Abstract
Previous studies demonstrated that thrombin is an important factor in brain injury after intracerebral hemorrhage. This study investigated the effect of thrombin on hydrocephalus development in a rat intraventricular hemorrhage (IVH) model. There were three parts in this study. First, male Sprague–Dawley rats had an injection of 200 μL saline, autologous blood or heparinized blood, into the right lateral ventricle. Second, rats had an injection of 50 μL saline or 3U thrombin into the right lateral ventricle. Third, rats had an injection of thrombin (3U) with a protease-activated receptor-1 (PAR-1) antagonist, SCH79797 (0.15 nmol), or vehicle into the right lateral ventricle. Lateral ventricle volumes were measured by magnetic resonance imaging and the brains were used for immunohistochemistry and western blot analyses. Intraventricular injection of autologous blood induced hydrocephalus from day 1 to 28. Heparinized blood injection resulted in less hydrocephalus at all time points compared with blood injection alone (P<0.05). Intraventricular injection of thrombin caused significant hydrocephalus, ventricular wall damage, and periventricular blood–brain barrier disruption. Thrombin-induced hydrocephalus was reduced by co-injection of the PAR-1 antagonist SCH79797 (P<0.05). In conclusion, thrombin contributes to hydrocephalus development after IVH and thrombin-induced hydrocephalus is through PAR-1.
Keywords: blood–brain barrier, hydrocephalus, protease-activated receptor-1, thrombin
Introduction
Spontaneous or secondary intraventricular hemorrhage (IVH) is a marker of poor prognosis for hemorrhagic stroke.1 Severe IVH, caused by extension from intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH), leads to hydrocephalus and has a greater risk of hemorrhage-associated morbidity.2 Therefore, it is critical to understand the mechanisms of IVH-induced hydrocephalus.
Thrombin is a serine protease and an essential component in the coagulation cascade. Experimental investigations have indicated that thrombin formation has a major role in ICH-induced injury.3, 4, 5 Thrombin is responsible for blood–brain barrier (BBB) disruption and early brain edema formation after ICH. Although thrombin inhibition has been shown to be neuroprotective in hemorrhagic stroke models,6, 7 the role of thrombin in IVH-induced hydrocephalus is unclear.
The physiologic actions of thrombin are either nonreceptor mediated (for example, the cleavage of fibrinogen to fibrin) or receptor mediated. A family of protease-activated receptors (PAR) has been identified, of which PAR-1, PAR-3, and PAR-4 are activated by thrombin. Protease-activated receptor-1 is considered as the main subtype and is known as a thrombin receptor.8 Protease-activated receptor-1 is also involved in thrombin-induced brain damage after ICH.9
In this study, we investigated the role of thrombin in hydrocephalus after IVH in rats. In addition, we evaluated the effect of SCH79797, a specific PAR-1 antagonist, on thrombin-induced hydrocephalus to clarify the role of thrombin receptor activation.
Materials and Methods
Animal Preparation and Intraventricular Injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. The University of Michigan has an Animal Welfare Assurance on file with the Office for Protection from Research Risks and is fully accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies follow the Guide for The Care and Use of Laboratory Animals (National Research Council) and comply with the ARRIVE guidelines for reporting in vivo experiments. A total of 68 male Sprague–Dawley rats (3-month old, Charles River Laboratories, Portage, MI, USA), weighing 270 to 300 grams, were used in this study. Animals were anesthetized with pentobarbital (50 mg/kg, intraperitoneally) and the right femoral artery was catheterized to monitor arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and glucose levels. Core body temperature was maintained at 37.5°C with a feedback-controlled heating pad. Rats were then positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). A cranial burr hole (1 mm) was drilled and a 26-gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.6 mm posterior, 4.5 mm ventral, and 1.6 mm lateral to the bregma). Blood, thrombin, or saline was injected using a micro infusion pump (World Precision Instruments, Sarasota, FL, USA). After injection, the needle was removed, the burr hole was filled with bone wax, and the skin incision was closed with sutures.
Experimental Groups
This study included three parts. Randomization was carried out. First, rats received a 200-μl injection of saline (n=8), autologous blood (n=8), or heparinized blood (5 units heparin, n=6) into the right lateral ventricle over 15 minutes. Serial magnetic resonance imaging (MRI) scanning was performed at days 1, 3, 8, 14, and 28. Rats were euthanized at day 28 after IVH. Second, rats received an intracerebroventricular injection of 3 units rat thrombin (Sigma-Aldrich, St Louis, MO, USA) in saline or saline alone (50 μL) in 7 minutes, and were euthanized at 24 hours after MRI scanning. The brains were used for western blotting (n=6 for each group) and brain histology (n=6 for each group). Third, rats had an intracerebroventricular injection of 3 units of thrombin in 50 μL saline with PAR-1 antagonist, SCH79797 (0.15 nmol), or vehicle. Rats were euthanized at 24 hours after MRI scanning and the brains were used for western blotting (n=6 for each group) and histologic analysis (n=5 for each group).
