Abstract
Objective
To examine calcitonin gene-related peptide (CGRP) gene expression under inflammatory conditions using trigeminal ganglia organ cultures as an experimental system. These cultures have increased proinflammatory signaling that may mimic neurogenic inflammation in the migraine state.
Background
The trigeminal nerve sends peripheral pain signals to the central nervous system during migraine. Understanding the dynamic processes that occur within the trigeminal nerve and ganglion may provide insights into events that contribute to migraine pain. A neuropeptide of particular interest is CGRP, which can be elevated and play a causal role in migraine. However, most studies have overlooked a second splice product of the CALCA gene, which encodes calcitonin (CT), a peptide hormone involved in calcium homeostasis. Importantly, a precursor form of calcitonin called procalcitonin (proCT) can act as a partial agonist at the CGRP receptor and elevated proCT has recently been reported during migraine.
Methods
We used a trigeminal ganglion whole organ explant model, which has previously been demonstrated to induce pro-inflammatory agents in vitro. Quantitative PCR and immunohistochemistry were used to evaluate changes in mRNA and protein levels of CGRP and proCT.
Results
Whole mouse trigeminal ganglia cultured for 24 h showed a 10-fold increase in CT mRNA, with no change in CGRP mRNA. A similar effect was observed in ganglia from adult rats. ProCT immunoreactivity was localized in glial cells. Cutting the tissue blunted the increase in CT, suggesting that induction required the close environment of the intact ganglia. Consistent with this prediction, there were increased reactive oxygen species in the ganglia and the elevated CT mRNA was reduced by antioxidant treatment. Surprisingly, reactive oxygen species were increased in neurons, not glia.
Conclusions
These results demonstrate that reactive oxygen species can activate proCT expression from the CGRP gene in trigeminal glia by a paracrine regulatory mechanism. We propose that this glial recruitment pathway may occur following cortical spreading depression and neurogenic inflammation to increase CGRP nociceptive actions in migraine.
Keywords: trigeminal ganglion, calcitonin gene-related peptide, migraine, procalcitonin, reactive oxygen species
Introduction
The trigeminal nerve serves as the major relay for sensory information from the face, anterior head, and intracranial structures such as meninges and cerebral vasculature1. The trigeminal ganglion contains the cell bodies for these sensory neurons. Sensory information from the trigeminal system arrives first at dedicated brain stem and upper spinal cord regions, then further processed in the thalamus and cortex where conscious perception of pain occurs2, 3. The trigeminal system is responsible for relaying pain messages in conditions such as migraine, trigeminal neuralgia, and temporomandibular joint dysfunction.
Migraine is a painful, debilitating neurological condition. The throbbing, unilateral headache pain typical of migraine is generally accompanied by other symptoms, such as nausea, vomiting, and increased sensitivity to light and sound4. The trigeminal sensory system is believed to be sensitized during migraine, possibly as a result of neurogenic inflammation5. Neurogenic inflammation describes a local, sterile inflammation that results from the release of neuropeptides and other chemical mediators from the peripheral ends of sensory neurons 6. One neuropeptide released during neurogenic inflammation is calcitonin gene-related peptide (CGRP). CGRP is a potent vasodilator7 and neuromodulator that likely plays a causal role in migraine pain8. CGRP levels are elevated during migraine pain9,10, although there is some controversy on this point11, 12. Importantly, administration of CGRP to migraine patients causes a delayed, migraine-like headache13. Additionally, clinical studies have demonstrated the efficacy of CGRP receptor antagonists in relieving migraine pain14, 15.
While the relationship between CGRP and migraine is well established, the role of a related peptide, procalcitonin (proCT) is less well understood. ProCT is a precursor form of the calcium homeostasis hormone calcitonin16, and is transcribed from the same gene as CGRP17. This precursor form can normally be detected at low levels in human serum and is greatly increased throughout the body during conditions of extreme inflammation, such as sepsis18. ProCT has partial agonist actions at the CGRP receptor19, so it may act in a similar manner as CGRP in migraine. ProCT elevation is generally associated with inflammatory conditions20, yet there is one report of increased plasma proCT during migraine21. The source and site of action for this peptide in migraine is yet to be determined22.
Since CGRP and proCT are alternative splice products from the same gene, Calca, their expression is likely regulated by similar mechanisms. Much has been discovered about the regulation of the Calca gene in recent years. Transcription factors USF1 and USF2 are activators of the gene23, but their ubiquitous expression pattern led to questions about how tissue specificity is maintained. We previously reported that the gene was kept silent in glia through an epigenetic mechanism24. Chemical removal of repressive promoter methylation and prevention of histone deacetylation resulted in increased expression from the Calca locus. Interestingly, the Calca gene can also be induced in otherwise non-expressing cells under inflammatory conditions. CGRP and proCT expression is elevated in a host of tissues during sepsis18, and expression of both transcripts can be induced in stem cell-derived adipocytes using an inflammatory cocktail25. Likewise, neuronal CGRP expression and release can also be induced by proinflammatory cytokines26. Edvinsson and colleagues recently demonstrated that whole organ explants of trigeminal ganglia have increased proinflammatory mediators along with increased Calca gene expression27, 28. In this study, we have used this organ explant model to investigate the cellular mechanisms underlying glial Calca gene activation.
Materials and Methods
Animals and tissue preparation
All experiments were performed in accordance with the rules and regulation of the University of Iowa Animal Care and Use Committee. Male C57B6/J mice (20-40 g, Jackson Laboratory) were euthanized by CO2 asphyxiation. Trigeminal ganglia were removed and placed in ice cold phosphate buffered saline (PBS). Ganglia were then either immediately fixed for immunohistochemistry (IHC), homogenized for RNA analysis, or placed in culture for 24 h. One ganglion per well was cultured in 1 ml media in a 12 well plate using DMEM High Glucose (Gibco) containing 10 units penicillin, 10 μg streptomycin, and 2.5 μg Amphotericin B. For all mouse experiments, between 2 and 8 animals were used for each independent experiment. An equal number of ganglia were used for each treatment, generally 2-4 ganglia per treatment. Adult Sprague Dawley rat (250-300 g, Charles River, Raleigh Facility) ganglia were harvested and prepared the same way as mouse tissues, except ganglia were cut into 3 mm sections before being placed into culture. For all rat experiments, one rat was used per experiment, with one ganglia being processed immediately and the other being placed in culture for 24 h. For experiments where ganglia were cut to reduce diameter, a McIlwain Tissue Chopper was used. For hypoxia treatment, cut ganglia were placed in a chamber containing 1% O2, 5% CO2, and 94% N2. Tempol (4-hydroxy-TEMPO, Sigma) was added to the media once, at the beginning of the 24 h incubation.
