Abstract
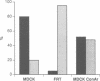
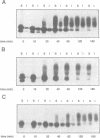
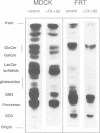
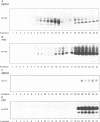
We studied the role of the association between glycosylphosphatidylinositol (GPI)-anchored proteins and glycosphingolipid (GSL) clusters in apical targeting using gD1-DAF, a GPI-anchored protein that is differentially sorted by three epithelial cell lines. Differently from MDCK cells, where both gD1-DAF and glucosylceramide (GlcCer) are sorted to the apical membrane, in MDCK Concanavalin A-resistant cells (MDCK-ConAr) gD1-DAF was mis-sorted to both surfaces, but GlcCer was still targeted to the apical surface. In both MDCK and MDCK-ConAr cells, gD1-DAF became associated with TX-100-insoluble GSL clusters during transport to the cell surface. In dramatic contrast with MDCK cells, the Fischer rat thyroid (FRT) cell line targeted both gD1-DAF and GlcCer basolaterally. The targeting differences for GSLs in FRT and MDCK cells cannot be accounted for by a differential ability to form clusters because, in spite of major differences in the GSL composition, both cell lines assembled GSLs into TX-100-insoluble complexes with identical isopycnic densities. Surprisingly, in FRT cells, gD1-DAF did not form clusters with GSLs and, therefore, remained completely soluble. This clustering defect in FRT cells correlated with the lack of expression of VIP21/caveolin, a protein localized to both the plasma membrane caveolae and the trans Golgi network. This suggests that VIP21/caveolin may have an important role in recruiting GPI-anchored proteins into GSL complexes necessary for their apical sorting. However, since MDCK-ConAr cells expressed caveolin and clustered GPI-anchored proteins normally, yet mis-sorted them, our results also indicate that clustering and caveolin are not sufficient for apical targeting, and that additional factors are required for the accurate apical sorting of GPI-anchored proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Evans W. H. Priority targeting of glycosyl-phosphatidylinositol-anchored proteins to the bile-canalicular (apical) plasma membrane of hepatocytes. Involvement of 'late' endosomes. Biochem J. 1990 Oct 1;271(1):193–199. doi: 10.1042/bj2710193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., De Loose M., Van Montagu M., Inzé D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1989 Jan;8(1):31–38. doi: 10.1002/j.1460-2075.1989.