Magnetic Resonance Imaging and Ventricle Volume Measurement
Rats were anesthetized with 2% isoflurane throughout MRI examination. Magnetic resonance imaging was performed in a 7.0-T Varian MR scanner (Varian, Palo Alto, CA, USA) with a T2 fast spin-echo sequence using a view field of 35 mm × 35 mm and 25 coronal slices. Ventricular volumes were calculated as described previously.10 Bilateral ventricles were outlined and the areas measured. Ventricular volume was calculated by summing the ventricle areas over all slices and multiplying by section thickness. All image analysis was performed using ImageJ program by a masked observer.
Ventricular Wall Damage Analysis
Ventricular wall damage was analyzed as described previously.11 Briefly, brain sections underwent hematoxylin and eosin staining. The length of the ependyma that was disrupted or detached from the periventricular parenchyma was divided by the total ventricular surface perimeter. All analyses were performed using ImageJ software by a masked observer.
Transmission Electron Microscopy
Transmission electron microscopy was performed as previously described.12 Anesthetized rats underwent intracardiac perfusion with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L Sorensen's buffer (pH 7.4). Brains were removed and a 1-mm thick coronal brain slice including ipsilateral ventricular wall was cut with a blade 2 mm posterior to the bregma. Slices were immersed in the same fixative overnight at 4°C and then post fixed with 1.0% OsO4 and dehydrated in graded ethyl alcohol. After dehydration, samples were infiltrated with propylene oxide, embedded in Epon, and sectioned. Ultra-thin sections were then stained with uranyl acetate and Reynold's lead citrate, and evaluated using a Philips CM 100 TEM and digitally imaged using a Hamamatsu (Hamamatsu City, Shizuoka, Japan) ORCA-HR camera.
Immunohistochemistry
Rats were anesthetized with pentobarbital (100 mg/kg, intraperitoneally) and perfused with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4% paraformaldehyde for 24 hours and then immersed in 30% sucrose for 2 to 3 days at 4°C. Brains were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and 18-μm thick slices cut using a cryostat. Immunohistochemical studies were performed using the avidin–biotin complex technique as previously described.13 The primary antibody was rabbit anti-HO-1(heme oxygenase-1) (1:400 dilution; Abcam, Cambridge, MA, USA).
Western Blot Analysis
Western blot analysis was performed as previously described.14 Briefly, brain tissue was immersed in western sample buffer and sonicated. Protein concentration was determined by the Bio-Rad (Hercules, CA, USA) protein assay kit and 50 μg protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham, Pittsburgh, PA, USA). Membranes were probed with the following primary antibodies: goat anti-mouse albumin (1:10,000 dilution; Bethyl Laboratories, Montgomery, TX, USA) and rabbit anti-HO-1 (1:2,000 dilution; Abcam). Antigen–antibody complexes were visualized with the ECL chemiluminescence system (Amersham) and exposed to a Kodak X-OMAT film (Rochester, NY, USA). The relative densities of bands were analyzed with NIH ImageJ.
Statistical Analysis
Values are given as means±s.d. Student's t-tests or Mann–Whitney U-tests were used to analyze the data. Differences were considered significant at P<0.05.
Results
Physiologic parameters, including mean arterial blood pressure, blood gases, pH, hematocrit, and glucose were in normal ranges. Mortality was zero in this study.