Immunohistochemistry
All tissue was fixed for 48 h at 4°C in 10% neutral buffered formalin. This time was selected based on reference29 and advice from the University of Iowa Central Microscopy Research Facility. Following fixation, tissue was cryoprotected using step-wise addition of sucrose over 1 day intervals to 30%. Tissue was snap frozen and cryosectioned at 10 μm onto Superfrost Plus slides. Since once ganglia were removed and cultured, it was impossible to maintain their anatomical position, ganglia were sectioned in either the sagittal or transverse plane without orientation to ophthalmic or mandibular regions. Sections were brought to room temperature and mounting media was removed by washing in PBS, pH 7.4. Sections were blocked in 5% normal goat serum (Sigma) or Mouse on Mouse (M.O.M.) Mouse Ig Blocking Reagent (Vector Laboratories) for 1 h at room temperature or overnight at 4°C. Primary antibodies were diluted in blocking solution or M.O.M. Diluent (Vector Laboratories) and incubated with sections for 1 h at room temperature. After washing in PBS, secondary antibodies were diluted in the same way as primary antibodies and incubated with sections for 30-60 min in the dark. Specific information about antibodies is provided in Table 1. All sections were mounted with Vectashield containing DAPI (Vector Laboratories). All sections were visualized using a Zeiss 710 Confocal Microscope. Images were processed using Image J software. The CGRP antibody (C8198, Sigma) has been previously validated using CGRP knockout mice30 and by peptide competition with synthetic CGRP prior to IHC31. Specificity of proCT antibody (ab53897, Abcam) for proCT compared to calcitonin has been previously reported24. Specificity of NeuN mAb A60 antibody (MAB377, Millipore) has been reported32. The beta-tubulin III antibody (2G10, ab78078, Abcam) has been described33. The GFAP antibody (PA5-16291, Pierce) was validated for IHC by selective staining of human astrocytoma (Pierce Antibodies). The glutamine synthetase mAb (GS-6, MAB302, Millipore) has previously been used to detect satellite glia in the trigeminal ganglion28. For all experiments, controls without primary antibodies were negative.
Table 1. Antibody specifications.
| Name and product code | Host | Dilution | Source |
|---|---|---|---|
| Anti-calcitonin gene-related peptide, #C 8198 |
Rabbit, whole antiserum |
1:8000 | Sigma |
| Anti-procalcitonin, ab53897 | Rabbit, polyclonal |
1:100 | Abcam |
| Anti-neuron specific beta III tubulin, ab78078 |
Mouse, monocloncal |
1:300 | Abcam |
| Anti-glial fibrillary acidic protein, PA5-16291 |
Rabbit, polyclonal |
1:100 | Pierce Antibodies |
| Anti-NeuN clone A60, MAB377 | Mouse, monocloncal |
1:1000 | Millipore |
|
| |||
| Conjugate and host | Against | Dilution | Source |
|
| |||
| Alexa Fluor 488 F(ab′)2 fragment of goat IgG (H+L) |
Mouse | 1:500 | Invitrogen |
| Alexa Fluor 568 F(ab′)2 fragment of goat IgG (H+L) |
Rabbit | 1:500 | Invitrogen |
| Alexa Fluor 488 F(ab′)2 fragment of goat IgG (H+L) |
Rabbit | 1:500 | Invitrogen |
In vitro reactive oxygen species (ROS) detection
Mouse trigeminal ganglia from freshly sacrificed animals or ganglia that had been cultured for 24 h were snap frozen, sectioned (10 μm) and maintained at −20°C until staining began. Fresh and 24 h samples were always frozen at the same time and processed in parallel. For dihydroethidium (DHE) (Life Technologies) labeling, 10 μM DHE was added directly to the sections in the dark. Slides were incubated at 37°C for 30 min, then washed with PBS and coverslipped. For CellROX (Life Technologies), slides were rinsed with PBS, then incubated with 50 μM CellROX DeepRed at 37°C for 30 min. Slides were rinsed with PBS, then fixed with 3.7% formaldehyde for 15 min. Slides were rinsed with PBS before blocking with M.O.M. block. After a brief incubation with M.O.M. diluent, anti-NeuN antibody (cloneA60, Millipore) was diluted 1:1000 in M.O.M. diluent and added to slices for 30 min. Slides were rinsed and incubated with goat anti-rabbit secondary antibody, conjugated to Alexa 488 (Life Technologies). Slides were rinsed 3 times with PBS, and then mounted with Vectashield containing DAPI. All images were captured using a Zeiss 710 confocal microscope.
