Abstract
Inflammation is associated with development of diseases characterized by altered nutrient metabolism. While an acute inflammatory response is host-protective and normally self-limited, chronic low-grade inflammation associated with metabolic diseases is sustained and detrimental. Resolution of inflammation involves termination of neutrophil recruitment, counter-regulation of pro-inflammatory mediators, stimulation of macrophage-mediated clearance and tissue remodeling. Specialized pro-resolving lipid mediators (SPM) -- resolvins, protectins and maresins -- are novel autacoids that resolve inflammation, protect organs, and stimulate tissue regeneration. Here, we review evidence that failure of resolution programs contributes to metabolic diseases and that SPM may play pivotal roles in their resolution.
Initiation and resolution of acute inflammation: Identification of the pro-resolving mediators
Acute inflammatory responses are protective for the host, yet when uncontrolled or inappropriately activated, acute inflammation can lead to persistent chronic inflammation that is unresolved and can promote organ fibrosis and dysfunction (Figure 1A) (Majno and Joris, 2004). While chronic inflammation is classically associated with arthritis and periodontitis (Gilroy, 2010; Karp, 2010; Ward, 2010), there is increasing evidence that uncontrolled inflammation is also associated with many other chronic diseases, such as asthma and neurological degenerative disorders, as well as metabolic diseases including diabetes, obesity and cardiovascular disease (reviewed in Nathan and Ding, 2010; Tabas and Glass, 2013). An acute inflammatory response is, by definition, divided into an initiation phase and a resolution phase. The initiation phase is accompanied by the cardinal signs of inflammation known to ancient civilizations as heat, swelling and pain (Majno and Joris, 2004) with eventual loss of function that are controlled for the most part by local chemical autacoids (Houck, 1979). A majority of these chemical messengers are in the form of peptides (cytokines, chemokines), proteins and lipid-derived mediators (prostaglandins, leukotrienes) that form chemical gradients that regulate leukocyte trafficking via chemotaxis and diapedesis from the blood stream into the injured tissue. The phagocytes contain invading microbes and clear tissue debris, or remove environmental toxins that appear in tissues as a result of barrier disruption. The repertoire of edema, polymorphonuclear neutrophil (PMN) infiltration and monocyte/macrophage accumulation ensues as a characteristic sequence of events during the initiation of the acute inflammatory response (Figure 1A) (Cassatella, 2003; Gilroy, 2010; Karp, 2010; Ward, 2010). Without an appropriate termination and clearance of phagocytes, the continued presence of activated leukocytes within tissues is associated with collateral tissue damage, amplification and persistence of tissue inflammation. Hence, controlling PMN infiltration, cessation and removal from tissues as well as macrophage accumulation/activation and removal could attenuate non-resolving chronic tissue inflammation.
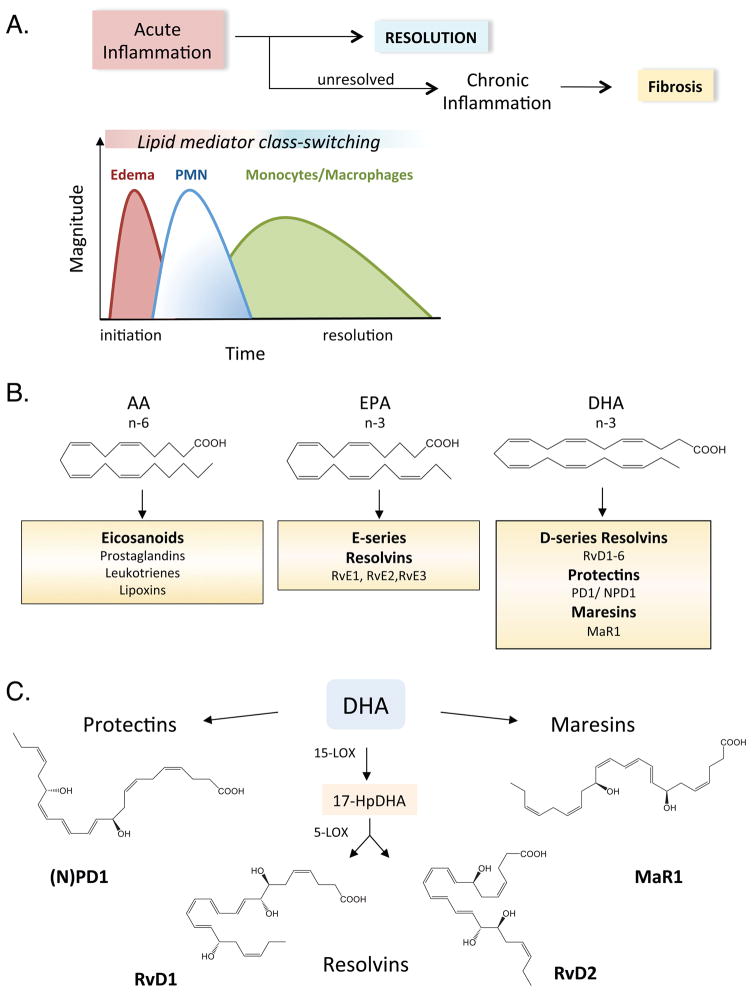
Figure 1. Specialized pro-resolving lipid mediator biosynthesis during resolution of inflammation.
(A) Complete resolution is the ideal outcome of inflammation, although if not properly regulated can lead to chronic inflammation, fibrosis and loss of function. Inflammation and its resolution involves a temporal series of leukocyte trafficking events coupled with lipid mediator class switching, in which pro-inflammatory lipid mediators signal the generation of pro-resolving lipid mediators. (B) Depiction of classic and novel lipid mediator families generated from essential omega-6 (n-6) and omega-3 (n-3) fatty acids, arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). (C) Abbreviated biosynthetic pathways and structures of resolvins, protectins and maresins generated enzymatically from DHA. Their complete stereochemical structures are established; see text for details. LOX-lipoxygenase, RvD1-resolvin D1, PD1-protectin D1, MaR1-maresin 1.
Many initiation phase pro-inflammatory mediators are well known (i.e. prostaglandins, pro-inflammatory cytokines) and popular anti-inflammatory treatments are directed toward either blocking and/or antagonizing these mediators in a quest to control unwanted excessive inflammation (Samuelsson et al., 1987; Flower, 2006; Dinarello et al., 2012). What controls or limits the number of leukocytes that congregate within inflammatory exudates and how are signaling events organized towards resolving the acute inflammatory response (i.e. removal of apoptotic PMN and cellular debris), promoting the return to homeostasis, which is the ideal outcome of an inflammatory challenge (Serhan et al., 2000; Levy et al., 2001; Serhan, 2004)? The resolution of inflammation and return to homeostasis was widely believed to occur via dissipation of initiating chemotactic signals in the acute inflammatory response (Majno and Joris, 2004). This dissipation can arise in part because of extensive negative feedback regulation of toll-like receptor signaling via induction of IkB-α, and A20, as well as transcriptional repressors, including activating transcription factor 3 (Atf3), for example (Olefsky and Glass, 2010). In addition, anti-inflammatory cytokines, such as IL-10, also blunt inflammatory gene transcription. However, in addition to a decrescendo of pro-inflammatory mediators, ample evidence now shows that resolution of contained inflammatory exudates is an actively programmed biochemical process regulated by temporal biosynthesis of novel chemical mediators during the resolution phase. As described below, these pro-resolving mediators not only counterregulate inflammatory gene transcription, but also directly block and limit excessive PMN migration and stimulate distinct cellular processes such as macrophage uptake of apoptotic PMN, microbes and cellular debris that are required for tissue homeostasis to be re-established (Serhan et al., 2000; Levy et al., 2001; Serhan et al., 2002; Serhan and Savill, 2005). These findings swiftly raised the possibility that failed resolution of an inflammatory challenge could potentially lead to recurring bouts of persistent tissue inflammation and diseases associated with chronic inflammation as well as the notion that the resolution phase is exciting new terrain for targeted innovative therapeutics (Serhan et al., 2007; Buckley et al., 2013).
Resolution is defined at the tissue level with the cessation of leukocyte infiltration in response to chemotactic signals, timely apoptosis of PMN, and the accompanied active clearance of apoptotic cells and debris by macrophages (Serhan, 2004; Ward, 2010). Using a systems approach with LC-MS/MS-based analysis of self-limited inflammatory exudates (ones that resolve to homeostasis on their own) formed in vivo in animal models, as well as in isolated human cells, the Serhan laboratory identified a novel genus of bioactive mediators that comprise four families of distinct structures, namely lipoxins, resolvins, protectins and maresins, biosynthesized within the resolution phase of acute inflammation (Serhan et al., 2000; Levy et al., 2001; Serhan et al., 2002; Hong et al., 2003). These new local mediators activate previously unappreciated pro-resolving mechanisms and their identification demonstrated that the resolution phase of acute inflammation is a biosynthetically active process (Serhan and Savill, 2005).