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown D. A. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992 Nov;2(11):338–343. [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Dupree P., Parton R. G., Raposo G., Kurzchalia T. V., Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993 Apr;12(4):1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Kobayashi T., Kurzchalia T. V., Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993 Jun 29;32(25):6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Garcia M., Mirre C., Quaroni A., Reggio H., Le Bivic A. GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J Cell Sci. 1993 Apr;104(Pt 4):1281–1290. doi: 10.1242/jcs.104.4.1281. [DOI] [PubMed] [Google Scholar]
- Green R. F., Meiss H. K., Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981 May;89(2):230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan L. A., Lisanti M. P., Rodriguez-Boulan E., Edidin M. Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cells. J Cell Biol. 1993 Jan;120(2):353–358. doi: 10.1083/jcb.120.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. C., Simons K., van Meer G. Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J. 1986 Mar;5(3):483–489. doi: 10.1002/j.1460-2075.1986.tb04237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E. Association of specific cell-surface glycoproteins with a triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798). Exp Cell Res. 1985 Jan;156(1):239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Birk R., Schmidt R. R., Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990 Feb 12;261(1):155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Parton R. G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
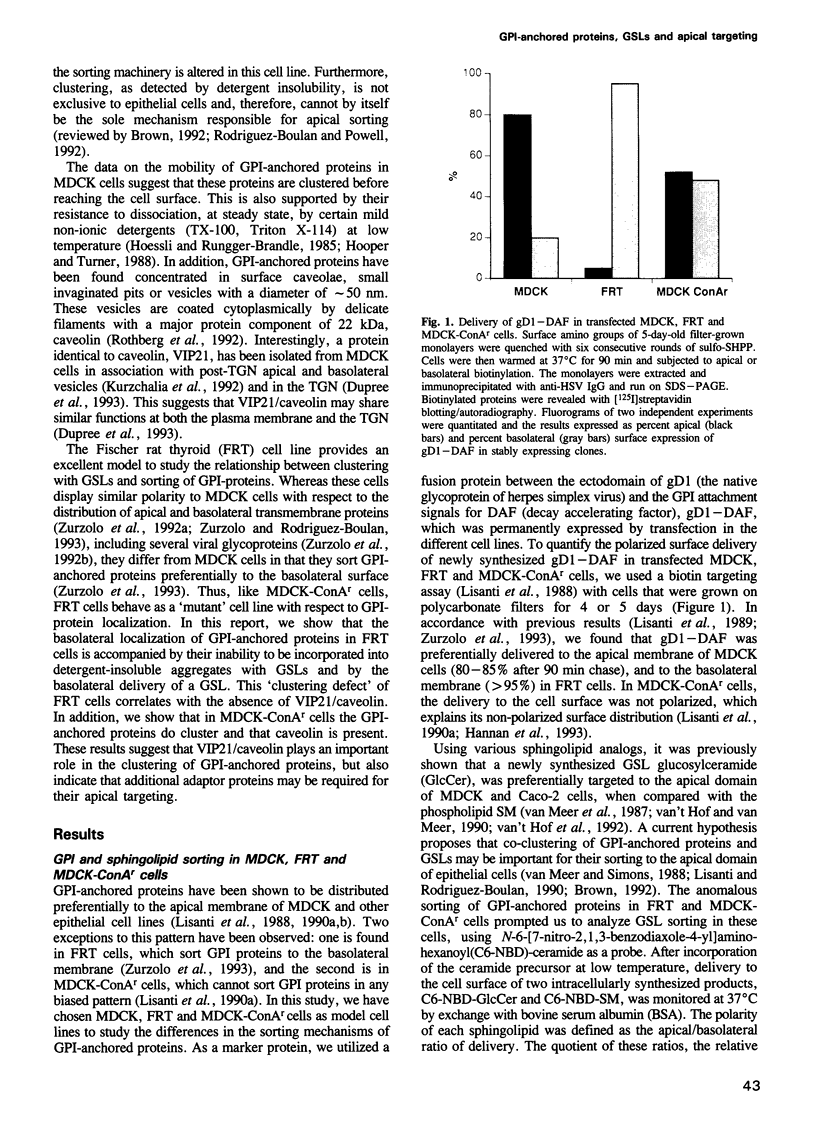
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Gilbert T., Hanzel D., Rodriguez-Boulan E. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7419–7423. doi: 10.1073/pnas.87.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
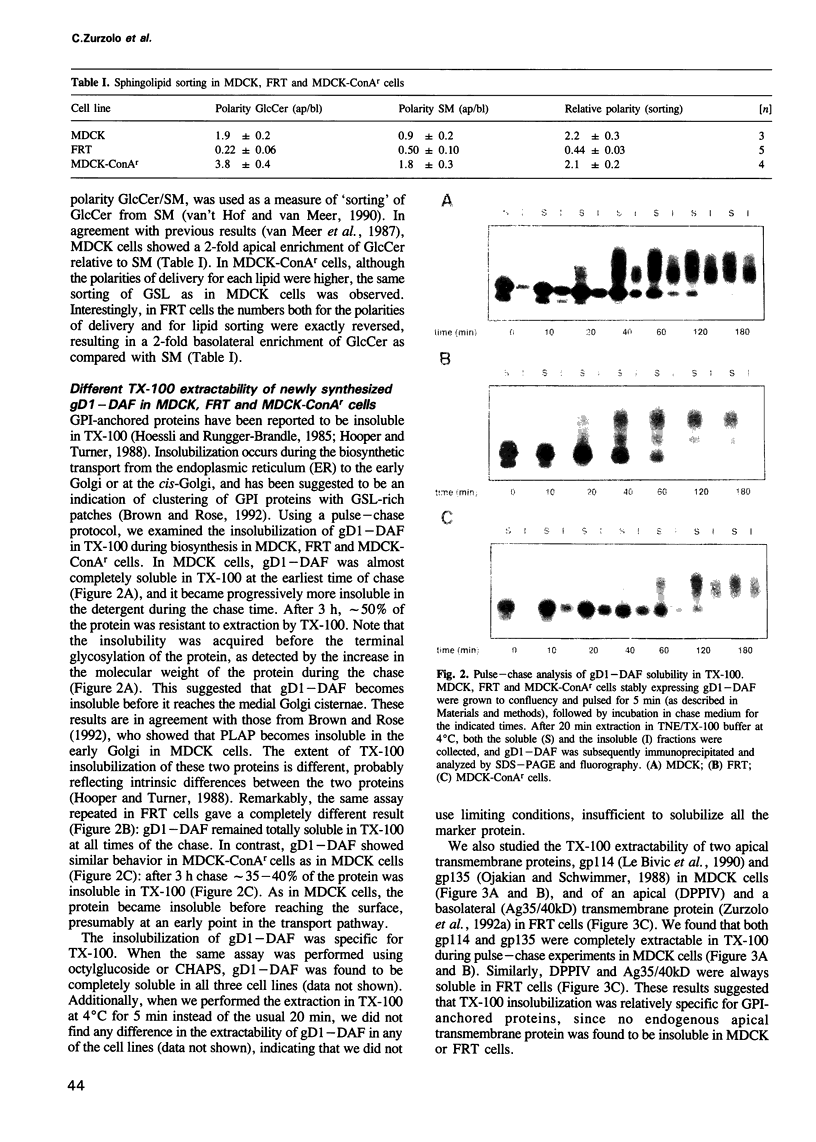
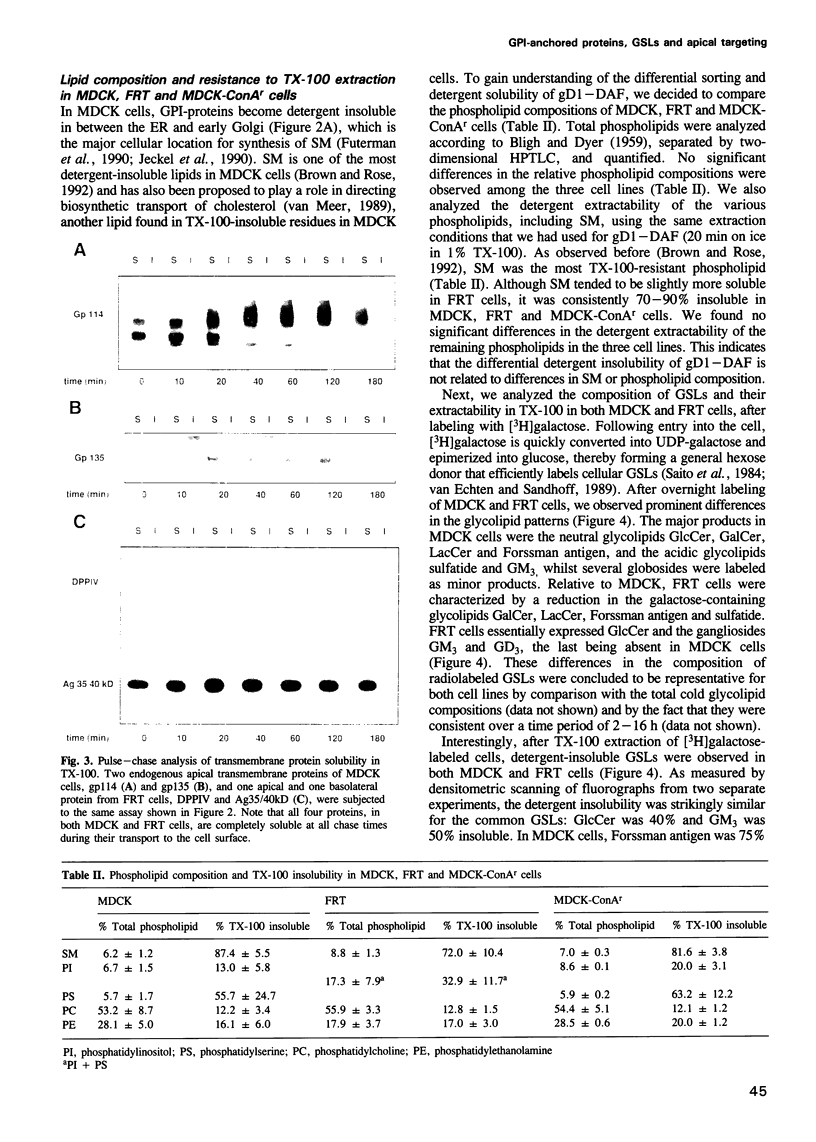