Quantitative RT-PCR (q-PCR)
RNA was isolated by first homogenizing in Trizol (Invitrogen) followed by alcohol precipitation and purification using RNeasy Micro columns (Qiagen). To address the possibility of RNA degradation, RNA gels were run in early experiments. Equal amounts of RNA based on A260 were loaded as serial dilutions on agarose gels and stained with ethidium bromide. The RNA was intact based on greater 28S than18S rRNA band intensities and comparable intensities between fresh and 24 h cultures. In all subsequent experiments, cycle numbers for the reference gene were used to verify the quality and amount of RNA used for each sample. The cycle numbers were similar (i.e. control and experimental samples had Cq values within 1 Cq of each other). For reverse transcription, 0.5 - 2 μg RNA was reverse transcribed using Quantitect Reverse Transcription kit (Qiagen). 10% of the reverse transcription reaction was used for qPCR. qPCR was carried out on a CFX Connect (BioRad) machine using IQ Sybr Green Supermix with the following cycling protocol: 95°C, 5 min; 40 cycles of 95°C, 15 sec; 60°C, 15 sec; 72°C, 20 sec. Following amplification, a melt curve was generated and analyzed for a single, uniform peak for each primer set. Primer sequences for peptidylprolyl isomerase A (PPIA, reference gene), CGRP, CT, and vascular endothelial growth factor (VEGF) are in Table 2. Other genes evaluated for use as a reference gene included beta actin, 18S rRNA, and hypoxanthine guanine phosphoribosyl transferase (HPRT). These transcripts were all found to change after 24 h of organ culture, and hence were not used as reference genes. PPIA levels were determined to be stable (control and test samples within 1Cq of each other). Relative expression levels of CT, CGRP, and VEGF were determined using the 2−ddCt method34. The Cq values for each experiment are as follows. For figure 1: mouse Cq values for PPIA: fresh, 14.0-15.6; 24h, 14.2-15.8; CGRP fresh, 18.3-21.1; 24h, 18.8-20.9, and CT fresh, 29.1-33.7; 24h, 26.2-27.7. Rat Cq values for PPIA fresh, 14.7-17.0; 24h, 14.6-16.7, CGRP fresh, 14.0-17.4; 24h, 15.4-19.5, and CT fresh, 29.7-30.4; 24h, 27.5-28.6. For Figure 3: PPIA: fresh, 15.6-16.4; 24h, 16.1-16.2; cut, 16.0-16.6; cut + hyp, 17.0-17.9; CGRP: fresh, 21.5-25.7; 24h 21.0-25.8; cut, 21.4-26.4; cut + hyp, 22.0-28.3; CT: fresh, 34.2-35.1; 24h, 30.5-31.6; cut, 32.1-34.0; cut + hyp, 33.8-34.0; VEGF: fresh, 22.6-23.1; 24h, 19.5-21.2; cut, 21.2-21.6; cut + hyp, 20.2-22.2. For Figure 4 Cq values: PPIA: fresh, 14.7-16.3; 24h, 15.1-16.8; tempol, 15.4-16.2; CT: fresh, 31.4-35.0; 24h, 29.3-33.1; tempol, 29.7-33.0.
Table 2. Primer sequences.
| Species | Gene | RefSeq ID | Forward primer (5′-3′) | Reverse Primer (5′-3′) | Product Size |
|---|---|---|---|---|---|
| Mouse | Vegfa | NM_009606.4 | CAGGCTGCTGTAACGATGAA | GCATTCACATCTGCTGTGCT | 140 |
| Mouse | Ppia | NM_008907.1 | CCACCGTGTTCTTCGACAT | CAGTGCTCAGAGCTCGAAAG | 114 |
| Mouse | Calca (CGRP) | NM_001033954.3 | CAGTGAAGAAGAAGTTCGCCTGCT | GCCCACATTGGTGGGAACAAAGTT | 223 |
| Mouse | Calca (CT) | NM_007587.2 | CCCTTTCCTGGTTGTCAGCATCTT | AGCATGCAGGTACTCAGATTCCCA | 258 |
| Rat | Ppia | NM_017101.1 | TTGCTGCAGACATGGTCAAC | TGTCTGCAAACAGCTCGAAG | 93 |
| Rat | Calca (CGRP) | NM_001033954.3 | AACCTTGGAAAGCAGCCCAGGCATG | GTGGGCACAAAGTTGTCCTTCACCA | 246 |
| Rat | Calca (CT) | NM_007587.2 | CCCTTTCCTGGTTGTCAGCATCTT | AGCATGCAGGTACTCAGATTCCCA | 258 |
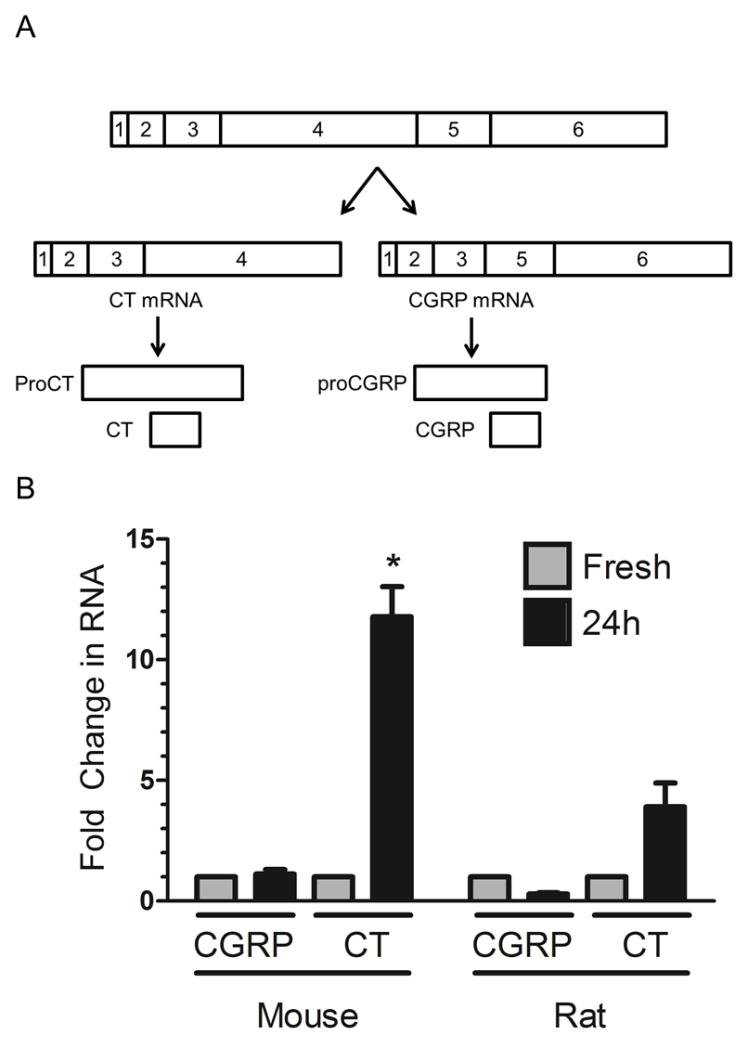
Figure 1. Organ culture induces CT mRNA in rat and mouse trigeminal ganglia.
A. Schematic of the alternative mRNAs from the Calca locus, coding for either calcitonin (CT) or calcitonin gene-related peptide (CGRP). The precursor peptides proCT and proCGRP and mature CT and CGRP peptides from each mRNA are shown relative to their corresponding mRNA sequences. B. Organ culture (24 h, black bars) leads to an increase in CT mRNA, with no change in CGRP mRNA in both mouse and rat relative to fresh tissue (gray bars). Mean +/−SEM from 5 (for CT) and 6 (for CGRP) independent experiments for mouse and 3 for rat. *, p<0.05 vs. fresh (Mann Whitney test).