Lipid mediator class-switching during inflammation and its resolution: Alpha signals Omega
Initiation of acute inflammation is controlled by a number of autacoids including lipid mediators, such as the eicosanoids-prostaglandins (PGs) and leukotrienes that are formed from arachidonic acid (AA; omega-6) and play key roles in regulating blood flow, endothelial permeability and PMN diapedesis (Samuelsson et al., 1987). Transendothelial migration and chemotaxis of PMN towards injured tissue and/or pathogens is governed in part by leukotriene B4 (LTB4) and chemokines (Figure 1A & B). Unexpectedly, we found that there is a temporal switch in lipid mediators from the initiation phase to resolution, that is, different lipid mediators are generated at different times during the evolution of the inflammatory response and these mediators coincide with distinct cellular traffic and events. While maximal levels of LTB4 occur as PMN infiltrate tissues, other eicosanoids, including pro-inflammatory cyclooxygenase products, PGE2 and PGD2, initiate a lipid mediator class switch; a mediator-circuit in exudates that activates leukocyte translational regulation of the enzymes required to produce pro-resolving lipid mediators (Levy et al., 2001). That is, PGE2 and PGD2 stimulate the biosynthesis of lipoxin A4 (LXA4), which appears in exudates at the time point at which PMN levels decline. Indeed, LXA4 serves as an endogenous “stop” signal that decreases PMN infiltration. Thus, signals that mediate resolution of a contained acute inflammatory response are tightly linked to mediators of the initiation phase, i.e. the beginning programs the end of inflammation (Serhan and Savill, 2005). Specialized pro-resolving lipid mediators (SPM) can be generated via transcellular biosynthesis and their appearance increases when macrophages are actively clearing apoptotic PMN (Dalli and Serhan, 2012). Interestingly, macrophage phagocytosis of apoptotic cells also leads to the biosynthesis of pro-resolving lipid mediators, which act in an autocrine manner to facilitate phagocytosis. This mechanism of biosynthesis, coupled with potent regulation of inflammatory gene transcription, is similar to that of other “find me” and “eat me” signals (e.g. adenosine, ATP, CX3CL1) generated by phagocytes that play a key role in the immunologically silent process of apoptotic cell clearance (Han and Ravichandran, 2011; Koroskenyi et al., 2011).
To emphasize specificity of their actions and specialized roles in inflammation, we coined the term “specialized pro-resolving mediators” (SPM). This genus includes several families of chemically and functionally distinct mediators, namely the lipoxins, resolvins, protectins and maresins, because they blunt PMN infiltration, decrease pro-inflammatory mediator production (both lipid mediators and cytokines), and stimulate macrophage-dependent uptake of apoptotic PMN, as well as bacterial clearance (Serhan and Savill, 2005; Chiang et al., 2012). Notably, SPM also regulate PMN apoptosis (see below) and stimulate chemokine scavenging (e.g., CCL3 & CCL5) during resolution via upregulation of CCR5 expression on apoptotic PMN and T cells (Ariel et al., 2006). This mechanism facilitates clearance of these chemokines from sites of inflammation, as the apoptotic cells and bound chemokines are cleared by macrophages and CCR5-dependent signaling is lost in apoptotic cells (Ariel et al., 2006). Systematic identification of these endogenous mediators indicated that they are novel structures and that the precursors for resolvins, protectins and maresins are omega-3 essential fatty acids EPA and DHA, while the lipoxins are generated from the omega-6 fatty acid, AA (Figure 1B). It should be noted that in addition to SPM described here, other important resolution agonists including glucocorticoid-induced annexin A1 are also important players (Perretti and D’Acquisto, 2009).
E-series resolvins (e.g. RvE1, RvE2 and RvE3) are produced from EPA, and DHA is precursor of three novel families of distinct SPM that include the D-series resolvins, protectins (including PD1 or neuroprotectin (N)PD1 when formed in neural tissues), and maresins (Serhan, 2007; Isobe et al., 2012)(Figure 1C). Of interest, there are also temporal relationships between different SPM families and individual mediators produced in response to different pathogens (e.g. viral vs. bacterial) triggers the production of different host SPM (Chiang et al., 2012; Koltsida et al., 2013; Morita et al., 2013). This suggests that, even within the SPM genus, there are distinct roles for individual SPM and that the complexities of their biosynthesis are just beginning to be appreciated.
It is extensively documented that in certain clinical settings omega-3 essential fatty acids (EPA and DHA) regulate both innate and acquired immune responses reviewed in (De Caterina, 2011; Calder, 2013). SPM are generated in humans taking omega-3 dietary supplements and SPM levels are increased above those produced normally in transgenic mice expressing an omega-3 fatty acid desaturase (fat-1) (Hudert et al., 2006; Mas et al., 2012). The identification of the SPM structures, mapping of their biosynthetic pathways and stereochemical assignments for each of the SPM, coupled with the elucidation of their potent actions with human cells and in numerous animal models of inflammation, collectively suggests that SPM formation may underlie some of the beneficial effects attributed to their precursors EPA and DHA (Serhan and Chiang, 2013). Like other small molecules, SPM evoke stereospecific bioactions mediated in the nanomolar range by their binding to specific G-protein coupled receptors (GPCRs; Figure 2). Systematic receptor screening approaches along with radioligand specific binding and results from competition studies identified GPCRs activated by SPM, namely, ChemR23/ERV for RvE1 and RvE2, and ALX/FPR2 and GPR32/DRV for LXA4 and RvD1 (Serhan and Chiang, 2013). Of interest, GPR32 is also activated by RvD5 and RvD3 (Dalli et al., 2013), while other pro-resolving mediators, including Annexin A1, activate ALX/FPR2 (Norling and Perretti, 2013). NPD1/PD1 displays specific binding to PMN and human epithelial cells where neither RvE1 nor LXA4 competes for PD1 specific binding, indicating that PD1 actions are likely mediated by separate receptors. Binding studies using specific receptor expression constructs corroborates their potent actions on isolated cell-types and in vivo, with Kd values in the picomolar-nanomolar range (Serhan and Chiang, 2013).
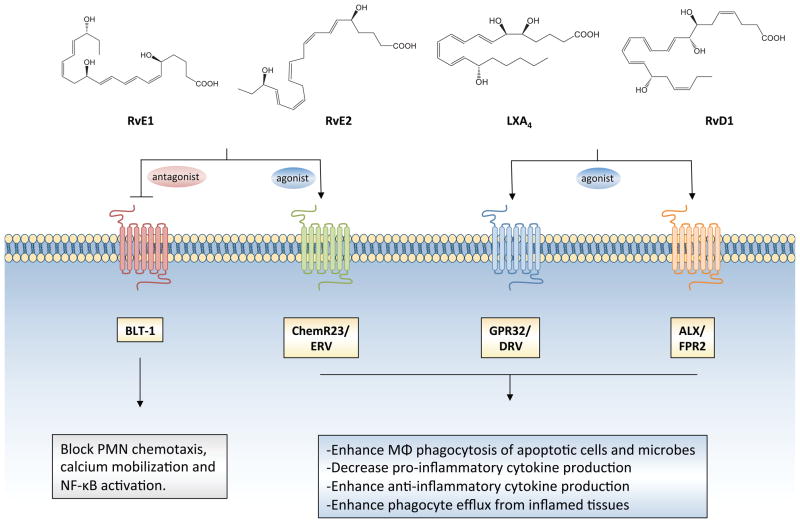
Figure 2. Pro-resolving receptors and biological actions of SPM.
E-series resolvins (RvE1 and RvE2) are agonists for ChemR23/ERV and antagonists for the leukotriene B4 receptor, BLT-1. Lipoxin A4 (LXA4) and D-series resolvins, RvD1, RvD3 and RvD5 (RvD1 structure shown) are agonists for GPR32/DRV1 and ALX/FPR2. Acting via these specific receptors, SPM elicit their biological actions on leukocytes, which include the attenuation of polymorphonuclear neutrophil (PMN) trafficking, activation and inflammatory gene transcription, as well as the stimulation of macrophage phagocytosis of both apoptotic cells and bacteria.
RvE1 and RvE2 are also endogenous receptor antagonists for the LTB4 receptor, BLT-1, which likely explains their ability to potently regulate PMN trafficking to sites of inflammation. The endogenous role of these specific GPCRs in transmitting SPM signals has now been elucidated in vivo in mice with transgenic overexpression of ChemR23 and ALX/FPR2, as well as mice deficient in Fpr2 (the murine isoform of ALX/FPR2) and this has provided insights into the biological role of pro-resolving receptors (Serhan and Chiang, 2013). It should be noted that the substrate precursors of SPM such as EPA and DHA do not activate these receptors (Figure 2) (Arita et al., 2005; Chiang et al., 2012; Dalli et al., 2013), but rather other receptors (i.e. GPR120), although these sensors are activated by these essential fatty acids at high micromolar concentrations. Other fatty acids, including monounsaturated fatty acids e.g., palmitoleic acid and oleic acid, also appear to activate GPR120 (Oh et al., 2010) and thus receptor-mediated actions of fatty acids remain to be elucidated.
During in vivo studies evaluating SPM bioactions, it was found that in addition to halting PMN recruitment and promoting macrophage phagocytosis, SPM also enhance phagocyte efflux from inflamed tissues to draining lymph nodes to aid in host defense. When leukocytes exit the inflamed site or exudate, they traverse perinodal adipose tissue en route to local lymph nodes (Schwab et al., 2007). Excessive and persistent inflammation during this lipo-passage or failure of leukocytes to reach the lymphatics (Schwab et al., 2007) and hence getting stuck while activated within adipose can lead to adipose inflammation that may contribute to metabolic syndrome (see below). Because dysregulated inflammation is associated with many etiologically diverse diseases, it is likely that SPM may play a critical role in preventing chronic inflammatory diseases and potentially organ fibrosis. We refer readers to detailed recent reviews covering SPM biosynthesis (Bannenberg et al., 2005), stereoselective receptor-dependent actions and total organic synthesis (Serhan, 2007; Serhan and Petasis, 2011; Serhan and Chiang, 2013), as a comprehensive analysis of these topics is outside of the scope of this review. Here, we evaluate recent evidence indicating that a failure of resolution and SPM biosynthesis contributes to chronic inflammation in the context of nutrient excess and that resolution agonists such as SPM improve several clinically-relevant outcomes in metabolic syndrome. This work posits that resolution agonists may represent a novel pharmacologic genus that are distinct from traditional anti-inflammatory therapies that impair host-defense. Importantly, it also suggests that SPM may be more effective from a therapeutic standpoint than parent omega-3 fatty acids, which are subject to multiple downstream metabolic checkpoints to elicit their biological effects.