- Lisanti M. P., Le Bivic A., Saltiel A. R., Rodriguez-Boulan E. Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J Membr Biol. 1990 Feb;113(2):155–167. doi: 10.1007/BF01872889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Rodriguez-Boulan E. Glycophospholipid membrane anchoring provides clues to the mechanism of protein sorting in polarized epithelial cells. Trends Biochem Sci. 1990 Mar;15(3):113–118. doi: 10.1016/0968-0004(90)90195-h. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Green R. F., Rodriguez-Boulan E. J. Lectin-resistant mutants of polarized epithelial cells. Mol Cell Biol. 1982 Oct;2(10):1287–1294. doi: 10.1128/mcb.2.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols G. E., Shiraishi T., Young W. W., Jr Polarity of neutral glycolipids, gangliosides, and sulfated lipids in MDCK epithelial cells. J Lipid Res. 1988 Sep;29(9):1205–1213. [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. K., Cunningham B. A., Edelman G. M., Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature. 1991 Sep 5;353(6339):76–77. doi: 10.1038/353076a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. Polarized assembly of enveloped viruses from cultured epithelial cells. Methods Enzymol. 1983;98:486–501. doi: 10.1016/0076-6879(83)98176-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kamen B. A., Anderson R. G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990 Dec;111(6 Pt 2):2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Saito M., Saito M., Rosenberg A. Influence of monovalent cation transport on anabolism of glycosphingolipids in cultured human fibroblasts. Biochemistry. 1985 Jun 4;24(12):3054–3059. doi: 10.1021/bi00333a038. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Skibbens J. E., Roth M. G., Matlin K. S. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989 Mar;108(3):821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Samelson L. E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992 Jun 15;267(17):12317–12322. [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Fasel N., Kraehenbuhl J. P. Polarity of endogenous and exogenous glycosyl-phosphatidylinositol-anchored membrane proteins in Madin-Darby canine kidney cells. J Cell Sci. 1990 May;96(Pt 1):143–149. doi: 10.1242/jcs.96.1.143. [DOI] [PubMed] [Google Scholar]