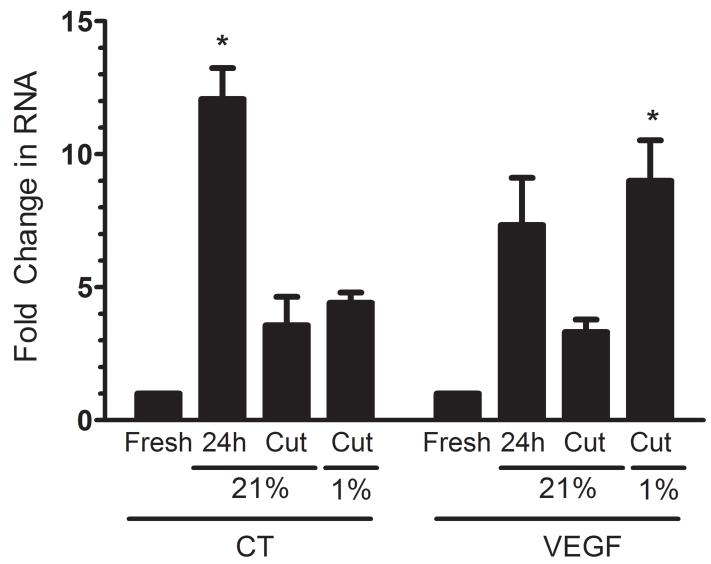
Figure 3. CT induction is diminished in organ slices.
Levels of CT and VEGF (hypoxia marker) RNA were determined in fresh and cultured (24 h) mouse ganglia, as well as ganglia that were cut and then cultured at normoxia (21% O2) or hypoxia (1% O2). Mean +/−SEM from 3 independent experiments. *, p < 0.05 vs. fresh (Kruskal Wallis test with Dunn’s posttest).
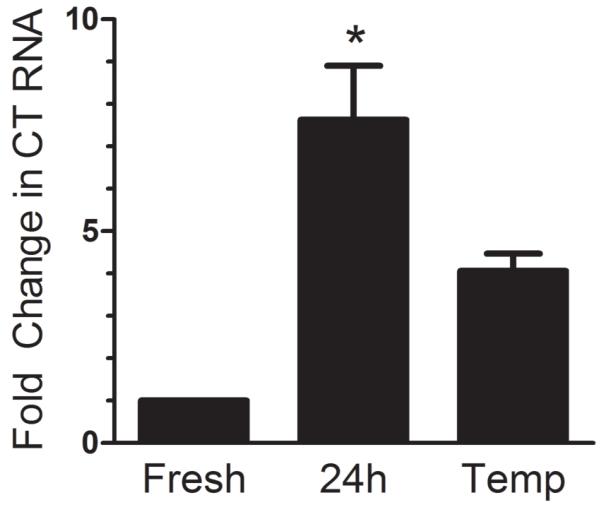
Figure 4. Treatment with an anti-oxidant attenuates CT induction.
Mouse trigeminal ganglia incubated with tempol (Temp, 1 mM) show a smaller increase in CT compared to untreated ganglia. Mean +/− SEM from 3 independent experiments. *, p < 0.05 vs. fresh (Kruskal Wallis test with Dunn’s posttest).
Statistical analysis
All data are given as mean +/− SEM. For comparisons between two groups, the non-parametric Mann Whitney test was used. When comparing 3 or more groups, Kruskal Wallis test was used, followed by Dunn’s test for post hoc analysis. For analysis of quantification of immunostaining, Fischer’s Exact Test was used. Differences were considered significant at p < 0.05.
Results
Increased CT mRNA in organ cultures of trigeminal ganglion
Trigeminal ganglia were removed from adult mice and rats and placed in organ culture overnight. Since the Calca gene encodes two alternative splice products, CT and CGRP (Figure 1A), we assessed RNA levels of both of these transcripts using quantitative RT-PCR. Following 24 h of organ culture, CT mRNA significantly increased in mouse tissue by ~12-fold and trended towards an increase in rat tissue (Figure 1B). In contrast, there was no significant change in CGRP mRNA in mouse and rat ganglia (Figure 1B). The housekeeping gene PPIA was used as the reference gene for all of these studies (see Methods).
Glial cells express proCT after organ culture
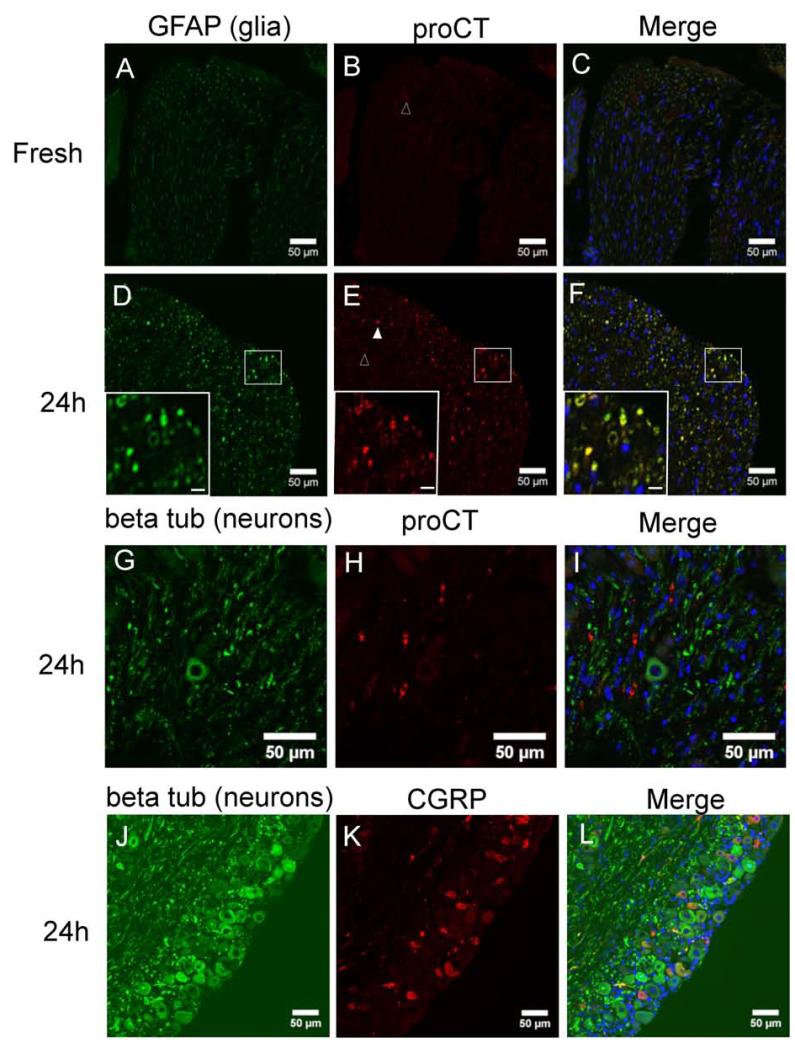
We next used immunohistochemistry to determine which cell type was responsible for the increased CT mRNA in the organ cultures (Figure 2). CT mRNA is initially translated into a 136 (mouse) or 141 (human) amino acid propeptide encoding proCT, which is then normally processed to yield the mature 32 amino acid peptide hormone CT (Figure 1A). ProCT staining was barely detectable in fresh ganglia (Figure 2B), but was readily observed after 24 h of organ culture (Figure 2E). The proCT antibody targets a portion of proCT that is unique to proCT and not present in mature CT24. Double immunolabeling experiments localized proCT staining to glial cells (Figure 2D-F). Identification of glia cells was based on their immunoreactivty for GFAP35. The location of proCT immunoreactivity is suggestive of Schwann cells, although we cannot rule out staining in satellite glia also. GFAP expression has been documented in both trigeminal satellite glia and Schwann cells. An antibody targeting glutamine synthetase was initially used to identify satellite glial cells28(not shown). However, staining for this marker disappeared at 24 h, consistent with other reports36, hence double labeling experiments with proCT were not possible. ProCT immunoreactivity was quantified by counting the number of bright and dim proCT puncta, as well as the number of nuclei per field. For fresh samples, only 15 dim puncta were found in regions containing a total of 697 nuclei. For 24 h cultured samples, there was a significant increase in the number of puncta, with 109 bright puncta and 459 dim puncta found in a region of 297 nuclei (p<0.0001). The puncta were usually not directly associated with a nucleus, which is consistent with expression in glia surrounding axon tracts. ProCT immunoreactivity did not co-localize with beta tubulin III, a neuronal marker (Figure 2G-I).