Defective resolution of inflammation in metabolic syndrome
Effector cells of the innate immune system, including PMN and macrophages, are sensitive to changes in nutrient tone and dysregulated systemic metabolism leads to chronic activation and inflammatory signaling in these cells (Lumeng and Saltiel, 2011). Likewise, robust inflammatory signaling occurs in non-immune cells such as endothelial cells and adipocytes during states of nutrient stress (Hotamisligil, 2006). As such, chronic inflammation is associated with several diseases characterized by systemic alterations in metabolism, such as obesity, diabetes and atherosclerosis (Rocha and Libby, 2009; Bornfeldt and Tabas, 2011). While persistent metabolic dysfunction drives inflammation, it is clear that inflammation in turn could disrupt metabolic homeostasis. Thus, it is plausible that a deficiency in the processes that normally resolve inflammation after the induction of an inflammatory response could underlie the viscous feed-forward cycle observed in chronic states of overnutrition.
Hypercholesterolemia
Hypercholesterolemia and sub-endothelial retention of cholesterol-rich oxidized lipoproteins in arteries lead to the recruitment and activation of macrophages, forming atherosclerotic lesions. Macrophage uptake of modified lipoproteins, coupled with defective cholesterol efflux through ATP-binding cassette transporters (i.e. ABCA1 and G1), leads to the formation of cholesterol-loaded macrophage foam cells that persist in the vessel wall, initiate robust pro-inflammatory signaling and eventually undergo post-apoptotic secondary necrosis due to defective egress or clearance by healthy phagocytes (Rocha and Libby, 2009; Bornfeldt and Tabas, 2011). It is currently held that deficient efferocytosis (apoptotic cell clearance) is related to the evolution of benign early fatty streaks (Merched et al., 2011) to more advanced lesions containing necrotic cores, suggesting that a failure to promote efferocytosis may be a key determinant of lesion progression (Bornfeldt and Tabas, 2011). While multiple mechanisms could underlie these defects, including a deficiency of receptors and bridging molecules involved in apoptotic cell uptake (i.e. Mertk, Mfge8), a lack of stimulatory mediators of efferocytosis could also play an important role (see below) (Bornfeldt and Tabas, 2011; Han and Ravichandran, 2011). In addition to macrophages, recent evidence indicates that PMN also contribute to plaque progression and destabilization (Weber et al., 2008). In humans and in animal models, atherosclerosis is associated with increased circulating PMN numbers, which contributes to plaque progression by increasing the generation of reactive intermediates from high levels of myeloperoxidase (MPO) and by promoting monocyte recruitment and dendritic cell (DC) activation (Della Bona et al., 2013). Moreover, accumulation of apoptotic PMN in plaques could result from deficient macrophage-mediated clearance. Thus, persistent recruitment of leukocytes coupled with a failure of phagocyte egress suggests a lack of endogenous pro-resolving mediators that are normally operative during acute inflammation.
Plasma levels of pro-resolving lipid mediator LXA4 (aspirin-triggered form; ATL) inversely correlate with the development of both peripheral and coronary atherosclerosis in humans (Ho et al., 2010). This inverse relationship between ATL and peripheral atherosclerosis remains significant even after adjusting for age, gender and high-sensitivity C-reactive protein (hsCRP). In mouse atherosclerosis models, macrophage-specific overexpression of a biosynthetic enzyme important in SPM biosynthesis (i.e. 12/15-LOX) decreases lesion formation in apolipoprotein E-deficient mice and macrophages isolated from these transgenic mice biosynthesize more LXA4 than WT (Merched et al., 2008). In macrophages, LXA4 and other SPM including RvD1 and PD1 blunt production of cytokines such as CCL2, IFNγ, KC (murine isoform of IL-8) and IL-1β. In contrast, phagocytosis of apoptotic cells is markedly enhanced by all three SPM. In addition to macrophage-targeted actions, SPM also reduce adhesion receptor expression (i.e. VCAM-1 and P-selectin) and chemokine production in isolated endothelial cells stimulated with TNFα. These atheroprotective actions were extended in more recent studies demonstrating that D-series resolvins are generated during vascular injury in vivo and that therapeutic administration of resolvins decreases intimal hyperplasia and leukocyte trafficking to injured arteries (Miyahara et al., 2013). In vitro studies demonstrated that SPM block proliferation, migration, monocyte adhesion and inflammatory signaling in human primary vascular smooth muscle cells (VSMC) in a receptor-dependent manner. Collectively, these findings demonstrate that the perpetuating inflammatory events that lead to advanced atherosclerosis may be related to defective biosynthesis of mediators that resolve local inflammation and promote efferocytosis. Importantly, they highlight that resolution agonists, e.g. resolvins, may be novel immunomodulators that could “resolve” chronic inflammation induced by hypercholesterolemia.
Non-esterified free fatty acids and triglycerides
Similar to hypercholesterolemia, high circulating levels of non-esterified free fatty acids (FFA) associated with obesity and type 2 diabetes also lead to profound activation of inflammatory signaling in both immune and non-immune cells. Liberation of FFA via lipolysis of triglyceride stores in insulin-resistant adipocytes drives macrophage recruitment to hypertrophied adipose tissue and promotes classical activation, while even acute elevation of FFA has been shown to increase macrophage accumulation in the heart (Ko et al., 2009; Gregor and Hotamisligil, 2011). Ectopic triglyceride accumulation in other tissues such as the skeletal muscle and liver is also associated with increased inflammatory signaling and leukocyte accumulation. Results from several studies demonstrate that pattern recognition receptors important in sensing exogenous pathogens are activated by FFA in micromolar concentrations. For instance, high levels of saturated FFA activate TLR2 and TLR4, which leads to immune cell activation, production of inflammatory cytokines and defective insulin signaling (Nguyen et al., 2007). Indeed, TLR4-null mice are protected against insulin resistance and adipose inflammation in response to acute FFA challenge (Shi et al., 2006). More recently, nod-like receptors (NLR), which are intracellular receptors and key components of inflammasome, are implicated as sensors of FFA. Activation of the NLRP3-ASC inflammasome by FFA in hematopoietic cells leads to production of IL1-β and IL-18, and mice deficient in inflammasome components, namely Pycard and Nlrp3, are protected from systemic insulin resistance and hyperglycemia in the context of obesity (Wen et al., 2011). Hence, nutrient sensing by pattern recognition receptors sustains inflammation, which contributes to the development of insulin resistance. It should be noted however that several other mechanisms have been proposed to mediate the diverse signaling roles of FFA, such as alterations in membrane fluidity, nuclear receptor activation and lipid raft formation (Hotamisligil, 2006; Li et al., 2009). Moreover, because circulating FFA encompass a diverse array of both saturated and unsaturated fatty acids, their overall inflammatory potential is likely to be regulated by the relative distribution of FFA species in vivo.
Consistent with increased inflammatory signaling via elevated FFA in leukocytes, we recently documented that saturated FFA promote PMN survival during acute inflammation (Hellmann et al., 2013). We utilized an acute model of sterile peritonitis because we established earlier a set of resolution indices (Bannenberg et al., 2005) that serve as useful criteria for establishing how specific components of resolution are modulated in different scenarios (e.g. experimental inflammation-modifying drugs, etc.). These indices include ψmax: which represents the magnitude of maximal PMN infiltration; Tmax: the time point at which PMN reach their maximum levels; T50: the time point at which PMN decline to half of their maximum value; and Ri (resolution interval): the period of time during which PMN decrease to half of their maximum value (Bannenberg et al., 2005). Using this system, we determined that despite similar infiltration of PMN during acute inflammation in obese-diabetic mice, PMN accumulate during the phase at which they normally undergo apoptosis and are cleared from the inflamed site (Figure 3A&B). Incubation of human PMN with saturated FFA, palmitic acid, promotes signaling via the extracellular signal-related kinase (ERK) pathway and decreases caspase-3 cleavage, effects that are similar to that of LPS-mediated activation of TLRs. As increased PMN survival is associated with delayed resolution of inflammation, these results suggest that chronically elevated FFA may modulate resolution of acute inflammation (Serhan et al., 2007). Interestingly, other studies have recently documented that pro-resolving lipid mediators, such as RvE1, override survival signaling induced by inflammatory mediators (i.e. serum amyloid A) and thus enhance resolution (El Kebir et al., 2012). Hence, in contrast to rapid apoptosis and clearance of PMN during acute inflammation in health, the altered metabolic environment in obesity and T2D leads to chronic leukocyte accumulation in part from prolonged survival at sites of inflammation, e.g., within the adipose tissue. This continued presence of activated PMN can precipitate unintentional tissue injury.
