Abstract
Chronic low back pain (cLBP) has a tremendous personal and socioeconomic impact, yet the underlying pathology remains a mystery in the majority of cases. An objective measure of this condition, that augments self-report of pain, could have profound implications for diagnostic characterization and therapeutic development. Contemporary research indicates that cLBP is associated with abnormal brain structure and function. Multivariate analyses have shown potential to detect a number of neurological diseases based on structural neuroimaging. Therefore, we aimed to empirically evaluate such an approach in the detection of cLBP, with a goal to also explore the relevant neuroanatomy. We extracted brain gray matter (GM) density from magnetic resonance imaging scans of 47 patients with cLBP and 47 healthy controls. cLBP was classified with an accuracy of 76% by support vector machine analysis. Primary drivers of the classification included areas of the somatosensory, motor, and prefrontal cortices—all areas implicated in the pain experience. Differences in areas of the temporal lobe, including bordering the amygdala, medial orbital gyrus, cerebellum, and visual cortex, were also useful for the classification. Our findings suggest that cLBP is characterized by a pattern of GM changes that can have discriminative power and reflect relevant pathological brain morphology.
Keywords: classification, low back pain, structural imaging, support vector machine
Introduction
An estimated 100 million Americans suffer from chronic pain, and chronic low back pain (cLBP) in particular is the most common cause for activity limitation in people <45 years (Andersson 1999; IOM 2011). The prevalence of cLBP has risen significantly, from 3.9% in 1992 to 10.2% in 2006, and is associated with an annual economic burden of $100–200 billion dollars (Freburger et al. 2009; IOM 2011). Further research is needed to improve diagnosis and treatment of this impactful condition, particularly given that the underlying pathology is often unclear.
The majority of cLBP cases (85–90%) are characterized as nonspecific, indicating that a specific pathology cannot be identified (van Tulder et al. 1997; Koes et al. 2006). In these cases, there is little or no evidence that identifying skeletal pathology improves outcomes (van den Bosch et al. 2004; Chou et al. 2007; Cohen et al. 2008). In fact, many individuals with cLBP show no abnormalities in spinal imaging, and there is evidence that abnormalities are equally common in those who have cLBP as in those who do not (Rubinstein and van Tulder 2008). Importantly, there is growing evidence that changes in brain structure and function are central to chronic pain disorders (Borsook et al. 2010). Whereas the acute pain system in the central nervous system has been well mapped to include regions such as the thalamus, somatosensory cortices, and the insula; chronic pain appears much more complex (Apkarian et al. 2005). The past literature has shown that chronic pain diseases such as cLBP, chronic regional pain syndrome, irritable bowel syndrome, fibromyalgia, and chronic migraine may arise due to dysfunction of central pain inhibitory or modulatory systems (Apkarian 1995; Ingvar 1999; Gracely et al. 2002; Giesecke et al. 2004; Davis et al. 2008; May 2008; Borsook et al. 2010; Seminowicz et al. 2010; Wood 2010; Younger et al. 2010). These changes, some detected by structural magnetic resonance imaging (MRI), may help further our understanding of the disease and the subsequent management of patients with cLBP.
Previous anatomical MRI research investigating cLBP has produced inconsistent findings. While one study reported that cLBP is associated with decreased gray matter (GM) density in the thalamus and dorsolateral prefrontal cortex (DLPFC; Apkarian et al. 2004), another reported that cLBP is associated with decreased GM density in the brainstem and somatosensory cortex and increased GM density in the basal ganglia and thalamus (Schmidt-Wilcke et al. 2006). These differences are difficult to interpret, but may be due to variables including methodology and clinical features of the population samples. For instance, the differing types of cLBP or medication state may lead to different brain changes (see Discussion). Given the inconsistency that is present in the few studies to date, it remains unclear which brain regions, if any, are characterized by pathological morphology in patients with cLBP.
To extend upon previous research, this study investigated GM density in unmedicated patients with non-neuropathic cLBP and in matched healthy controls. Although previous studies have used voxel-based morphometry (VBM) to investigate individual brain regions, this study used a novel multivariate machine learning approach. Machine learning algorithms such as support vector machines (SVM) have been recently adopted in the field of structural and functional neuroimaging to characterize and classify. At least 3 such studies have employed this technique, to noninvasively identify Huntington's disease with 83% accuracy (Kloppel et al. 2009), Alzheimer's disease with up to 89.3% accuracy (Vemuri et al. 2008), and acute painful stimuli with 81% accuracy (Brown et al. 2011). In the present study, we adopted SVMs to detect distributed brain features that may better to characterize cLBP.
Our over-riding goals in this study were: 1) to accurately classify patients with cLBP from healthy individuals on the basis of structural changes in the brain and 2) to investigate pathological changes distributed across multiple brain regions. To accomplish these goals, we used SVM analysis to identify a whole-brain pattern of GM density that best distinguishes cLBP patients from controls. To compare these results with classical analysis methods, a separate whole-brain VBM analysis was also conducted to investigate GM changes in individual brain regions.