Figure 2. ProCT immunoreactivity in mouse glial cells.
Fresh tissue (A-C) has only faint proCT immunoreactivity (red, B). Cultured ganglia (24 h, D-F) contain proCT immunoreactivity (red, E) that co-localizes with GFAP (green, D), a glial cell marker. GFAP immunoreactivity also increases after 24 h. Open and filled arrowheads indicate cells defined as faint and bright proCT positive cells, respectively. Inset regions of panels D-F are higher magnificent images of the region outlined with a white box. Inset scale bar: 5 μm. G-I: ProCT (red, H) does not co-localize with the neuronal marker beta tubulin III (green, G). J-L: CGRP immunoreactivity (red, K) is found in a subset of neurons (labeled with beta tubulin III, green, J). No change in staining intensity or relative amount of CGRP positive neurons was noted after 24 h. All panels: nuclei are labeled with DAPI. Panels C, F, I, L: merged images. Scale bar: 50 μM.
CGRP immunoreactivity was present only in neurons in both fresh ganglia (not shown) and 24 h cultured tissue (Figure 2J-L). Consistent with the qPCR data, there was no change in staining intensity or relative number of CGRP positive neurons after 24 h. In fresh ganglia, 26% of neurons (56 out of 214) were CGRP positive. In 24 h cultured ganglia, there was no statistical difference from fresh ganglia, with 24% of neurons (27 out of 114) being CGRP positive.
Organ slices show smaller induction of CT mRNA
When the ganglia are removed and placed in culture, oxygenation can only take place via passive diffusion through the media and tissue. We hypothesized that the ganglia were hypoxic in culture due to the relatively large size of the tissue. This notion was supported by an increase in transcript levels of VEGF, a marker of hypoxia (Figure 3). To test whether hypoxia was responsible for the induction of CT, we cut the ganglia to increase oxygen availability. This decreased the radius of the ganglia from 684 +/− 19 μm to 244 +/− 23 μm. As predicted, cutting the tissue blunted the increase in VEGF. CT levels were also decreased when the ganglia was cut (Figure 3). To directly test the dependence of CT induction on hypoxia, we then placed the cut ganglia into a hypoxia (1% O2) chamber. While hypoxia treatment of the cut ganglia was able to restore the induction of VEGF in the culture, CT levels remained low (Figure 3). This suggests that hypoxia alone is not sufficient to induce the gene in this organ culture model; rather, the result suggests that a paracrine signaling mechanism that requires cell-cell contacts may be important for CT induction.
Antioxidant treatment blunts culture induced CT expression
To test if the induction of CT expression in organ culture was mediated by a soluble factor, we first performed conditioned media experiments. Media removed from trigeminal ganglia cultures was added to primary glia prepared from rat trigeminal ganglia. Conditioned media alone was not able to induce expression in trigeminal glia (data not shown). We next considered signaling mechanisms that could act only on cells intimately close to their origin. Reactive oxygen species (ROS) are unstable signaling molecules, and generally act in an autocrine or paracrine manner37. We tested whether ROS in cultured ganglia were responsible for the increase in CT mRNA by adding the antioxidant tempol to the culture media. Ganglia cultured in the presence of tempol show a smaller increase in CT mRNA than cultures with no antioxidant treatment (Figure 4). This supports a model of paracrine signaling in the organ culture induction of CT and links ROS to the induction of CT mRNA.