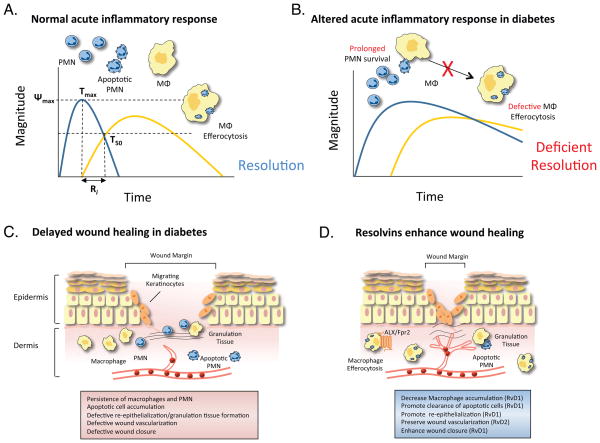
Figure 3. Altered resolution of inflammation in diabetic wounds.
(A) Cellular events during a normal acute inflammatory response, depicting the rapid macrophage-dependent clearance of apoptotic neutrophils (PMN). Established resolution indices are shown, with ψmax representing maximum PMN infiltration, Tmax representing the time point at which PMN reach their maximum levels, T50 representing the time point at which PMN decline to half of their maximum value and Ri representing the resolution interval, the period of time during which PMN decrease to half of their maximum value; see text for details. (B) Altered resolution of acute inflammation in the context of obesity and diabetes in which PMN apoptosis is delayed and macrophage efferocytosis is defective. (C) Depiction of defects in the wound-healing program in diabetes, including persistent leukocyte and apoptotic cell accumulation and defective wound closure. (D) Enhancement of wound healing in diabetes by SPM by promoting macrophage-mediated apoptotic cell clearance and re-epithelialization.
Along with promoting low-grade inflammation, nutrient excess also paradoxically impairs host defense and promotes leukocyte dysfunction. Indeed, results from several studies show that both obesity and diabetes are associated with increased susceptibility to respiratory, skin, odontogenic and post-surgical nosocomial infections (Falagas and Kompoti, 2006). As noted, innate immune cells such as macrophages and PMN play critical roles in bacterial containment via phagocytosis and lysosomal-dependent bacterial killing. Defects in macrophage phagocytosis have been established in animal models of obesity and diabetes and exposure of macrophages to saturated FFA induces defects in both FcR-mediated phagocytosis and apoptotic cell uptake (O’Brien et al., 2002; O’Brien et al., 2006; Li et al., 2009; Khanna et al., 2010; Hodgson et al., 2011). We recently demonstrated that macrophage-mediated clearance of apoptotic thymocytes, as well as IgG-opsonized zymosan, is defective in obese-diabetic mice (Hellmann et al., 2013; Tang et al., 2013). In these studies, we also observed a persistence of apoptotic PMN and zymosan in the peritoneum during acute sterile inflammation. As macrophage-mediated clearance of apoptotic cells is required to resolve inflammation, defects in this specific process in obesity and diabetes suggests that endogenous mediators involved in active stimulation of immunologically silent efferocytosis are diminished (Figure 3A& B). These results in the setting of acute sterile inflammation largely recapitulate findings in models of chronic inflammation, such as atherosclerosis (see above), suggesting that this and related acute models may be useful in identifying molecular events in inflammation that are dysregulated by nutrient excess.
Although the precise molecular mechanisms underlying defects in macrophage phagocytosis induced by saturated FFA are not complete, recent results indicate that autocrine production of prostanoids, such as PGE2 and PGD2 may play a role. These mediators have roles in blunting macrophage phagocytosis by receptor-mediated activation of cAMP pathway, effects that are in part due to downstream activation of phosphatase and tensin homolog on chromosome 10 (PTEN) and subsequent inactivation of phosphatidyl inositol 3-kinase (PI3K) (Canetti et al., 2007). Our recent results, demonstrate that FFA drive inflammatory signaling that leads to the upregulation of COX-2 and subsequent production of PGE2 and PGD2 in macrophages and that autocrine actions of these prostanoids are causally related to defective phagocytosis induced by FFA (Hellmann et al., 2013). Li et al. found that the ratio of omega-6 to omega-3 polyunsaturated fatty acids in macrophages may be an important determinant of defective phagocytosis induced by FFA (Li et al., 2009). While altered uptake of apoptotic cells was evident in macrophages incubated with FFA, these defects were restored by the addition of EPA and DHA. While alterations in membrane fluidity could play a role in the regulation of phagocytosis at higher local concentrations by these diverse fatty acids (Calder, 2013), it is also possible that substrate diversion to anti-inflammatory/pro-resolving lipid mediators may in part underlie the protective effects of omega-3 PUFA in this context because SPM are biosynthesized during macrophage phagocytosis (Chiang et al., 2012; Serhan and Chiang, 2013). In support of this view, defects in diabetic macrophage phagocytosis are acutely reversed by RvD1 in a receptor-dependent manner (Tang et al., 2013). Activation of ALX/Fpr2 by RvD1 blocks cAMP accumulation via coupling ALX/Fpr2 to Gαi and subsequent inhibition of adenylate cyclase (Krishnamoorthy et al., 2010; Hellmann et al., 2013; Tang et al., 2013). As cAMP-PKA signaling impairs phagosome formation at the level of PI3K, it is likely that chronic activation of this pathway may underlie an inability of macrophages to undergo phagocytosis in the context of nutrient excess. Indeed, FFA alter tyrosine phosphorylation of the regulatory p85 subunit of PI3K in macrophages, which is related to defective efferocytosis (Li et al., 2009). This dysfunction and its correction by SPM is potentially of high significance because defects in phagocytosis lead to apoptotic cell accumulation and bacterial proliferation and are thus associated with atherosclerosis, autoimmunity and increased susceptibility to infections. These results also highlight that temporal imbalances between pro-inflammatory vs. pro-resolving lipid mediators sustains inflammation.
Hyperglycemia
The defining metabolic feature of both T1D and T2D is hyperglycemia, due to deficient insulin production or systemic insulin resistance, respectively. Because of the highly integrated nature of glucose and lipid metabolism, it is not surprising that the changes in lipid metabolism in states of obesity and nutrient excess lead ultimately to systemic hyperglycemia. The common downstream effector in these conditions of nutrient stress is uncontrolled inflammation, which likely explains the strong association of diabetes with increased risk of inflammatory diseases, such as cardiovascular disease and cancer. With respect to cardiovascular disease, recent studies suggest that hyperglycemia regulates myelopoiesis and sustained monocyte recruitment to atherosclerotic lesions (Nagareddy et al., 2013). Interestingly, this enhanced myelopoiesis results from increased production of inflammatory mediators (S100A8) released by PMN. Other recent results demonstrate that macrophages isolated from T1D mice exhibit an inflammatory phenotype associated with increased expression of long-chain acyl-CoA synthetase 1 (ACSL1) and that myeloid deletion of Acsl1 decreases atherosclerosis in the context of type I diabetes (Kanter et al., 2012). Mechanistically, hyperglycemia induces ACSL1 in macrophages, which results in increased arachidonic acid (AA)-CoA esters and the production of inflammatory lipid mediators, such as PGE2. Production of PGE2 enhances inflammatory signaling in macrophages and is markedly reduced in Acsl1-deficient macrophages. These results are in alignment to those obtained with macrophages incubated with FFA reviewed above, indicating that sustained production of pro-inflammatory lipid mediators may be a common downstream pathway in macrophages activated by nutrient stress.
Similar to elevated levels of FFA, hyperglycemia also promotes leukocyte dysfunction. Hyperglycemia impairs PMN chemotaxis, yet it increases the production of superoxide and inflammatory cytokines. In Akita mice, which are a model of human T1D, increased PMN adherence to the microvasculature and associated tissue damage were observed in a model of ligature-induced bone loss (Gyurko et al., 2006). In macrophages, hyperglycemia impairs phagocytosis of both apoptotic cells and bacteria, and non-obese diabetic (NOD) mice have a deficiency in the clearance of apoptotic cells (Abrass and Hori, 1984; O’Brien et al., 2002; O’Brien et al., 2006). Given that both hyperglycemia and elevated FFA initiate inflammatory signaling that gives rise to PGE2 production in macrophages (see above), these results suggest that autocrine actions of pro-inflammatory lipid mediators may underlie phagocyte defects in the context of nutrient excess. Thus, leukocyte dysfunction leading to persistent inflammation and altered resolution are common features of nutrient excess and play a causal role in promoting chronic metabolic disease.
Delayed resolution of inflammation in diabetic wounds
Complete resolution of tissue injury involves a temporally coordinated program that is evolutionarily conserved. Following tissue damage from blunt trauma, surgical incision, burn, or ischemia, a prompt response is mounted that has the ultimate goal of returning tissue(s) to their previous state. While in some tissues and organisms the regeneration process is complete, in most cases a fibrotic scar is left at the site of injury after the host-protective inflammatory response has resolved (Figure 1A). Following primary hemostasis, there is early infiltration of PMN, which carry an armament for destroying foreign invaders. As noted above, endogenous counter-regulatory mediators, such as SPM, regulate the magnitude of the PMN infiltrate appropriate for the level of injury and/or pathogen load. Subsequent infiltration of macrophages leads to clearance of apoptotic cells and tissue debris, and macrophages also assist with pathogen eradication and participate in notifying the adaptive immune system of a potential threat through differentiation into antigen presenting cells (i.e. monocyte-derived DCs). Macrophages persist in wounds for an extended period of time and exist in multiple phenotypic states to carry out specific tasks in the wound-healing program, such as tissue remodeling, revascularization and fibrosis (Mosser and Edwards, 2008; Brancato and Albina, 2011). It has recently been shown that so-called “inflammatory” monocytes, which in mice are defined as Ly6ChiCCR2hiCX3CR1low, have considerable plasticity and differentiate into reparative monocytes/macrophages during resolution of fibrosis and tissue regeneration (Ramachandran et al., 2012; Godwin et al., 2013; Nahrendorf and Swirski, 2013; Wynn et al., 2013). Ultimately, blood supply is restored to the injured tissue and carefully orchestrated tissue fibrosis ensues. In the case of cutaneous wounds, re-epithelialization and the reinstatement of barrier function proceeds with the extent of scar tissue formation related to the depth of the wound.