Heparinized Blood Induced Less Ventricle Enlargement after Injection into the Lateral Ventricle
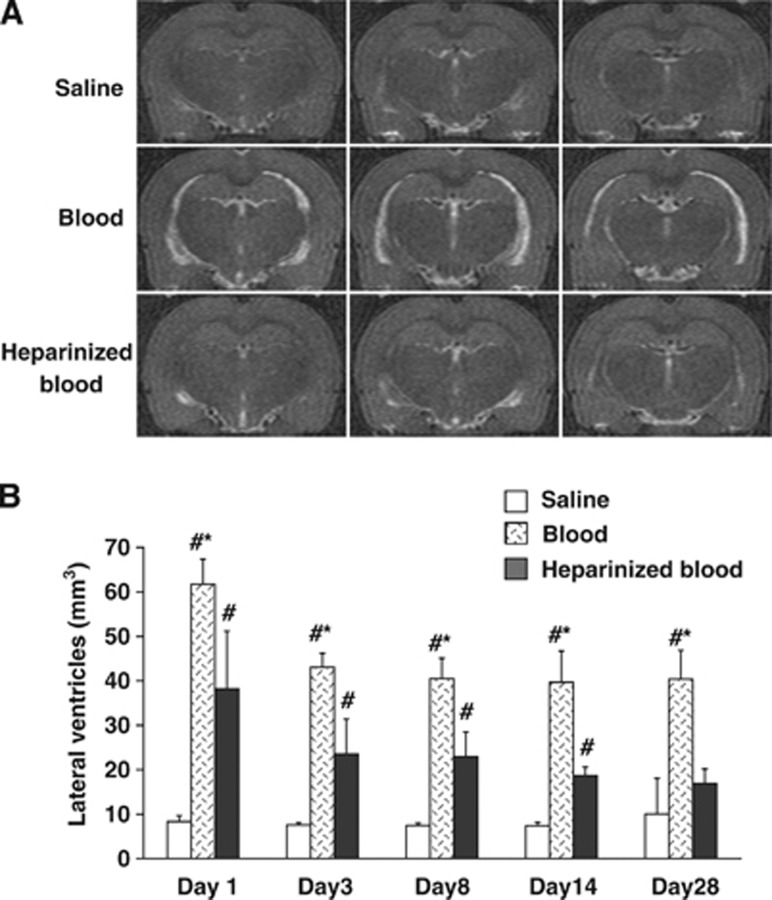
Our previous data found that intracerebroventricular injection of blood causes hydrocephalus.10 This was confirmed in the current study where injection of autologous arterial blood resulted in obvious hydrocephalus on MRI (Figure 1A). Lateral ventricular volumes in IVH rats were significantly larger than in rats receiving saline injection from day 1 to 28 (P<0.01, Figure 1B). To examine whether the coagulation cascade has a role in IVH-induced hydrocephalus, heparin was co-injected with blood. This resulted in less hydrocephalus at all time points compared with rats receiving blood injection alone (P<0.05, Figure 1B).
Figure 1.
Heparin reduced intraventricular hemorrhage hydrocephalus. (A) T2-weighted magnetic resonance imaging (MRI) showing coronal brain sections at day 1 after injection of 200 μl of saline, blood, or heparinized blood into the right lateral ventricle. (B) Quantification of lateral ventricle volume from MRI images at time points from day 1 to day 28. Values are expressed as the means±s.d., n=6 to 8, #P<0.01 versus saline group and *P<0.05 versus heparinized blood group.