- Zurzolo C., Le Bivic A., Quaroni A., Nitsch L., Rodriguez-Boulan E. Modulation of transcytotic and direct targeting pathways in a polarized thyroid cell line. EMBO J. 1992 Jun;11(6):2337–2344. doi: 10.1002/j.1460-2075.1992.tb05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Lisanti M. P., Caras I. W., Nitsch L., Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993 Jun;121(5):1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Polistina C., Saini M., Gentile R., Aloj L., Migliaccio G., Bonatti S., Nitsch L. Opposite polarity of virus budding and of viral envelope glycoprotein distribution in epithelial cells derived from different tissues. J Cell Biol. 1992 May;117(3):551–564. doi: 10.1083/jcb.117.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Rodriguez-Boulan E. Delivery of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1993 Apr 23;260(5107):550–556. doi: 10.1126/science.8386394. [DOI] [PubMed] [Google Scholar]
- van 't Hof W., van Meer G. Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J Cell Biol. 1990 Sep;111(3):977–986. doi: 10.1083/jcb.111.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Modulation of ganglioside biosynthesis in primary cultured neurons. J Neurochem. 1989 Jan;52(1):207–214. doi: 10.1111/j.1471-4159.1989.tb10918.x. [DOI] [PubMed] [Google Scholar]
- van Meer G., Burger K. N. Sphingolipid trafficking--sorted out? Trends Cell Biol. 1992 Nov;2(11):332–337. [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988 Jan;36(1):51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]
- van Meer G., Stelzer E. H., Wijnaendts-van-Resandt R. W., Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987 Oct;105(4):1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993 Aug;5(4):661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- van Meer G., van 't Hof W. Epithelial sphingolipid sorting is insensitive to reorganization of the Golgi by nocodazole, but is abolished by monensin in MDCK cells and by brefeldin A in Caco-2 cells. J Cell Sci. 1993 Mar;104(Pt 3):833–842. doi: 10.1242/jcs.104.3.833. [DOI] [PubMed] [Google Scholar]
- van't Hof W., Silvius J., Wieland F., van Meer G. Epithelial sphingolipid sorting allows for extensive variation of the fatty acyl chain and the sphingosine backbone. Biochem J. 1992 May 1;283(Pt 3):913–917. doi: 10.1042/bj2830913. [DOI] [PMC free article] [PubMed] [Google Scholar]