Several steps in the intricately orchestrated wound healing program are disrupted in the context of metabolic disease, and defective wound healing (accompanied by tissue necrosis and infection) is one of the most prominent clinical manifestations of diabetes (Jeffcoate and Harding, 2003). Because of peripheral neuropathies and both macrovascular and microvascular disease, diabetics often sustain wounds in the extremities. Insufficient blood supply increases susceptibility to wounds and also impairs wound healing and thus clinical management of tissue vascularization through vascular and endovascular surgical procedures is often necessary to prevent chronic ischemia. Both obesity and diabetes are associated with increased risk of infection in open wounds, which can be related to defects in leukocyte-mediated pathogen killing and containment (Figure 3C). In fact, diabetic wounds are usually characterized by excessive leukocyte accumulation, indicating that leukocyte dysfunction and failure to promote phagocyte egress may be important determinants of delayed wound healing in diabetes (Figure 3C) (Wetzler et al., 2000; Khanna et al., 2010).
We recently reported that SPM biosynthetic pathways are perturbed in wounds of obese-diabetic mice. In a murine model of cutaneous excisional wound healing, obese-diabetic mice had significant defects in wound closure and this was associated with decreased conversion of DHA to intermediates and pathway biomarkers involved in the biosynthesis of maresins, resolvins and protectins, e.g. 14-HDHA and 17-HDHA (Tang et al., 2013). Leukocytes and apoptotic cells accumulate in the wounds of diabetic mice and because clearance of apoptotic cells is a primary feature of active resolution of inflammation, we asked whether SPM would enhance apoptotic cell clearance and wound closure. Indeed, local application of RvD1 significantly decreases apoptotic cells and macrophages in diabetic wounds, and this translates into enhanced wound closure and granulation tissue formation (Figure 3D). RvD1 rescues defective clearance of apoptotic cells in the thymus of diabetic mice, indicating that correcting phagocyte defects is a primary protective action of SPM in diabetes. In addition to the clearance of apoptotic cells, RvD1 rescues defective FcR-mediated phagocytosis, suggesting that SPM can also serve as effective adjunctive therapeutics to antibiotics in the context of diabetic wound infection. Along these lines, co-treatment of wild-type mice with ciproflaxacin and RvD5 significantly enhances survival in the context of Escherichia coli infection, compared with antibiotic treatment alone, while RvD1, RvD5 and PD1 enhance vancomycin-mediated clearance of Staphylococcus aureus during skin infection (Chiang et al., 2012). Taken together, these findings suggest that defective SPM biosynthesis in diabetes may be related to increased susceptibility to wound infection and prolonged inflammation.
In addition to macrophage phagocytosis, SPM regulate other distinct cellular events during the wound-healing program and may have actions on non-immune cells as well. For instance, RvD1 enhances human keratinocyte migration in vitro, which is a critical step in wound closure (Norling et al., 2011). Lipoxygenase-mediated biosynthesis of MaR1, which is the newest member of the SPM genus, was recently uncovered during tissue regeneration in brown planaria (D. tigrina) subject to surgical injury (Serhan et al., 2012). Add back of synthetic MaR1 significantly enhances tissue regeneration suggesting that SPM play an evolutionarily conserved role in wound remodeling and tissue regeneration. As noted, resolvins, specifically RvD1, RvD2 and RvD5, potently regulate bacterial containment by phagocytes and, in support of this notion, RvD2 was found to enhance survival in a rodent model of cutaneous burn injury and sepsis (Bohr et al., 2013; Kurihara et al., 2013). Treatment of burn wounds with RvD2 largely prevents dermal necrosis and preserves the vasculature, whereas untreated mice show progressive tissue necrosis and thrombosis in the deep dermal vascular network. Mechanistically, RvD2 rescues defective directional migration of PMN isolated from burn wounds and simultaneously decreases pro-inflammatory cytokine production by PMN. These findings suggest that SPM display diverse roles in wound healing programs and that they may be effective mediators in modulating innate cells (e.g. phagocytes) that could rescue defective wound healing in the context of metabolic disease and prevent tissue loss and susceptibility to infection.
Non-resolving adipose tissue inflammation
Adiposity is a dominant risk factor for the metabolic syndrome and related co-morbidities. In mammals, the two types of fat tissue are white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is characterized by the presence of adipocytes containing large unilocular lipid droplets, whereas BAT is mainly composed by multiloculated adipocytes containing large numbers of mitochondria (Rosen and Spiegelman, 2006). WAT is widely distributed through the body and its main function is to store excess energy as triglycerides. In contrast, BAT is located in discrete pockets and is specialized to generate heat by dissipating chemical energy and counteracting hypothermia (Rosen and Spiegelman, 2006). However, BAT is difficult to find in adult humans, since brown fat pads existing within the posterior neck in neonatal humans to provide cold adaptive nonshivering thermogenesis for newborns are lost soon after birth (Cristancho and Lazar, 2011). WAT is mainly recognized for its role as a major secretory organ responsible for lipolysis and release of fatty acids in the circulation.
WAT is the major source of fatty acids that are used as energy substrates for generating ATP (Redinger, 2009). Until the last decade, energy storage and lipolysis were seen essentially as the unique roles of WAT. Nowadays, WAT is regarded as a highly active metabolic tissue and an important endocrine organ involved in the balance of body homeostasis beyond the paradigm of fuel storage. Indeed, since cloning in 1994 of the “ob” gene coding for leptin (Zhang et al., 1994), an ever-increasing number of hormones, signalling peptides and lipid mediators secreted from WAT, now numbering more than 50 different molecular entities, have been identified. These molecules, collectively known as adipokines, are widely recognized as key regulators of inflammation and immune functions, blood pressure homeostasis and metabolic processes such as appetite and satiety, as well as glucose and lipid metabolism.
Adiposity develops when food availability exceeds the metabolic demand (“nutrient excess”). In this setting, adipocytes expand nearly 1,000-fold in volume and 10-fold in diameter to store the excess of fuel as triglycerides (Redinger, 2009). In the setting of obesity, an imbalance between oxygen supply and demand in enlarged adipocytes inadvertently triggers tissue hypoxia, which initiates a cascade of events leading to chronic “low-grade” inflammation in the adipose tissue (Eltzschig and Carmeliet, 2011). This “low-grade” inflammation can be regarded as a long-term inflammatory response triggered by nutrients and metabolic surplus, and therefore is also known as “metabolic-triggered inflammation” or “metainflammation” (Hotamisligil, 2006). “Metabolic inflammation” is usually not systemic but it may affect disease processes in target organs such as the liver and pancreas and increase the incidence of classical inflammatory diseases such as rheumatoid arthritis (Jeppesen, 2011; Gukovsky et al., 2013). It involves molecules and pathways similar to those of classical inflammation, but in this case these signals play a dual role as inflammatory mediators as well as regulators of energy storage and metabolism. A rise in pro-inflammatory adipokines such TNFα, IL-6, IL-1β, MCP-1, leptin and resistin, accompanied by a reduction in the anti-inflammatory and insulin-sensitizing adipokine, adiponectin, has been reported to signal the onset of metabolic dysfunction (Ouchi et al., 2011). This unbalanced secretion of inflammatory adipokines results in the recruitment of circulating inflammatory cells, especially macrophages, into WAT, thus perpetuating an inflammatory vicious cycle.
The ability of adipose tissue to produce bioactive local mediators derived from enzymatic oxygenation of polyunsaturated fatty acids was first described in the late 1960s (Shaw and Ramwell, 1968). PGE2 is an abundant COX product in adipose tissue, where it plays a role in adipogenesis and lipolysis, although its role in WAT inflammation remains unclear. Of interest, 5-LOX products are identified to play a pro-inflammatory role in adipose tissue. Indeed, all enzymes necessary for the formation of 5-LOX products (5-LO, 5-LO-activating protein (FLAP), LTA4 hydrolase, and LTC4 synthase), as well as receptors involved in LT signalling (BLT-1, BLT-2, CysLT1, and CysLT2), are present in adipose tissue (Horrillo et al., 2010). FLAP overexpression and excessive generation of 5-LOX products are common findings in adipose tissue of obese patients and animals with insulin resistance (Back et al., 2007; Horrillo et al., 2010; Chakrabarti et al., 2011). In obese adipose tissue, a direct connection between LTB4 and enhanced release of the adipokines, MCP-1 and IL-6 has been established and mice deficient in LTB4 receptor, BLT-1, show reduced monocyte recruitment to hypertrophied adipose tissue (Horrillo et al., 2010; Spite et al., 2011). Consistently, inhibition of the 5-LOX pathway with a selective FLAP inhibitor or genetic deletion of BLT-1 alleviates adipose tissue inflammation and insulin resistance in obesity (Horrillo et al., 2010; Spite et al., 2011). In addition to 5-LOX, inflammatory lipid mediators generated from AA via 12/15-LOX (i.e. 12-HETE) also play an important role in development of adipose tissue inflammation in obesity (for review see (Cole et al., 2013)). This further emphasizes that lipid mediator pathways are skewed toward continued production of inflammatory lipid mediators in the context of nutrient excess and that the “lipid mediator tone” (see below) may be an important determinant as to whether inflammation resolves or persists in obesity.
