |||
| 24 | ≥300 | 11.43 | 7.87 | 0.002 | 0.004 | |||
| MCP1 | 88 | 0-29 | 1046.16 | 840.77 | 0.02 | 0.06 | ||
| 48 | 30-299 | 1616.26 | 1365.40 | 0.09 | 0.003 | |||
| 23 | ≥300 | 1979.55 | 908.23 | 0.03 | 0.38 | |||
p-value derived from linear regression; trend p-value derived from treating ACR groups as linear variable with values 1, 2, and 3
log-transformed data; p-value derived from linear regression; trend p-value derived from treating ACR groups as linear variable with values 1, 2, and 3
There were 52 cases of persistent proteinuria as defined above, with the time of persistent proteinuria ranging from day 7 to day 273 (median time of proteinuria among all patients who met this definition was 48 days (25th and 75th percentiles, 25 and 72 days, respectively). Average urinary cytokine level was modeled as a time-dependent covariate, and in addition to examining the actual cytokine levels, we also log-transformed the levels as described above. Similar to the conclusions drawn when restricting ACR to day 100, increasing mean levels of urinary IL-6 and IL-15 were associated with an increased risk of development of persistent proteinuria (Table 5).
Table 5.
Association between mean cytokine level and the risk of persistent proteinuria at any time post-HCT.
| Cytokine | Direction of association, p-value1 | Direction of association, p-value2 |
|---|---|---|
| IL6 | Positive, 0.01 | Positive, .002 |
| gp130 | Negative, 0.92 | Negative, 0.99 |
| sIL6r | Negative, 0.84 | Positive, 0.63 |
| IL10 | Positive, 0.69 | Positive, 0.35 |
| IL15 | Positive, 0.003 | Positive, 0.002 |
| MCP1 | Positive, 0.51 | Positive, 0.12 |
p-value from Wald test, Cox regression, Mean cytokine level modeled as a time-dependent covariate based on actual cytokine value
p-value from Wald test, Cox regression, Mean cytokine level modeled as a time-dependent covariate based on log-transformed cytokine value
Association between urinary cytokine levels and CKD
Among patients who survived to one year, the association of mean urinary cytokine levels with CKD, as defined by one-year GFR < 60 mL/min/1.73m2, is summarized in Table 6. We similarly compared the mean of the average urinary cytokine levels before day 100 according to 1 year GFR as summarized in Table 5. Urinary cytokine levels before day 100 bore no statistically significant relationship to development of CKD at 1-year, with the possible exception of MCP1 (Table 5).
Table 6.
Average urinary cytokine levels in pg/mL during the first 100 days and association with CKD at one-year post-HCT among one year survivors.
| Cytokine | GFR Group | N | Mean Level | Median Level | p-value1 | p-value2 |
|---|---|---|---|---|---|---|
| IL6 | < 60 | 27 | 20.36 | 10.55 | 0.56 | 0.86 |
| ≥ 60 | 89 | 25.50 | 11.42 | |||
| gp130 | < 60 | 27 | 10385.89 | 11483.15 | 0.99 | 0.68 |
| ≥ 60 | 89 | 10388.12 | 9395.90 | |||
| sIL6r | < 60 | 27 | 809.05 | 548.14 | 0.81 | 0.54 |
| ≥ 60 | 89 | 846.21 | 573.76 | |||
| IL10 | < 60 | 27 | 2.09 | 2.00 | 0.84 | 0.74 |
| ≥ 60 | 89 | 2.06 | 2.00 | |||
| IL15 | < 60 | 27 | 6.29 | 3.92 | 0.16 | 0.18 |
| ≥ 60 | 91 | 9.80 | 4.85 | |||
| MCP1 | < 60 | 27 | 1290.87 | 1168.96 | 0.21 | 0.02 |
| ≥ 60 | 89 | 1041.93 | 705.32 | |||
p-value from linear regression, based on cytokine values
p-value from linear regression, based on log-transformed cytokine values
Blood and urinary cytokine levels in patients with proteinuria at day 100
Cytokine levels in the blood were analyzed in 51 patients with proteinuria at day 100 post-HCT. When levels in blood were assessed for correlation with the corresponding levels in urine on the same day, the associations were relatively weak (IL-10, R2 = .03; IL-15, R2 = 0.34; IL-6, R2 = 0.009; MCP-1 R2 = 0.01; gp130, R2 = 0.005; sIL6R, R2 = 0.007).