Intraventricular Thrombin Injection Caused Hydrocephalus and Periventricular Injury
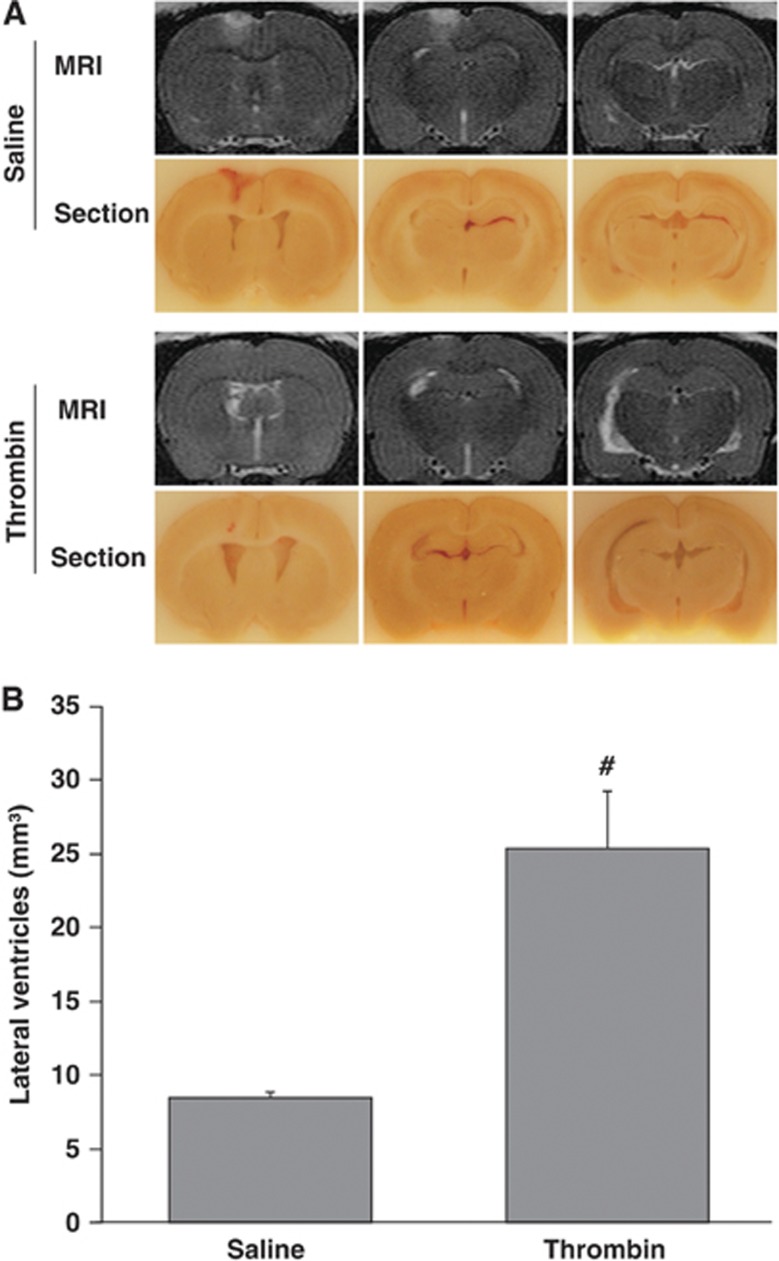
To test whether thrombin has a role in hydrocephalus development, thrombin (3 units) was injected into the right lateral ventricle. Control animals received an injection of the same volume of saline. At 24 hours, thrombin caused significant ventricular enlargement (25.4±3.9 versus 8.4±0.4 mm3 in saline control, P<0.01, Figure 2) and more severe ventricular wall damage (damage of ependymal layer, 17.9±1.7 versus 2.8±0.4% in saline control, P<0.01, Figure 3A).
Figure 2.
Intraventricular injection of thrombin caused hydrocephalus. T2-weighted MRI and frozen coronal brain sections (A) and the measurement of ventricular volume with T2-weighted MRI (B) at 24 hours after injection of 50 μl of saline or thrombin (3U) into the right lateral ventricle. Values are expressed as the means±s.d., n=6, #P<0.01 versus saline group.
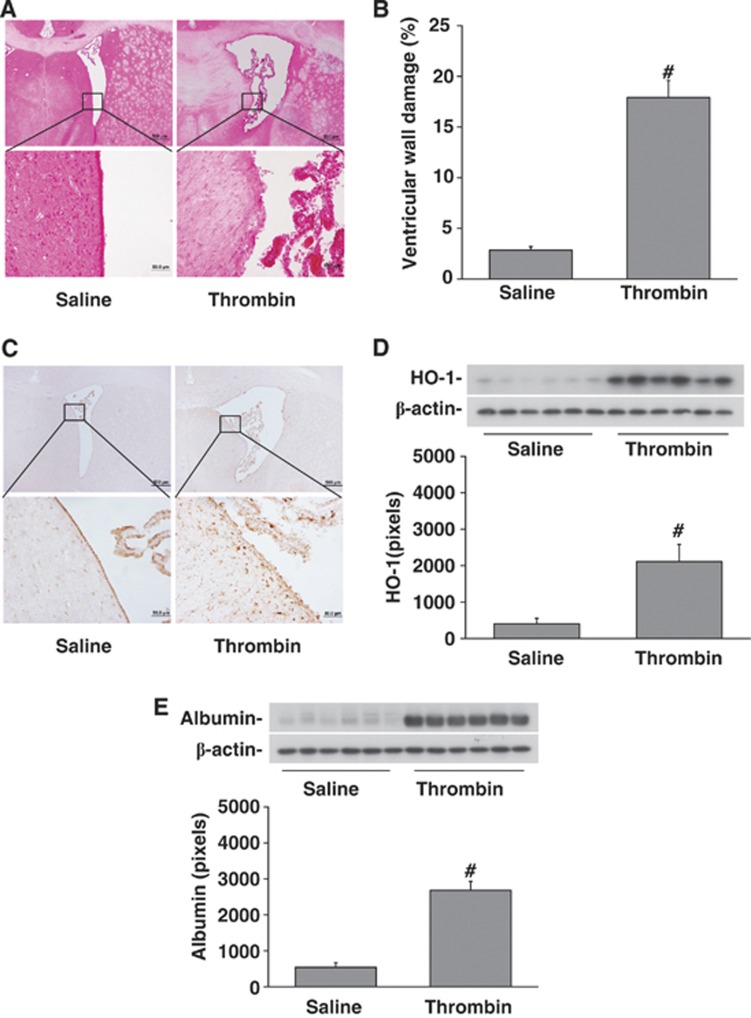
Figure 3.