In addition to heightened production of pro-inflammatory lipid- and peptide-derived chemical mediators (i.e. LTB4, IL-6, TNFα, and MCP-1), unresolved inflammation in obese adipose tissue also appears to be associated with impaired biosynthesis of anti-inflammatory mediators. Indeed, obesity entails a consistent reduction in the circulating levels of adiponectin, one of the few adipokines that possesses anti-inflammatory and insulin-sensitizing properties (Trayhurn and Wood, 2005). Notably, a deficit in SPM levels is also present in adipose tissues from patients with metabolic syndrome and experimental models of obesity and insulin resistance. In particular, recent studies have uncovered the existence of a marked deficit in PD1 and its precursor 17-HDHA in subcutaneous fat from patients with peripheral vascular disease in whom the inflammatory status in adipose tissue is remarkably exacerbated, compared with healthy subcutaneous fat (Claria et al., 2013). In this study, LC-MS/MS-based metabolo-lipidomic analyses of fat from selected human anatomic locations identified unique signature profiles in the content of bioactive lipid mediators. Importantly, these analyses demonstrated a heterogeneous capacity for SPM biosynthesis among different adipose tissue depots, with higher activation of resolution circuits in perivascular fat compared with subcutaneous fat (Claria et al., 2013). This is relevant for vascular pathologies because for its tissue mass and anatomic proximity surrounding systemic vessels, perivascular adipose tissue plays an emerging role in vascular biology homeostasis. A failure in the endogenous anti-inflammatory peptide annexin A1 to respond to increased systemic inflammation has also been demonstrated in human obesity (Kosicka et al., 2013). At the experimental level, a deficit in tissue SPM levels (RvD1, PD1 and 17-HDHA) has been characterized in inflamed visceral and subcutaneous fat compartments from ob/ob obese and db/db obese/diabetic mice (Gonzalez-Periz et al., 2009; Neuhofer et al., 2013). Since SPM autacoids are made locally, act in their surrounding tissue milieu and are metabolically inactivated, the loss of SPM in obese adipose tissue can be the consequence of omega-3 fatty acid deficiency in the tissue, which are substrates for SPM biosynthesis (Canetti et al., 2007). Along these lines, transgenic restoration of omega-3 fatty acids reversed the inefficient resolution capacity in adipose tissue from obese mice (White et al., 2010). Alternatively, the loss of SPM in obesity may reflect accelerated tissue SPM conversion and clearance to inactive further metabolites because 15-PG-dehydrogenase/eicosanoid oxidoreductase, a key enzyme in SPM inactivation, is markedly up-regulated in obese adipose tissue (Claria et al., 2012). In adipose tissue, both RvD1 and RvD2 are each further metabolically converted to oxo-resolvin products, of which some appear inactive (Claria et al., 2012). It is noteworthy that SPM deficiencies in obesity appears to be a generalized defect in all metabolic tissues, because in addition to adipose, deficiencies are noted in liver, cutaneous wounds and skeletal muscle (White et al., 2010; Tang et al., 2013). Collectively, these findings are consistent with the notion that unresolved chronic “low grade” inflammation in obese adipose tissue is the result of inappropriate resolution-capacity allowing the inflammatory response to proceed without controlled checkpoints.
Consistent with the notion that defective SPM biosynthesis promotes adipose tissue inflammation, administration of exogenous SPM successfully rescues the impaired resolution capacity of obese adipose tissue. In this regard, the administration of nanogram doses of RvD1 to db/db obese/diabetic mice improves glucose tolerance, decreases fasting blood glucose, and increases insulin-stimulated Akt phosphorylation in adipose tissue (Hellmann et al., 2011). This SPM also reduces the formation of crown-like structures rich in inflammatory macrophages in adipose tissue. Similarly, intraperitoneal injection (nanogram amounts) of RvE1 to obese ob/ob mice confers significant insulin-sensitizing effects by mechanisms related to the AMPK-adiponectin axis and the induction of GLUT-4 and IRS-1 expression (Gonzalez-Periz et al., 2009). Also, 17-HDHA treatment (intraperitoneal injection of nanogram doses) reduces adipose tissue expression of inflammatory cytokines (MCP-1, TNFα, IL-6 and osteopontin), increases adiponectin expression and improves glucose tolerance in parallel to insulin sensitivity in db/db obese/diabetic mice (Neuhofer et al., 2013). Ex vivo, in fat explants, both RvD1 and RvD2 each rescue the impaired phenotype of obese adipose tissue by enhancing expression and secretion of adiponectin in parallel with decreasing the secretion of pro-inflammatory adipokines/cytokines including leptin, TNFα, IL-6 and IL-1β (Claria et al., 2012). Using adipose tissue explants from aging female mice as a model of age-associated adipose inflammation, Börgeson et al. recently discovered that LXA4 in nanomolar concentrations decreases IL-6 while restoring GLUT-4 and IRS-1 expression, indicating improved inflammation and insulin sensitivity (Borgeson et al., 2012). Further investigations reveal that LXA4 preserves Akt signaling and glucose uptake in cultured adipocytes. In human monocyte-adipocyte co-incubations, both RvD1 and RvD2 reduce MCP-1 and LTB4-stimulated monocyte adhesion to adipocytes as well as monocyte transadipose migration. These interactions between monocytes and adipocytes are likely events in the progression of inflamed adipose tissue (see above). Importantly, RvD1 stimulates macrophage phagocytosis and enhances the phagocytic activity of macrophages isolated from the adipose tissue stromal vascular cell fraction (Titos et al., 2011). A summary of the main actions of SPM uncovered to date in adipocytes and macrophages from the stromal vascular cell fraction is illustrated in Figure 4. Together, these findings suggest that the lack of intrinsic capacity of adipose tissue to produce endogenous “stop signals” required in the resolution of inflammation is a critical factor(s) that can contribute to obesity-linked inflammation and insulin resistance.
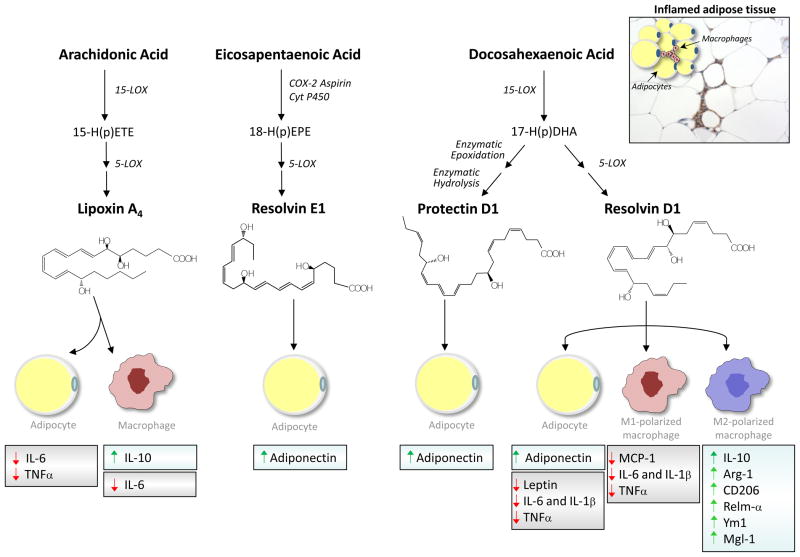
Figure 4. Biosynthesis and biological role of SPM in adipose tissue.
In the adipose tissue, the major long chain polyunsaturated fatty acids, arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid, and, are endogenously converted into potent anti-inflammatory and pro-resolving SPM lipoxin A4, resolvin E1, protectin D1 and resolvin D1. In inflamed adipose tissue, these mediators evoke cell-specific regulatory actions on adipokine expression and/or secretion by adipocytes and macrophages. Resolvin D1 also promotes macrophage polarization toward the anti-inflammatory M2 phenotype. A section of adipose tissue immunostained with an F4/80 antibody, depicting a remarkable infiltration of macrophages forming “crown-like” structures within surrounding adipocytes, is shown in the upper right corner. COX-2: cyclooxygenase-2; Cyt P450: cytochrome P450; LOX: lipoxygenase; IL: interleukin; TNFα: tumor necrosis factor α; MCP-1: monocyte chemoattractant protein-1.