Reactive oxygen species accumulate following organ culture
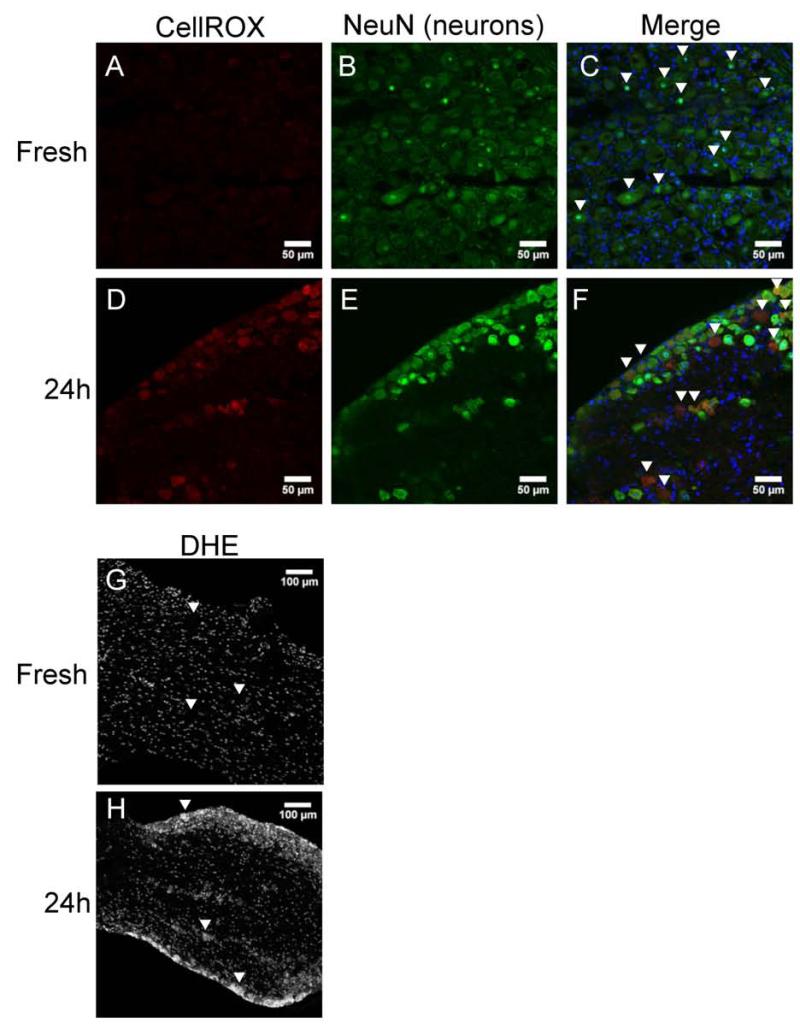
We next sought to determine the source of ROS after 24 h of organ culture. To qualitatively assess levels of ROS in the tissue, we employed two ROS indicator dyes: CellROX Deep Red and dihydroethidium (DHE) (Figure 5). CellROX Deep Red is a non-specific ROS indicator. It lacks fluorescence when it is reduced, but emits light in the far red range following oxidation by ROS. Similarly, DHE changes its emission from blue to red wavelengths when it is oxidized by superoxide ions (O2−). Fresh tissue shows no signal from the CellROX Deep Red (Figure 5A), but following 24 h of organ culture, a strong signal is present (Figure 5D). We determined the cell type with the increased ROS signal was neurons based on double-labeling with the neuronal marker NeuN (Figure 5D-F). Fresh tissue labeled with DHE shows a low level of basal staining in the nucleus of each cell (Figure 5G). The nucleophilic ethidium portion of the molecule causes the molecule to preferentially localize there38. After 24 h of organ culture, the cytoplasm of large, neuronal shaped cells show an increased signal (Figure 5H). These data show an accumulation of ROS within neurons in trigeminal ganglia organ cultures.
Figure 5. Reactive oxygen species accumulate in neurons following 24 h mouse organ culture.
A-F: CellROX Deep Red staining demonstrates the presence of ROS in neurons (based on double labeling with NeuN, indicated with arrowheads) of cultured (24 h), but not fresh, ganglia. Images are representative of 3 independent experiments. Sections are stained with DAPI to detect nuclei. Scale bar: 50 μM. G and H: dihydroethidium (DHE) staining shows superoxide presence in neurons (based on size and location, indicated with arrowheads). Scale bar: 100 μM
Discussion
In this study we have used trigeminal ganglia organ cultures to model an inflammatory state mimicking neurogenic inflammation. While its significance remains controversial, it is generally accepted that neurogenic inflammation can occur during cortical spreading depression and is likely to occur during migraine39-41. The pioneering work by Edvinsson and colleagues established the inflammatory state within the trigeminal ganglia organ cultures and importantly established that the CGRP gene is activated under these conditions28. We reasoned that this activation might involve actions by cytokines based on our previous studies with trigeminal neuron cultures26. Furthermore, activation was coincident with activation of MAP kinases, which we have shown to activate the CGRP gene enhancer in neurons42, 43. Surprisingly, we found a novel induction of the CGRP gene in glia yielded an alternative peptide from the CGRP gene, proCT. While the fundamental observation of Calca gene induction is in full agreement with Tajti et al.28, we did not observe the previously reported increase in CGRP28; possibly due to differences in culture conditions and analytical methods examining RNA and peptides. A major finding of our study was that induction of glial expression of proCT was dependent on ROS signaling from neurons, which provides a mechanism by which activation of the CGRP gene may occur in response to inflammatory signals in migraine.
Recent studies have raised the possibility that proCT may play a role in migraine since it is elevated in serum during the attack21. Based on cellular studies, proCT is likely to act as a partial agonist at the CGRP receptor19. In contrast, unlike mature CT, proCT does not activate the CT receptor19. The binding affinity of proCT at the CGRP receptor has not been determined; however, proCT begins to activate recombinant CGRP receptors at levels around 10−9 M (~13 ng/ml)19. Serum levels of proCT during migraine have been reported at around 0.05 ng/ml21. Concentrations at the sites of production are certainly higher than these serum levels. Nonetheless, the serum levels are low compared to proCT levels used as a marker for sepsis (0.5 ng/ml to 6 ng/ml)44, and level of over 4000 ng/ml reported in a patient following myocardial infarction45. Thus, while it seems reasonable that sufficient proCT can be generated in vivo to activate the CGRP receptor, whether these levels are reached during migraine remains speculative.