Intraventricular injection of rat thrombin induced brain injury. Rats received an intracerebroventricular injection of thrombin (3U) or saline and were killed at 24 hours. (A) Hematoxylin and eosin staining of coronal sections showing damage to the ventricular wall after thrombin injection (lower micrographs are magnified images of the boxes in the upper micrographs) compared with saline injection. The bar graph quantifies the ventricular wall damage (% of ventricular wall damaged). (B) Immunoreactivity and protein levels of heme oxygenase-1 (HO-1) (western blot) with quantification. Thrombin increased HO-1 expression. (C) Periventricular albumin levels (western blot with quantification) to assess blood–brain barrier (BBB) disruption. Thrombin caused periventricular BBB disruption. Values are mean±s.d., n=6, #P<0.01 versus saline group.
Intraventricular injection of thrombin caused significant upregulation of HO-1, a stress protein, in the periventricular area at 24 hours (2105±476 versus 399±158 pixels in saline control, P<0.01, Figure 3B). The periventricular levels of albumin, an indicator of BBB leakage, were significantly elevated in the brain after thrombin injection (2675±260 versus 536±136 pixels in saline control, P<0.01, Figure 3C).
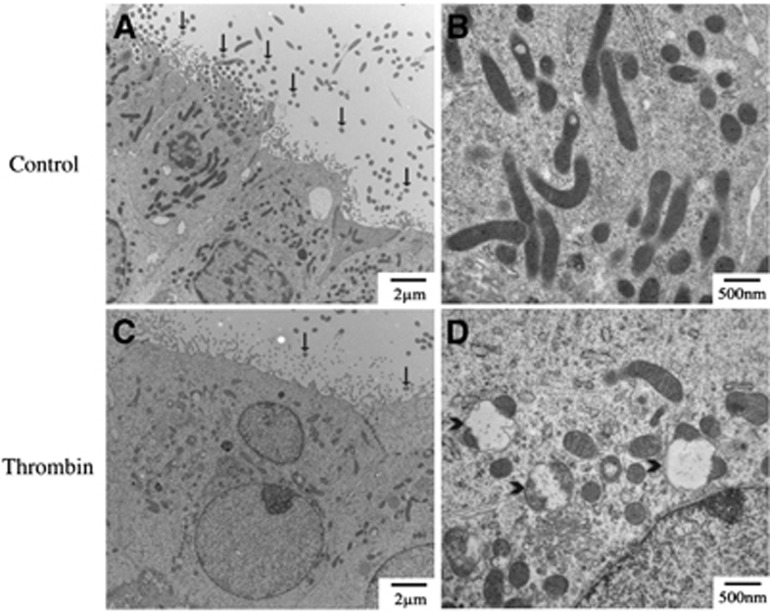