An intriguing aspect of adipose tissue inflammation is that the inflammatory response in this organ/tissue appears to be a unique process driven in large part by tissue macrophages (Lumeng and Saltiel, 2011). Indeed, the presence of an increased number of adipose tissue-infiltrating macrophages, which form the characteristic “crown-like structures” that surround necrotic adipocytes and scavenge adipocyte debris, is a hallmark of obesity (Cancello et al., 2005). Although enlarged adipocytes were initially thought to be the cellular source of pro-inflammatory mediators in obesity, it was later established that infiltrated macrophages in obese fat are the major driver of exacerbated production of pro-inflammatory mediators (Odegaard and Chawla, 2013). The contribution of other inflammatory cell types, including T lymphocytes and eosinophils, to adipose tissue inflammation and homeostasis has also been acknowledged (Odegaard and Chawla, 2013). In addition to the augmented infiltration of macrophages into adipose tissue, obesity also induces a phenotypic switch in these cells toward the classically activated M1 phenotype (Lumeng and Saltiel, 2011). Depending on the disease stage and the signals they are exposed to, macrophages are broadly characterized by their activation (polarization) status according to the M1/M2 classification system (Mosser and Edwards, 2008). Based on this classification, the M1 designation is reserved for classically activated macrophages following stimulation with IFNγ and LPS, whereas the M2 designation is applied to the alternatively activated macrophages after in vitro stimulation with IL-4 and IL-13. M1 macrophages display enhanced pro-inflammatory biosynthetic capacity and increased superoxide anion (O2−) levels (Mosser and Edwards, 2008). Conversely, M2 macrophages produce less pro-inflammatory cytokines and are essential for tissue repair and resolution of inflammation. M1/M2 macrophage polarization can be easily monitored in rodents by assessing the expression of selected markers. M1-associated genes include inducible nitric oxide synthase (iNos) and classical pro-inflammatory mediators such as Tnfα, Il-1β, Il-6 and Ccl2. M2 macrophages display up-regulation of scavenger, mannose (CD206) and galactose (Mgl-1) receptors, arginase 1 (Arg-1), which antagonizes iNOS activity, and Il-10, as well as up-regulation of other genes such as chitinases Ym1 and Ym2, and resistin-like molecule (Relm)-α (also known as FIZZ) (Mosser and Edwards, 2008). Notably, Alox15 is an IL-4/1L-13 responsive gene and recent transcriptomics analyses have demonstrated that Alox15 is highly upregulated in macrophages isolated during the resolution of acute inflammation (Stables et al., 2011). Along these lines, Th2 skewed human peripheral blood monocytes express high levels of Alox15 and produce PD1 from endogenous DHA (Ariel et al., 2005). Moreover, M2 macrophages produce elevated SPM, including MaR1, PD1 and RvD5, than M1 polarized macrophages in vitro, as assessed with metabolipidomic analysis (Dalli and Serhan, 2012).
In line with SPM protective actions against adipose tissue inflammation and in addition to their increased biosynthesis by M2 macrophages, SPM skew adipose tissue macrophages toward an M2 phenotype. RvD1 up-regulates a panel of M2 markers including Il-10, CD206, Relm-α and Ym1 in macrophages from obese adipose tissue (Figure 4) (Titos et al., 2011). RvD1 also remarkably stimulates Arg-1 expression while promoting non-phlogistic macrophage phagocytosis and attenuating IFNγ/LPS-induced Th1 cytokine secretion (Titos et al., 2011). Similar findings have been reported on the ability of RvD1 to improve insulin sensitivity by increasing the percentage of macrophages expressing the M2 marker, Mgl-1, in adipose tissue from obese-diabetic mice (Hellmann et al., 2011). The ability of SPM to modify macrophage plasticity has also been demonstrated by Schif-Zuck et al. (2011). Administration of RvD1 or RvE1 to mice enhances appearance of CD11blow macrophages during acute peritonitis by reducing the number of engulfment-related events required for macrophage deactivation and by reducing the ability of peritoneal macrophages to produce pro-inflammatory cytokines upon LPS stimulation. Together, the ability of SPM to modulate plasticity of tissue macrophages offers new opportunities to facilitate the resolution of adipose tissue inflammation in metabolic diseases.
Steatohepatitis: Failed resolution of metabolic liver disease?
Non-alcoholic fatty liver disease (NAFLD) is a condition ranging from steatosis or simple accumulation of triglycerides in the cytoplasm of hepatocytes, to steatosis combined with inflammation (steatohepatitis or NASH) in the absence of excessive alcohol consumption (Day, 2011). NAFLD is considered the hepatic manifestation of the metabolic syndrome as their prevalence is coincidental in the Western society (Day, 2011). The strong association between NAFLD and obesity has been well documented in the European DIONYSOS study cohort (3,000 participants) in which NAFLD was present in 25% of participants with a normal weight (BMI 20.0–24.9 kg/m2), 67% of overweight participants (BMI 25.0–29.9 kg/m2) and 94% of participants with obesity (BMI ~30 kg/m2) (Bedogni et al., 2005). Although hepatic steatosis is generally an asymptomatic pre-morbid condition, it increases the vulnerability of the liver to progress to more advanced and irreversible forms of liver disease (Day, 2011). Steatotic livers are indeed more susceptible to tissue-damaging effects of oxidative stress and inflammatory mediators, which pave the way for progressive liver damage into NASH, fibrosis and ultimately cirrhosis (Day, 2011). In addition to increased FFA release and exacerbated production of pro-inflammatory adipokines by adipose tissue (Tilg and Moschen, 2008; Gregor and Hotamisligil, 2011), hepatic insulin resistance and hepatic steatosis in obesity are also driven by the activation of classical inflammatory lipid mediator-pathways. This view is consistent with earlier observations reporting that omega-6-derived eicosanoids, especially LTB4, play a role in the progression of metabolic diseases. Indeed, leukotrienes would promote hyperlipidemia-dependent vascular complications and represent a risk factor in atherosclerosis (Zhao et al., 2004). Given that the sequence of molecular and cellular events underlying atherosclerosis (i.e. lipid accumulation, mounting inflammation and progression to fibrous plaque in the arterial vessel wall) is fundamentally similar to that described for NAFLD, it was not surprising that leukotrienes also contribute to metabolic liver disease. Indeed, the 5-LOX pathway is markedly activated in patients and animals with NAFLD (Puri et al., 2009; Horrillo et al., 2010). Consistently, either pharmacological or genetic inhibition of the leukotriene pathway protects against HFD-induced inflammatory liver injury, insulin resistance and TNFα–induced hepatocyte cell death (Horrillo et al., 2010; Martinez-Clemente et al., 2010; Spite et al., 2011).
Omega-3 fatty acid precursors and SPM exert opposite roles in hepatic steatosis to those described for omega-6-derived eicosanoids. In this regard, the administration of an omega-3-enriched diet for 5 weeks significantly alleviates hepatic steatosis in ob/ob mice, an experimental model of obesity-induced insulin resistance and fatty liver disease (Gonzalez-Periz et al., 2009). This anti-steatotic effect is associated with improved insulin tolerance and changes in the expression of specific adipocyte-derived factors (i.e. adipokines) that orchestrate the interaction between adipose tissue and the liver (Gonzalez-Periz et al., 2009). In parallel with this anti-steatotic effect, omega-3-enriched diets also ameliorate inflammatory liver injury in mice (Gonzalez-Periz et al., 2006). The hepatoprotective actions of omega-3 diets are associated with increased generation of SPM in liver tissue (i.e. PD1 and 17S-HDHA). These SPM are able to attenuate DNA damage and oxidative stress in hepatocytes and to reduce TNFα release in macrophages (González-Périz et al., 2006). A proof of concept of the beneficial role of omega-3 fatty acids in metabolic liver disease has recently been gathered in mice with transgenic expression of the C. elegans fat-1 gene, which encodes an omega-3 desaturase capable of generating omega-3 fatty acids from the omega-6 type (see above) (Lopez-Vicario et al., 2013). These fat-1 mice have a more balanced omega-6/omega-3 ratio and are protected from obesity-induced hepatic insulin resistance, steatosis and inflammation. Of interest, transgenic fat-1 mice show increased formation of resolvins (Hudert et al., 2006). Consistently, we reproduce the protective actions of omega-3s against inflammatory injury observed in fat-1 hepatocytes by incubating wild-type hepatocytes with nanomolar concentrations of RvD1 (Lopez-Vicario et al., 2013). Little information is available on the role of other SPM (i.e. lipoxins, maresins) in metabolic liver disease, although the liver is a rich source of LXA4 and 15-epi-LXA4 (Titos et al., 1999), formation of which attenuates IL-8 secretion by hepatocytes (Planaguma et al., 2002) and LXA4 protects mice against hepatotoxin-induced hepatic fibrosis (Puri et al., 2009).
Concluding remarks
While partial gastrectomy and other types of bariatric surgery are successful in weight loss (Carlin et al., 2013), new approaches are needed. Recent efforts have uncovered extensive interactions between immunity and metabolism and have led to the identification of salient mechanisms underlying the initiation of inflammatory signaling in the context of metabolic diseases. Less is known about the resolution of inflammation or SPM biosynthesis in the context of metabolic diseases. As discussed in the present review, some of the features of active resolution appear to be deficient in metabolic disease and treatment with specific SPM improves metabolism and immunity. Nevertheless, several key questions remain: Is resolution impaired in states of nutrient excess or insulin resistance? How do the pro-resolving mediators impact intermediary metabolism and how are these mediators in turn affected by insulin resistance? How do metabolic diseases perturb lipid mediator class switching? Is this merely a problem of substrate ratios in the diet and utilization (e.g. AA vs. EPA and DHA), or are there unappreciated changes in expression or the regulation of the enzymes involved in SPM biosynthesis? How does nutrient metabolism regulate SPM biosynthesis at the molecular level within adipose tissues? As SPM circuits are active in adipose tissue and are decreased in obesity (Claria et al., 2013), what are the roles of specific SPM in lean non-inflamed adipose tissue homeostasis?