Association between urinary cytokine levels and mortality
Consistent with the association of increasing IL-15 with increased ACR and persistent proteinuria, we also saw an increased risk of overall mortality with increasing average urinary IL-15 (p<.0001 for average IL-15, p=.12 for average log-transformed IL-15). An increased risk of overall mortality was also seen with increasing IL-6, although the association was not statistically significant (p=.08 for average IL-6, p=.12 for average log-transformed IL-6). Suggestive positive associations were also seen for gp130 (p=.03 for average gp130, p=.07 for average log-transformed gp130) and sIL6r (p=.06 for average sIL6r, p=.03 for average log-transformed sIL6r). Increasing average MCP-1 was associated with an increased risk of mortality (p=.001), but this association was reduced when the values were log-transformed (p=.30).
Discussion
We found increased urinary levels of IL-6, IL-15 and to a lesser extent MCP-1 to be associated with the development of proteinuria in the first 100 days post-transplant. The lack of correlation between these urinary cytokines and simultaneously collected plasma cytokines suggest that intra-renal inflammation and not merely clearance underlies these results. Interleukin-6 is considered a prototypic proinflammatory cytokine. It has an unique receptor system involving 2 functional proteins, IL-6R (which is specific for IL-6) and a common signal transducer of cytokines, gp130 16. In proliferative glomerular diseases such as IgA nephropathy, urinary IL-6 excretion has also been reported to predict worse kidney outcomes 17. In patients with diabetes, the data are mixed as to the associations with urinary and serum IL-6 levels and albuminuria and decline in renal function 18. Though we found an association with urinary IL-6 levels and development of albuminuria and proteinuria, we did not find an association between urinary IL-6 levels or its receptors and development or progression of CKD at 1 year in this patient population.
IL-15 is a pro-inflammatory cytokine produced by multiple tissues including kidney epithelial and proximal tubular cells. It has both paracrine and autocrine effects in the kidney and IL-15 plays an important role in the cross talk between activated macrophages and natural killer cells and establishment of an innate immune response19. In a mouse model of nephrotoxic serum nephritis, mice deficient in IL-15 had an increase in tubular epithelial cell apoptosis and an increase in MCP-1 expression compared to wild-type IL-15+ mice and normal control mice. In addition, renal interstitial macrophage and CD4+ T-cell infiltrates were more prominent in the IL-15 deficient mice with nephritis compared to wild-type mice. The authors conclude that the decrease in IL-15 expression in tubular epithelial cells after injury suggests a protective role against immunologic insults for IL-15 to the kidney in normal conditions. This loss of protection by IL-15 at time of injury is mediated by a lack of induction of anti-apoptotic molecules and a lack of inhibition of MCP-1 expression in the kidney20. Increased expression of IL-15 has been found in renal allografts with rejection compared to grafts without rejection 19. Tubular epithelial cells produce IL-15 in response to injury21. The increased levels of urinary IL-15 in the HCT population may reflect increased inflammation within the kidney and subsequent tubular injury.
Urinary MCP-1 is also found to be increased in acute kidney injury in both humans and animal models (28). Progressive increases in serum MCP-1 and renal cortical expression of MCP-1 along with a lack of response from anti-inflammatory cytokines such as IL-10 are thought to lead to progression to CKD in animal models(28). In studies of patients with diabetic nephropathy, increased urinary levels of MCP-1 were found in diabetic patients with nephrotic range proteinuria and advanced tubulointerstitial lesions. Increased expression of MCP-1 was observed in the interstitium and in tubular epithelial cells, endothelial cells and mononuclear infiltrates in patients with more advanced lesions. In this study, patient with minimal change nephrotic syndrome and membranous nephropathy had lower levels of MCP-1 suggesting that proteinuria in and of itself was not associated with increased urinary MCP-1 levels and that activation of resident tubular or endothelial cells and interstitial infiltrates is needed for MCP-1 expression (29). We found elevations in urinary MCP-1 associated with the development of CKD at 1 year in this patient population. Perhaps the injury that occurs post-transplant leads to an early increase in IL-15 in the kidney but as tubular epithelial cells die, IL-15 is lost allowing for the uninhibited activity of MCP-1 which leads to ongoing inflammation and progression to CKD.