Thrombin-induced ependymal cell damage was examined by transmission electron microscopy (Figure 4). Thrombin caused swelling of ependymal nucleus and mitochondria, and loss of cilia.
Figure 4.
Electron micrographs showing the ultrastructure of the ipsilateral lateral ventricle wall 24 hours after intracerebroventricular injection of saline (A, B) or thrombin (C, D). (A, C) Low power micrographs of the ependyma. (B, D) Higher power micrographs of ependymal mitochondria. Note the swollen mitochondria (arrowheads) and changes in cilia (arrows) after thrombin injection.
SCH79797, a Specific PAR-1 Antagonist, Blocked Thrombin-Induced Ventricular Enlargement and Brain Injury
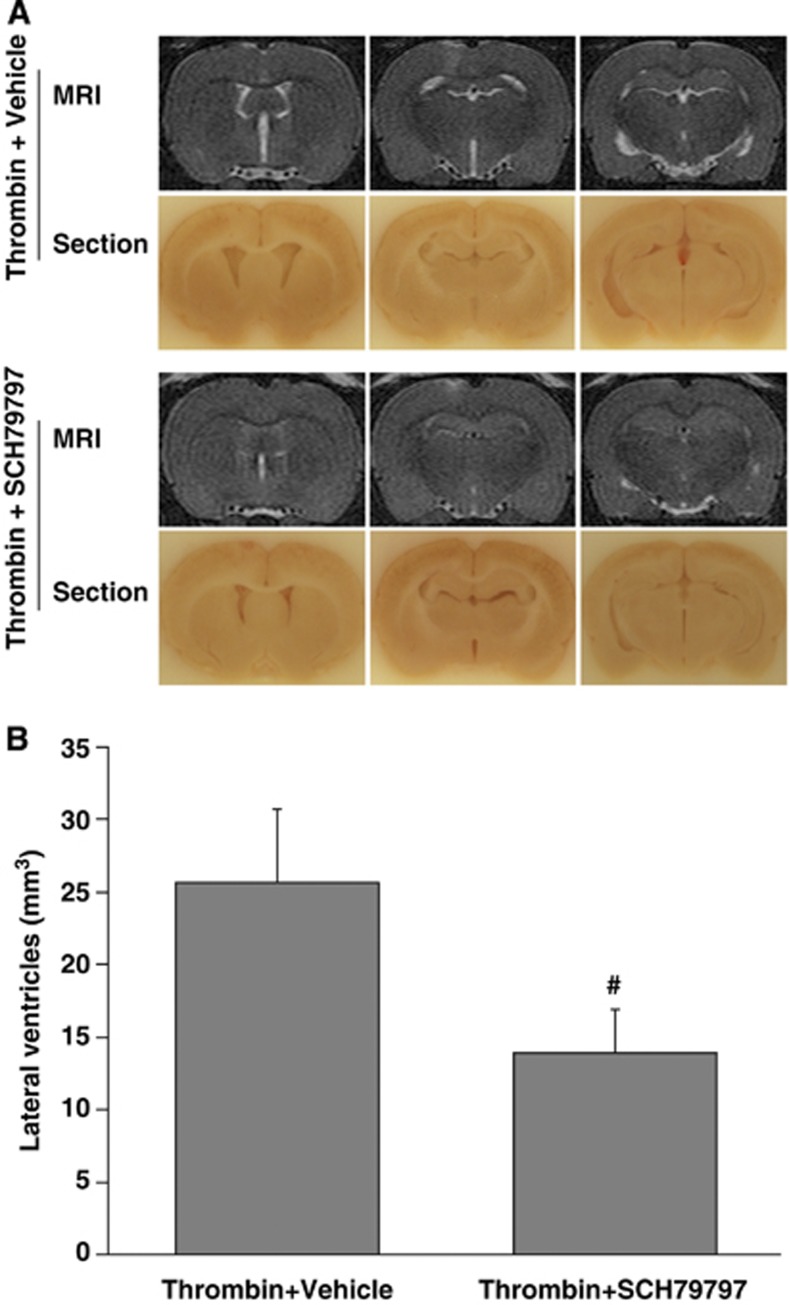
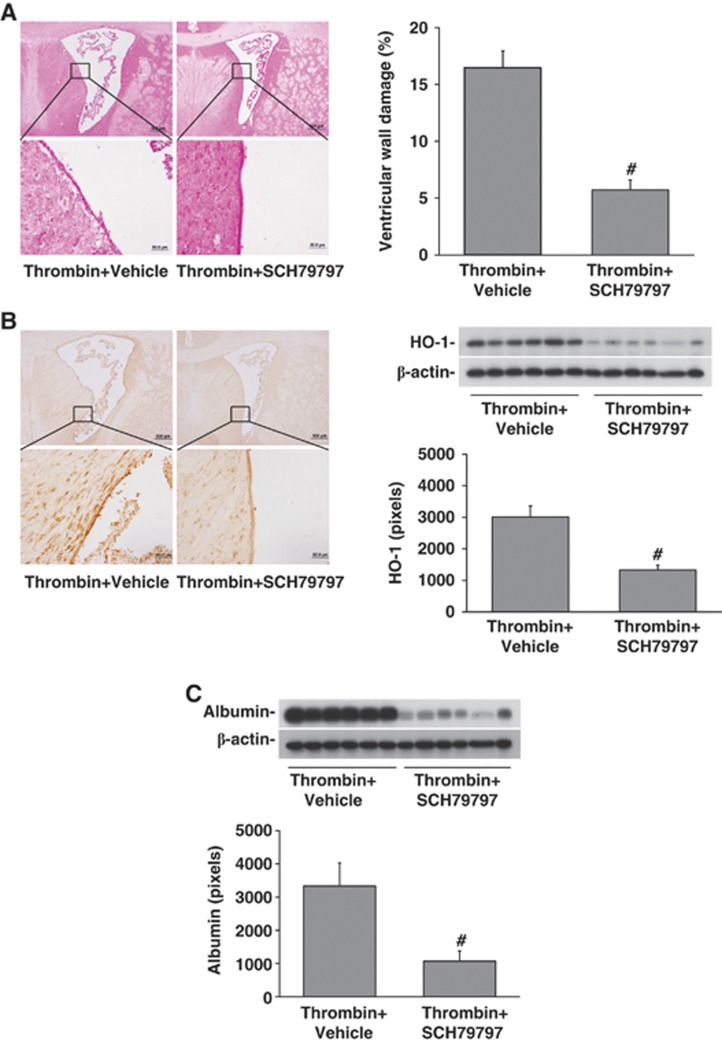
To examine whether PAR-1 has a role in thrombin-induced hydrocephalus, SCH79797 was used. Co-injection SCH79797 with thrombin resulted in less ventricle enlargement (13.9±3.0 versus 25.7±5.1 mm3 in vehicle-treated group, P<0.01, Figure 5) and ventricular wall damage (5.7±0.9 versus 16.4±1.5% in vehicle-treated rats, P<0.01, Figure 6A). In addition, SCH79797 co-injection reduced thrombin-induced brain HO-1 upregulation (1335±148 versus 3010±353 pixels in vehicle-treated group, P<0.01, Figure 6B) and BBB disruption (albumin levels: 1077±303 versus 3336±696 pixels in vehicle-treated group, P<0.01, Figure 6C) in the ipsilateral periventricular area.