The physiological function of proCT during inflammation has been extensively studied in the sepsis field; however, there are limited reports documenting the specific cellular activity of this peptide. In an in vivo model of sepsis, exogenous administration of proCT leads to increased mortality, while immunoneutralization with a blocking antibody increased survival46, suggesting a physiological role for proCT. ProCT may also be involved in the response to ischemia-reperfusion injury that occurs subsequent to cardiac arrest. Increased levels of proCT following cardiac arrest are associated with increased duration of hypoxia and poor neurological outcome47. Whether proCT is driving any of the pathological processes following injury has not yet been studied. ProCT also has pro-inflammatory properties in vitro, leading to an upregulation of proinflammatory cytokines such as IL-6, IL-1β, and TNF-α48, 49. Many of these same cytokines are increased in jugular blood during migraine attacks50. The significance of this inflammatory response in migraine remains an open question, but is likely to be important given the clinical success of anti-inflammatory drugs, such as aspirin and COX-2 inhibitors51, 52
This is the first report of accumulation of ROS in neurons of the trigeminal ganglion. In this system, ROS act in a paracrine manner to upregulate proCT in glial cells. How might elevated ROS in one cell affect a nearby cell? One mechanism can be by transfer of a relatively long-lived ROS species (such as H2O2 or a lipid peroxide) between adjacent cells by diffusion across membranes or via gap junctions. Or alternatively, in the model that we favor, ROS in neurons may trigger a signaling cascade that leads to release of CGRP and cytokines, such as TNFa, that act on nearby glia to activate the CGRP gene, possibly by phosphorylation of USF1/2 transcription factors. This latter mechanism is supported by evidence of elevated cytokines and MAP kinase activity in the organ cultures mentioned earlier28. At this point, specific signaling pathways that induce proCT following ROS production are unknown. Neurons are likely to be affected by changes in their local redox environment. Recently, redox sensitive residues were identified on an M-type potassium channel expressed in the trigeminal ganglion53. Modification of these residues by peroxide and superoxide radical increase excitability and thus lower thresholds for pain transmission along the trigeminal nerve. Another study showed that ROS were able to directly sensitize sensory neurons via sensitization of TRPA1 as well as increase CGRP release54. CGRP can then lead to increased Substance P release, triggering more ROS production along with neurogenic inflammation6. In both of these models, ROS signals would ultimately lead to relaxed chromatin surrounding the normally silent CGRP gene in glia24. Future studies will be needed to evaluate the mechanisms underlying ROS-induced epigenetic changes.
There are several strands of evidence that link ROS and migraine. First, ROS are produced in the cortex during cortical spreading depression (CSD) that is associated with migraine55. This report has recently been confirmed and extended to the trigeminal system. Shatillo et al. demonstrated CSD induction of ROS by measuring a relatively long-lived peroxidation product in the cortex, meninges, and, importantly, the trigeminal ganglion54. There are hints in the literature that CGRP may play some role in CSD56, 57. A second link between migraine and ROS is the use of nitroglycerin to induce migraine. Nitroglycerin is an established migraine trigger58 that is converted to the free radical nitric oxide in vivo59. Endogenous nitric oxide levels are also reported to be increased in jugular venous plasma during migraine60. Third, neurogenic inflammation and local signaling in the trigeminal ganglia may also be sufficient to generate ROS. It has been demonstrated that sensory neurons contain all of the machinery necessary to produce ROS61. For example, substance P can induce ROS production via its actions at the NK1 receptor62. Substance P and CGRP are frequently released from the same neuron63, 64, and CGRP can induce substance P release from trigeminal ganglia neurons in vitro65. Finally, while hypoxia alone did not account for the upregulation of proCT, hypoxia is known to induce ROS 66. A combination of hypoxia with paracrine signals may be required for full induction in the organ culture. Also, while hypoxia is unlikely to occur within the trigeminal nerve under physiological conditions, a prolonged hypoxia occurs in the cortex following CSD67, and hypoxia is an established trigger of migraine68
In conclusion, these results demonstrate that ROS can induce the Calca gene in trigeminal ganglia glia by a paracrine mechanism. Glial production of the CGRP-like peptide proCT adds a new potential biomarker of migraine. Furthermore, these findings raise the possibility that cortical glia may also be recruited by ROS-mediated mechanisms to express the Calca gene, for example following neurogenic inflammation, hypoxia, or CSD.
Acknowledgements
The authors wish to thank Chantal Allamargot and the University of Iowa Central Microscopy Research Facility for training and assistance with IHC and imaging. We also wish to thank Frederick Domann and Trenton Place for helpful discussions and technical assistance with hypoxia experiments. This work was supported by National Institutes of Health grant NS075599 and a National Research Service Award F31NS074728 to ACR.
Abbreviations
- proCT
procalcitonin
- CGRP
calcitonin gene-related peptide
- IHC
immunohistochemistry
- DHE
dihydroethidium
- VEGF
vascular endothelial growth factor
- ROS
reactive oxygen species
- CSD
cortical spreading depression
- q-PCR
quantitative real time PCR
- PPIA
peptidylprolyl isomerase A
Footnotes
Authors declare no conflict of interest
References
- 1.Ray BS, Wolff HG. Experimental studies on headache - Pain-sensitive structures of the head and their significance in headache. Arch Surg-Chicago. 1940;41:813–856. [Google Scholar]
- 2.Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci. 2011;31:14204–14217. doi: 10.1523/JNEUROSCI.3285-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edvinsson L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia. 2011;31:737–747. doi: 10.1177/0333102411398152. [DOI] [PubMed] [Google Scholar]
- 4.Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 5.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 8.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacology & Therapeutics. 2009;124:309–323. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain. 2009;10:137–143. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 14.Connor KM, Shapiro RE, Diener HC, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–977. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 16.Becker KL, Nylen ES, White JC, Muller B, Snider RH., Jr. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. Journal Clinical Endocrinology Metabolism. 2004;89:1512–1525. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- 17.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 18.Domenech VS, Nylen ES, White JC, et al. Calcitonin gene-related peptide expression in sepsis: postulation of microbial infection-specific response elements within the calcitonin I gene promoter. Journal of Investigative Medicine. 2001;49:514–521. doi: 10.2310/6650.2001.33628. [DOI] [PubMed] [Google Scholar]
- 19.Sexton PM, Christopoulos G, Christopoulos A, Nylen ES, Snider RH, Jr., Becker KL. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med. 2008;36:1637–1640. doi: 10.1097/CCM.0b013e318170a554. [DOI] [PubMed] [Google Scholar]