Mapping of lipid mediator networks during resolution of inflammation has increased our understanding of the process of resolution itself and has informed new therapeutic strategies for treating inflammation. Although animal studies show that dietary intake of omega-3 fatty acids reduces inflammation, while at the same time improving systemic metabolism and increasing SPM biosynthesis, human studies have in some cases failed to demonstrate consistently a beneficial effect of omega-3s on systemic metabolism (De Caterina, 2011). This discrepancy highlights the need for a more thorough understanding of the impact of nutrient excess on local SPM biosynthesis and SPM levels. It also raises the possibility that use of SPM might represent a more targeted therapeutic approach than simply increasing omega-3 fatty acids in the diet (Tabas and Glass, 2013). These fatty acids are precursors of lipid mediators that are several orders of magnitude more potent and display stereospecific biological roles in regulating inflammation (Serhan and Petasis, 2011; Serhan and Chiang, 2013). Moreover, the anti-inflammatory effects of omega-3 fatty acids are clearly regulated at multiple levels (e.g., receptors and enzymatic activities), which might be independently affected by disease. Hence, direct treatment with lipid mediators, rather than the parent omega-3-fatty acids, may be a more effective in targeting inflammatory responses. The notion that non-selective or off-target effects of omega-3s might be problematic is supported by results suggesting that while high intake of omega-3 fatty acids is beneficial in some contexts, it can increase the appearance of auto-oxidation products and may also increase the risk of certain types of cancer (Brasky et al., 2013), or it is possible that these disease populations are at risk because they are unable to produce SPM.
In resolution, all SPM have two main functions: they stop/limit further PMN entry and stimulate macrophage intake and clearance of apoptotic cells, debris and bacteria; hence, additional research is needed to identify how different SPMs are biosynthesized in response to inflammatory challenges within adipose tissues and how individual SPM differ in their individual actions. Therefore, it will be of interest to determine whether different SPM regulate distinct events in chronic inflammation, and to identify how individual SPM differ in their ability to regulate discrete processes in tissue-specific resolution program(s) and tissue regeneration. In summation, it should be pointed out that certain therapeutic strategies impact resolution, either positively (e.g., carbon monoxide, cyclin-dependent kinase inhibitors) or negatively (e.g., selective COX-2 and LOX inhibitors) (Chiang et al., 2013) and thus determining how the resolution of inflammation in obesity is impacted may be an essential criterion in developing future therapeutic interventions aimed at combating inappropriate inflammation in metabolic disease.
Acknowledgments
The authors of this review gratefully acknowledge support by National Institutes of Health grants HL106173 and GM103492 (to M.S.), GM095467 and GM038765 (to C.N.S.), and Ministerio de Economía y Competitividad (SAF 12/32789) and Generalitat de Catalunya (2009SGR1484) (to J.C.).
Abbreviations
- SPM
Specialized pro-resolving mediator
- PMN
Polymorphonuclear neutrophil
- LC-UV-MS/MS
liquid chromatography-ultraviolet spectrometry-tandem mass spectrometry
- LTB4
leukotriene B4 (5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid)
- LXA4
lipoxin A4 (5S, 6R, 15S-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- PD1/NPD1
protectin D1/neuroprotectin D1 (10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid)
- PGE2
9-oxo-11a, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid
- RvE1
Resolvin E1 (5S, 12R, 18R-trihydroxy-eicosa-6Z, 8E, 10E, 14Z, 16E pentaenoic acid)
- RvE2
Resolvin E2 (5S, 18R-dihydroxy-eicosa-6E, 8Z, 11Z, 14Z, 16E, pentaenoic acid)
- RvE3
Resolvin E3 (17,18-dihydroxy-eicosa-5Z, 8Z, 11Z, 13E, 15E, pentaenoic acid)
- RvD1
Resolvin D1 (7S, 8R, 17S-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD2
Resolvin D2 (7S, 16R, 17S-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid)
- RvD3
Resolvin D3 (4S, 11R, 17S- trihydroxy-docosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD5
Resolvin D5 (7S, 17S-dihydroxy-docosa-4Z, 8E, 10Z, 13Z, 15E, 19Z- hexaenoic acid)
- WAT
White adipose tissue
- NAFLD
non alcoholic fatty liver disease
Footnotes
Disclosure: C.N.S. is an inventor on patents (resolvins) assigned to BWH and licensed to Resolvyx Pharmaceuticals. C.N.S. was scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. C.N.S.’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrass CK, Hori M. Alterations in Fc receptor function of macrophages from streptozotocin-induced diabetic rats. J Immunol. 1984;133:1307–1312. [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back M, Sultan A, Ovchinnikova O, Hansson GK. 5- Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res. 2007;100:946–949. doi: 10.1161/01.RES.0000264498.60702.0d. [DOI] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, Roche HM, Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Jr, Goodman GE, Minasian LM, Parnes HL, et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Canetti C, Serezani CH, Atrasz RG, White ES, Aronoff DM, Peters-Golden M. Activation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of FcgammaR phagocytosis by prostaglandin E2 in alveolar macrophages. J Immunol. 2007;179:8350–8356. doi: 10.4049/jimmunol.179.12.8350. [DOI] [PubMed] [Google Scholar]
- Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, Schram JL, Kole KL, Finks JF, Birkmeyer JD, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791–797. doi: 10.1097/SLA.0b013e3182879ded. [DOI] [PubMed] [Google Scholar]
- Cassatella MA, editor. The Neutrophil. Basel: Karger; 2003. [Google Scholar]
- Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, Williams MD, Bevard MH, Vandenhoff GE, Keller SR, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol Endocrinol Metab. 2011;300:E175–187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AMK, Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique pro-resolving mediator--heme oxygenase-1 circuits. J Immunol. 2013;190:6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–1149. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Lieb DC, Dobrian AD, Nadler JL. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013;104–105:84–92. doi: 10.1016/j.prostaglandins.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med. 2011;11:176–178. doi: 10.7861/clinmedicine.11-2-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- Della Bona R, Cardillo MT, Leo M, Biasillo G, Gustapane M, Trotta F, Biasucci LM. Polymorphonuclear neutrophils and instability of the atherosclerotic plaque: a causative role? Inflamm Res. 2013;62:537–550. doi: 10.1007/s00011-013-0617-0. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147:S182–S192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW. Resolution of acute inflammation and wound healing. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York: Cambridge University Press; 2010. p. 17. [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, Horrillo R, Ferre N, Deulofeu R, Arroyo V, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased Saturated Fatty Acids in Obesity Alter Resolution of Inflammation in Part by Stimulating Prostaglandin Production. J Immunol. 2013 doi: 10.4049/jimmunol.1203369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson KA, Morris JL, Feterl ML, Govan BL, Ketheesan N. Altered macrophage function is associated with severe Burkholderia pseudomallei infection in a murine model of type 2 diabetes. Microbes Infect. 2011;13:1177–1184. doi: 10.1016/j.micinf.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, Lopez-Parra M, Ferre N, Titos E, Moran-Salvador E, Deulofeu R, Arroyo V, Claria J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184:3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Houck JC, editor. Chemical Messengers of the Inflammatory Process. Amsterdam: Elsevier/North-Holland Biomedical Press; 1979. [Google Scholar]
- Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- Jeppesen J. Low-grade chronic inflammation and vascular damage in patients with rheumatoid arthritis: don’t forget “metabolic inflammation”. J Rheumatol. 2011;38:595–597. doi: 10.3899/jrheum.110006. [DOI] [PubMed] [Google Scholar]
- Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–724. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL. Links between innate and adaptive immunity. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York: Cambridge University Press; 2010. p. 28. [Google Scholar]
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN, Andreakos E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5:762–775. doi: 10.1002/emmm.201201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroskenyi K, Duro E, Pallai A, Sarang Z, Kloor D, Ucker DS, Beceiro S, Castrillo A, Chawla A, Ledent CA, et al. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. J Immunol. 2011;186:7144–7155. doi: 10.4049/jimmunol.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicka A, Cunliffe AD, Mackenzie R, Zariwala MG, Perretti M, Flower RJ, Renshaw D. Attenuation of plasma annexin A1 in human obesity. FASEB J. 2013;27:368–378. doi: 10.1096/fj.12-213728. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, Fagan SP, Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vicario C, Gonzalez-Periz A, Rius B, Moran-Salvador E, Garcia-Alonso V, Lozano JJ, Bataller R, Cofan M, Kang JX, Arroyo V, et al. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2013 doi: 10.1136/gutjnl-2012-303179. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. New York: Oxford University Press; 2004. [Google Scholar]
- Martinez-Clemente M, Ferre N, Gonzalez-Periz A, Lopez-Parra M, Horrillo R, Titos E, Moran-Salvador E, Miquel R, Arroyo V, Funk CD, et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology. 2010;51:817–827. doi: 10.1002/hep.23463. [DOI] [PubMed] [Google Scholar]
- Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics. 2011;4:12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, Hochbrugger EE, Fraisl P, Cinti S, Serhan CN, et al. Impaired Local Production of Proresolving Lipid Mediators in Obesity and 17-HDHA as a Potential Treatment for Obesity-Associated Inflammation. Diabetes. 2013;62:1945–1956. doi: 10.2337/db12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Norling LV, Perretti M. Control of myeloid cell trafficking in resolution. J Innate Immun. 2013;5:367–376. doi: 10.1159/000350612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–115. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- O’Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51:2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Planaguma A, Titos E, Lopez-Parra M, Gaya J, Pueyo G, Arroyo V, Claria J. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinger RN. Fat storage and the biology of energy expenditure. Transl Res. 2009;154:52–60. doi: 10.1016/j.trsl.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.1005.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shaw JE, Ramwell PW. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem. 1968;243:1498–1503. [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187:1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Claria J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A(4) Am J Physiol. 1999;277:C870–877. doi: 10.1152/ajpcell.1999.277.5.C870. [DOI] [PubMed] [Google Scholar]
- Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Ward PA. Acute and chronic inflammation. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York: Cambridge University Press; 2010. p. 1. [Google Scholar]
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59:3066–3073. doi: 10.2337/db10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]