Increased serum cytokine levels have also been described in patients with complications after HCT and specifically in patients who develop GVHD after HCT. The inflammatory and cytokine cascades that are activated by GVHD can also affect the kidney without invoking the direct T-cell mediated epithelial cell injury that is implicated in GVHD involving skin, gut and liver 22, 23. In support of the hypothesis that GVHD-related organ damage can result from exposure to pro-inflammatory cytokines, increased plasma cytokine levels correlate with post-transplant complications and organ dysfunction in the HCT population 24, 25. In animal models of GVHD, tissue destruction by acute GVHD does not require alloantigen expression on target epithelium for cellular cytotoxicity; injury can be mediated by inflammatory cytokines 26.
This study confirms the strong association between elevated urinary ACR levels at day-100 and mortality 27, a relationship that appears to be proportional to the degree of albuminuria (see Figures 1 and 2). Development of proteinuria and albuminuria at any time point after HCT, not just at day 100, also showed a significant increase in the risk of mortality and non-relapse mortality following HCT. We also found an association of mortality with levels of MCP-1 and the IL6 receptors gp130 and sIL6r before day 100, suggesting that stimulation of multiple cytokines may be involved in the increased risk of mortality in patients with albuminuria post-HCT. Another possible explanation for these findings is that a shift in the cytokine profile may occur from the early inflammatory injury to the kidney causing albuminuria to a more chronic pathway.
In summary, elevated urinary levels of IL-6, IL-15 and MCP-1 are associated with development of albuminuria and proteinuria in the months following transplant, suggesting early intrarenal inflammation as an important pathogenetic mechanism. Albuminuria and proteinuria are associated with an increased risk of overall mortality in patients post-HCT. We speculate that pro-inflammatory cytokines in the urine are a reflection of the inflammatory milieu of GVHD in the kidney which might be mediated by two different mechanisms: the kidney could be a direct target of T-cell mediated renal damage or the chronic systemic inflammatory state of GVHD could lead secondarily to cytokine-mediated nephropathy. Renal biopsies are needed to clarify these findings and guide therapy, especially in patients with proteinuria around day 80 post-HCT. Biopsies may help to determine if increased urinary cytokine levels correlate with increased expression of these cytokines in renal tissue and to determine potential etiologies for the increase in cytokine levels such as tubulitis, interstitial inflammation , endothelial and/or glomerular injury that might also be suggestive of GVHD in the kidney.
Acknowledgements
This research was supported by the National Institutes of Health (NIDDK) 1R01DK080860-01 Chronic kidney disease in survivors of hematopoietic cell transplant received by Dr. Hingorani.
Footnotes
The authors have no conflict of interest to report.