Figure 5.
SCH79797 attenuated thrombin-induced hydrocephalus. (A) T2-weighted magnetic resonance imaging (MRI) images and frozen brain sections at 24 hours after intracerebroventricular injection of 50 μL of thrombin (3U in saline) with SCH79797 (0.15 nmol) or vehicle (saline). (B) Lateral ventricle volumes calculated from the MRI images. Values are expressed as the means±s.d., n=6, #P<0.01 versus vehicle.
Figure 6.
SCH79797 reduced thrombin-induced periventricular injury. (A) Hematoxylin and eosin staining of the periventricular brain 24 hours after intracerebroventricular injection of 3U rat thrombin with or without SCH79797. Boxes show the areas examined at higher power in the lower micrographs. Ventricular wall damage was determined and expressed as a % of total ventricular wall. (B) Effects of thrombin with and without SCH79797 on periventricular heme oxygenase-1 (HO-1)as determined by immunohistochemistry and western blot. (C) Effects of thrombin with and without SCH79797 on periventricular blood–brain barrier disruption as assessed by albumin western blot. Values are mean±s.d., n=6, #P<0.01 versus vehicle.
Discussion
The major findings of this study are as follows: (1) thrombin contributed to hydrocephalus development after IVH; (2) intraventricular injection of thrombin caused hydrocephalus and periventricular injury; and (3) SCH79797, a specific PAR-1 antagonist, reduced the ventricular enlargement caused by thrombin.
Thrombin, a serine protease generated by the cleavage of prothrombin, is an essential component of the coagulation cascade and produced immediately in the brain after IVH.
Thrombin and iron are two major factors causing brain injury after ICH.3 Our previous study showed that iron has a role in IVH-induced hydrocephalus with deferoxamine, an iron chelator, reducing hydrocephalus.10 The present study demonstrated that coagulation cascade activation and thrombin formation after hemorrhage are also critical in hydrocephalus development after IVH. Thus, heparin could ameliorate IVH-induced hydrocephalus and intracerebroventricular thrombin could induce hydrocephalus. Thrombin-induced hydrocephalus is associated ventricular wall damage and BBB disruption. In the current study, 3 units rat thrombin was injected into the lateral ventricle. It is known that 0.2 mL of whole blood produces ∼52 to 72 units of thrombin and intracerebral infusion of 5 units of thrombin causes marked edema in the rat.15 Thus, the amount of thrombin injected intracerebroventricularly in this study was less than that which can be produced by the blood in the IVH model. However, it should be noted that thrombin may be slowly released from a hematoma and preliminary experiments with higher amounts of rat thrombin caused significant mortality. We noticed that autologous whole blood resulted in more severe hydrocephalus than that induced by thrombin alone. It suggests that other factors, including obstruction of cerebrospinal fluid (CSF) circulation and iron, may also contribute to hydrocephalus development after IVH.10
At present, the mechanisms associated with ventricular dilatation induced by thrombin are uncertain. According to previous studies, acute hydrocephalus after IVH may be associated with impaired CSF circulation because of blood clots obstructing CSF outflow pathways, or the direct result of the hematoma mass effect.16, 17, 18 An early primate study designed to examine CSF absorption resistance after SAH suggested an effect of coagulation on CSF absorption. It found that injection of autologous non-heparinized blood into the cisterna magna significantly retarded CSF absorption, whereas CSF absorption was less affected and resumed to normal levels soon after heparinized blood injection.19 While such an effect might account for the impact of heparin on hydrocephalus in the current study, our finding that injection of thrombin in the absence of blood causes hydrocephalus suggests that thrombin has other effects. Intracerebroventricular injection of thrombin caused ependymal damage and there is increasing evidence that ependymal damage may lead to hydrocephalus development.10, 11, 20 In addition, multiple mouse genetic models have shown hydrocephalus when ependymal cilia are absent or dysfunctional.21 Therefore, ependymal damage and abnormal ependymal cilia may have a role in hydrocephalus induced by thrombin.