- 20.Buhaescu I, Yood RA, Izzedine H. Serum procalcitonin in systemic autoimmune diseases--where are we now? Semin Arthritis Rheum. 2010;40:176–183. doi: 10.1016/j.semarthrit.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Turan H, Horasanli B, Ugur M, Arslan H. Procalcitonin levels in migraine patients. Can J Neurol Sci. 2011;38:124–128. doi: 10.1017/s0317167100011161. [DOI] [PubMed] [Google Scholar]
- 22.Eftekhari S, Edvinsson L. Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Therapeutic Advances Neurological Disorders. 2010;3:369–378. doi: 10.1177/1756285610388343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park KY, Russo AF. Control of the calcitonin gene-related peptide enhancer by upstream stimulatory factor in trigeminal ganglion neurons. J Biol Chem. 2008;283:5441–5451. doi: 10.1074/jbc.M708662200. [DOI] [PubMed] [Google Scholar]
- 24.Park KY, Fletcher JR, Raddant AC, Russo AF. Epigenetic regulation of the calcitonin gene-related peptide gene in trigeminal glia. Cephalalgia. 2011;31:614–624. doi: 10.1177/0333102410391487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–2708. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- 26.Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansen KA, Edvinsson L. Neurogenic inflammation: a study of rat trigeminal ganglion. J Headache Pain. 2010;11:485–495. doi: 10.1007/s10194-010-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajti J, Kuris A, Vecsei L, Xu CB, Edvinsson L. Organ culture of the trigeminal ganglion induces enhanced expression of calcitonin gene-related peptide via activation of extracellular signal-regulated protein kinase 1/2. Cephalalgia. 2011;31:95–105. doi: 10.1177/0333102410382796. [DOI] [PubMed] [Google Scholar]
- 29.Dapson RW. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotechnic & Histochemistry. 2007;82:133–140. doi: 10.1080/10520290701567916. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Fisher JT, Lynch TJ, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121:3144–3158. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 33.Spano AJ, Frankfurter A. Characterization of anti-beta-tubulin antibodies. Methods Cell Biology. 2010;95:33–46. doi: 10.1016/S0091-679X(10)95003-6. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Jessen KR, Morgan L, Stewart HJ, Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990;109:91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- 36.Belzer V, Shraer N, Hanani M. Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol. 2010;6:237–243. doi: 10.1017/S1740925X1100007X. [DOI] [PubMed] [Google Scholar]
- 37.Zangar RC, Bollinger N, Weber TJ, Tan RM, Markillie LM, Karin NJ. Reactive oxygen species alter autocrine and paracrine signaling. Free Radical Biology & Medicine. 2011;51:2041–2047. doi: 10.1016/j.freeradbiomed.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucana CD, Giavazzi R, Nayar R, et al. Retention of vital dyes correlates inversely with the multidrug-resistant phenotype of adriamycin-selected murine fibrosarcoma variants. Experimental Cell Research. 1990;190:69–75. doi: 10.1016/0014-4827(90)90145-z. [DOI] [PubMed] [Google Scholar]
- 39.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurological Sciences. 2006;27(Suppl 2):S86–90. doi: 10.1007/s10072-006-0577-z. [DOI] [PubMed] [Google Scholar]
- 42.Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23:807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durham PL, Russo AF. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Molecular Endocrinology. 1998;12:1002–1009. doi: 10.1210/mend.12.7.0135. [DOI] [PubMed] [Google Scholar]
- 44.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clinical Infectious Diseases. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 45.Lateef A, Khoo SM, Lee KH. Procalcitonin in hypoxic brain damage. Intensive Care Medicine. 2005;31:494. doi: 10.1007/s00134-004-2541-9. [DOI] [PubMed] [Google Scholar]
- 46.Nylen ES, Whang KT, Snider RH, Jr., Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001–1006. doi: 10.1097/00003246-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Annborn M, Dankiewicz J, Erlinge D, et al. Procalcitonin after cardiac arrest - an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation. 2013;84:782–787. doi: 10.1016/j.resuscitation.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Araujo M, Doi SQ, Palant CE, Nylen ES, Becker KL. Procalcitonin induced cytotoxicity and apoptosis in mesangial cells: implications for septic renal injury. Inflammation Research. 2013 doi: 10.1007/s00011-013-0646-8. [DOI] [PubMed] [Google Scholar]
- 49.Liappis AP, Gibbs KW, Nylen ES, et al. Exogenous procalcitonin evokes a pro-inflammatory cytokine response. Inflammation Research. 2011;60:203–207. doi: 10.1007/s00011-010-0255-8. [DOI] [PubMed] [Google Scholar]
- 50.Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 51.Diener HC, Lampl C, Reimnitz P, Voelker M. Aspirin in the treatment of acute migraine attacks. Expert Rev Neurother. 2006;6:563–573. doi: 10.1586/14737175.6.4.563. [DOI] [PubMed] [Google Scholar]
- 52.Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002;22:491–512. doi: 10.1046/j.1468-2982.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- 53.Ooi L, Gigout S, Pettinger L, Gamper N. Triple cysteine module within M-type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. J Neurosci. 2013;33:6041–6046. doi: 10.1523/JNEUROSCI.4275-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shatillo A, Koroleva K, Giniatullina R, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Viggiano A, Viggiano E, Valentino I, Monda M, Viggiano A, De Luca B. Cortical spreading depression affects reactive oxygen species production. Brain Res. 2011;1368:11–18. doi: 10.1016/j.brainres.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 56.Tozzi A, de Iure A, Di Filippo M, et al. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A. 2012;109:18985–18990. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colonna DM, Meng W, Deal DD, Busija DW. Calcitonin gene-related peptide promotes cerebrovascular dilation during cortical spreading depression in rabbits. Am J Physiol. 1994;266:H1095–1102. doi: 10.1152/ajpheart.1994.266.3.H1095. [DOI] [PubMed] [Google Scholar]
- 58.Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacological Sciences. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Foster MW, Zhang J, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 61.Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Autonomic Neuroscience: Basic & Clinical. 2009;151:90–97. doi: 10.1016/j.autneu.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linley JE, Ooi L, Pettinger L, et al. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci U S A. 2012;109:E1578–1586. doi: 10.1073/pnas.1201544109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y, Takami K, Kawai Y, et al. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- 64.Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides. 1985;6:747–754. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magalhaes J, Ascensao A, Soares JM, et al. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. Journal Applied Physiology. 2005;99:1247–1253. doi: 10.1152/japplphysiol.01324.2004. [DOI] [PubMed] [Google Scholar]
- 67.Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 68.Schoonman GG, Sandor PS, Agosti RM, et al. Normobaric hypoxia and nitroglycerin as trigger factors for migraine. Cephalalgia. 2006;26:816–819. doi: 10.1111/j.1468-2982.2006.01112.x. [DOI] [PubMed] [Google Scholar]