REFERENCES
- 1.Cohen EP, Lawton CA, Moulder JE. Bone marrow transplant nephropathy: radiation nephritis revisited. Nephron. 1995;70(2):217–22. doi: 10.1159/000188587. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39(4):223–9. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 3.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6(1):89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 4.Guinan EC, Tarbell NJ, Niemeyer CM, Sallan SE, Weinstein HJ. Intravascular hemolysis and renal insufficiency after bone marrow transplantation. Blood. 1988;72(2):451–5. [PubMed] [Google Scholar]
- 5.Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease (ESRD)after bone marrow transplantation: poor survival compar ed to other causes of ESRD. Nephron. 1998;79(4):408–12. doi: 10.1159/000045085. [DOI] [PubMed] [Google Scholar]
- 6.Carella AM, Champlin R, Slavin S, McSweeney P, Storb R. Mini-allografts: ongoing trials in humans. Bone Marrow Transplant. 2000;25(4):345–50. doi: 10.1038/sj.bmt.1702204. [DOI] [PubMed] [Google Scholar]
- 7.Doroshow JHaS, T.W. Pharmacologic Basis for High-dose Chemotherapy. In: Appelbaum FR, Blume KG, Forman SJ, Negrin RS, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. Wiley-Blackwell; Malden: 2009. pp. 289–315. [Google Scholar]
- 8.Leather HL, Wingard JR. Bacterial Infections. In: Appelbaum FR, Blume KG, Forman SJ, Negrin RS, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. Wiley-Blackwell; Malden: 2009. pp. 1325–1345. [Google Scholar]
- 9.Zaia JA. Cytomegalovirus Infections. In: Appelbaum FR, Blume KG, Forman SJ, Negrin RS, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. Wiley-Blackwell; Malden: 2009. pp. 1367–1381. [Google Scholar]
- 10.Brown JMY. Thomas' Hematopoietic Cell Transplantation. Wiley-Blackwell; 2009. Fungal Infections after Hematopoietic Cell Transplantation. pp. 1346–1366. [Google Scholar]
- 11.Brown JWMY. Fungal Infections after Hematopoietic Cell Transplantation. In: Frederick R, Appelbaum SJF, Robert S, Negrin, Karl G, Blume, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. Blackwell Science; 2009. pp. 1346–1366. [Google Scholar]
- 12.Ito J. Herpes Simplex Virus Infections. In: Appelbaum FR, Blume KG, Forman SJ, Negrin RS, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. Wiley-Blackwell; Malden: 2009. pp. 1382–1387. [Google Scholar]
- 13.Ho DY, Arvin AM. Thomas’ Hematopoietic Cell Transplantation. Wiley-Blackwell; 2009. Varicella-zoster Virus Infections. pp. 1388–1409. [Google Scholar]
- 14.Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100(6):1977–83. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 15.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. 114. Pediatrics. 2004;(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 16.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–52. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 17.Harada K, Akai Y, Kurumatani N, Iwano M, Saito Y. Prognostic value of urinary interleukin 6 in patients with IgA nephropathy: an 8-year follow-up study. Nephron. 2002;92(4):824–6. doi: 10.1159/000065465. [DOI] [PubMed] [Google Scholar]
- 18.Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19(4):789–97. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, et al. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. The Journal of clinical investigation. 2002;109(7):951–60. doi: 10.1172/JCI14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiler M, Rogashev B, Einbinder T, Hausmann MJ, Kaneti J, Chaimovitz C, et al. Interleukin-15, a leukocyte activator and growth factor, is produced by cortical tubular epithelial cells. J Am Soc Nephrol. 1998;9(7):1194–201. doi: 10.1681/ASN.V971194. [DOI] [PubMed] [Google Scholar]
- 22.Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101(9):1936–46. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 23.Krenger W, Hill GR, Ferrara JL. Cytokine cascades in acute graft-versus-host disease. Transplantation. 1997;64(4):553–8. doi: 10.1097/00007890-199708270-00001. [DOI] [PubMed] [Google Scholar]
- 24.Takatsuka H, Takemoto Y, Okamoto T, Fujimori Y, Tamura S, Wada H, et al. Thrombotic microangiopathy following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;24(3):303–6. doi: 10.1038/sj.bmt.1701890. [DOI] [PubMed] [Google Scholar]
- 25.Seconi J, Watt V, Ritchie DS. Nephrotic syndrome following allogeneic stem cell transplantation associated with increased production of TNF-alpha and interferon-gamma by donor T cells. Bone Marrow Transplant. 2003;32(4):447–50. doi: 10.1038/sj.bmt.1704151. [DOI] [PubMed] [Google Scholar]
- 26.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–81. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 27.Hingorani SR, Seidel K, Lindner A, Aneja T, Schoch G, McDonald G. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biol Blood Marrow Transplant. 2008;14(12):1365–72. doi: 10.1016/j.bbmt.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