The cellular signaling effects of thrombin are through three receptors, PAR-1, -3 and -4, but PAR-1 is generally the main thrombin receptor. In the current study, we found that SCH79797, a specific PAR-1 antagonist, significantly reduced intracerebroventricular thrombin-induced ventricular dilatation, ependymal damage, and BBB disruption. Our results indicate that thrombin-induced hydrocephalus is, at least in part, mediated by PAR-1. Because of the role of thrombin in stopping bleeding, direct thrombin inhibitors that block fibrinogen cleavage are an unattractive therapeutic target for limiting IVH-induced hydrocephalus. Our study suggests that PAR-1 antagonists, that do not directly affect coagulation, have similar effects on hydrocephalus development and might be a better therapeutic target. Recently, Yan et al22 found that SCH79797 also reduces brain microvascular injury after SAH. Future studies should determine whether SCH79797 can reduce IVH-induced hydrocephalus.
The effects of intracerebroventricular thrombin were not limited to the ependymal layer. Thrombin also caused periventricular upregulation of HO-1, a stress protein. Heme oxygenase-1 has a complex role after brain hemorrhage. The exact mechanisms of thrombin-induced HO-1 upregulation are still unclear, but HO-1 is regulated by hypoxia-inducible factor-1 alpha and our previous study found that thrombin is able to upregulate that transcription factor.23 Increased brain HO-1 levels may contribute to brain iron overload, resulting in brain damage after ICH and SAH.3, 11 However, HO-1 could be protective.24 Therefore, the precise role of HO-1 in IVH and hydrocephalus needs to be determined. Thrombin also caused a marked increase in periventricular albumin, a marker of BBB disruption. Liu et al.25 also found that intraventricular injection of thrombin (20 U from bovine plasma) in rat caused BBB breakdown and Sugawara et al7 found that argatroban, a thrombin inhibitor, ameliorated BBB disruption, brain, inflammation, and apoptosis in a rat SAH model. Whether the BBB dysfunction after intracerebroventricular thrombin might contribute to hydrocephalus development is uncertain. It has been suggested that some CSF absorption may occur across the BBB,26 but this is controversial. The BBB disruption may contribute to periventricular parenchymal cell damage and inflammation.
The current study focused on the role of thrombin in adult IVH. However, germinal matrix hemorrhage is common in premature infants, where it is a major cause of cerebral palsy. In a rodent neonatal germinal matrix hemorrhage model, Lekic et al27 found that IVH resulted in an increase of thrombin and hydrocephalus. The role of thrombin and PAR-1 in hydrocephalus after neonatal IVH should be the subject of further investigation.
In conclusion, the present study revealed that thrombin contributes to hydrocephalus development after IVH and thrombin-induced hydrocephalus and periventricular injury is through thrombin receptor, PAR-1.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants NS-057539, NS-073959, NS079157 and NS084049 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the stich trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117–121. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke. 2002;33:3012–3018. doi: 10.1161/01.str.0000037673.17260.1b. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009;40:1530–1532. doi: 10.1161/STROKEAHA.108.531699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective. J Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44:547–550. doi: 10.1161/STROKEAHA.112.662312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Xi G, Jin H, He Y, Keep RF, Hua Y. Thrombin-induced autophagy: a potential role in intracerebral hemorrhage. Brain Res. 2011;1424:60–66. doi: 10.1016/j.brainres.2011.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–383. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Kuker W, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kim Y, Kissler J, Delsing P, Gilsbach JM, Schroder JM, et al. Morphological changes following experimental intraventricular haemorrhage and intraventricular fibrinolytic treatment with recombinant tissue plasminogen activator. Acta Neuropathol. 2000;100:561–567. doi: 10.1007/s004010000219. [DOI] [PubMed] [Google Scholar]
- Donauer E, Reif J, al-Khalaf B, Mengedoht EF, Faubert C. Intraventricular haemorrhage caused by aneurysms and angiomas. Acta Neurochir. 1993;122:23–31. doi: 10.1007/BF01446982. [DOI] [PubMed] [Google Scholar]
- Blasberg R, Johnson D, Fenstermacher J. Absorption resistance of cerebrospinal fluid after subarachnoid hemorrhage in the monkey; effects of heparin. Neurosurgery. 1981;9:686–691. doi: 10.1227/00006123-198112000-00012. [DOI] [PubMed] [Google Scholar]
- Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3:25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, et al. Role of sch79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44:1410–1417. doi: 10.1161/STROKEAHA.113.678474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wu J, Keep RF, Hua Y, Hoff JT, Xi G. Hypoxia-inducible factor-1alpha accumulation in the brain after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22:689–696. doi: 10.1097/00004647-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by src after thrombin-induced injury. Ann Neurol. 2010;67:526–533. doi: 10.1002/ana.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145–165. doi: 10.1007/s10143-004-0326-9. [DOI] [PubMed] [Google Scholar]
- Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, et al. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol. 2012;236:69–78. doi: 10.1016/j.expneurol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]