Materials and Methods
Participants
Structural MRI scan data were acquired from 94 participants: 47 with cLBP and 47 healthy controls who were individually gender and age matched within 2 years. Participants with cLBP were included if they had axial low back pain, without radicular symptoms, that had persisted for greater than 6 months. Participants with a current or previous history of a major psychiatric disorder, including depression and anxiety, were excluded. No participants were taking prescription pain medications at the time of study, however, up to 4g acetaminophen per day, low-dose aspirin, and occasional acetaminophen and ibuprofen use were allowed. In addition, patients reporting substance abuse within the past 6 months of enrollment were excluded. All data were collected from volunteers who had provided written, informed consent with procedures approved by the Stanford University Institutional Review Board.
Image Acquisition
High-resolution T1-weighted structural images of the brain were conducted on 2 General Electric 3-T scanners at Stanford University using a 3-dimensional inversion recovery fast spoiled gradient-recalled (3D IR-FSPGR) pulse sequence. Imaging parameters were: time repetition = 8.18 s, time echo = 1.71 ms, 124 slices, 1.5-mm slice thickness, 0.86 × 0.86 mm or less in-plane resolution.
Preprocessing
Preprocessing was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) in MATLAB (MathWorks, Natick, MA). Images were segmented into GM tissue classes, bias corrected, and normalized to the Montreal Neurological Institute (MNI) template using nonlinear transformations based on SPM8's Unified Segmentation scheme (Ashburner and Friston 2000). A signal was preserved throughout the normalization procedure, and GM density was acquired using supplied tissue probability priors. A mask generated from the average of all images was used to remove non-GM voxels. All images were spatially smoothed with an 8-mm full width at half maximum Gaussian kernel. Total GM volume was also calculated for each subject.
SVM Analysis
Principal Component Transformation
Since each of the 94 MRI scans was originally described by hundreds of thousands of voxels, many possible models can separate the data, resulting in high variance and poor generalizability. Thus, feature reduction was a necessary step before the classification.
Principal component analysis (PCA) is a multivariate analysis technique that finds orthogonal vectors to best explain variance in data, allowing the representation of the data in a reduced, principal component (PC) space (Howley et al. 2006). This allows better model estimation with minimal loss of generalizability, and since the original data can be reobtained from the PC's, PCA is a loss-less transformation. PCA was performed on the training data and all primary PCs (eigenvectors), with nonzero eigenvalues were used to transform the test set into PC space before classification, ensuring that the test set did not play a role in identifying PCs.
Classification
A support vector machine is a supervised learning algorithm used successfully in many classification problems. It is supervised in that a training step is required to generate the model before testing on novel examples. During the training step, the SVM attempts to separate the data represented in a high dimensional space by drawing an optimal separating hyperplane (decision boundary) that maximizes the margin between the 2 groups. Readers are encouraged to refer to other sources for more in-depth review of SVMs (Burges 1998; Cristianini and Shawe-Taylor 2000; Pereira et al. 2009).
A linear SVM was used, and the regularization parameter (C) was selected with a standard grid search. The analysis was performed in MATLAB with in-house implementations and LIBSVM (Chang and Lin 2011).
Leave-Pair-Out Cross-Validation
The validation of a predictive model involves training on a labeled (known) dataset and testing on an unlabeled (unknown) set. When data are scarce, cross-validation can provide a good estimate for the generalizability of a classifier (Kohavi 1995). The dataset was randomly divided into pairs of 1 patient and 1 control, and a leave-pair-out cross-validation (LPOCV) technique was applied where the model is trained on all but one pair and subsequently tested on the remaining pair (Airola et al. 2011). This ensures that each training set has an equal class representation. This procedure was performed until each pair has been left out. During each model training stage, PCA was performed on the training set (resulting in N−1 = 91 components), and the data in PC space were entered into a linear SVM. The test pair in the PC space was then classified. Performance was assessed on the basis of accuracy, sensitivity, specificity, positive predictive value, and negative predictive value. Since the training data are randomly divided into pairs, slightly different models may form depending on this division. Thus, LPOCV was run 100 times, and the results were averaged to give final performance measures.
Significance Assessment
The significance of performance measurements and discriminative regions were tested using an empirical Monte Carlo permutation test as described by Mourao-Miranda et al. (2005). One thousand iterations of LPOCV were run as described above. In each iteration, class labels were randomly permuted and the entire cross-validation procedure performed. The number of times where a performance measure from this permutation test is greater than or equal to the observed performance, divided by 1000, represents a P-value. In addition, each iteration provides a data point in a null distribution of weights. These weights, after transformation to brain space, were used to determine the significance of the regions found to be discriminative. Regions significant to P < 0.001 and the false discovery rate (FDR) P < 0.05 for performance and weight measurements, respectively, were reported at a cluster size of 10 voxels.
VBM Analysis
A univariate whole-brain VBM analysis was also performed using preprocessed data generated above. T-tests were performed on the voxel level to detect regional differences (minimum cluster size = 10 voxels, P = 0.001 uncorrected for multiple comparisons).
Results
Subject Data
The healthy control group consisted of 47 volunteers (25 males and 22 females) with a mean age of 37.7 years (standard deviation [SD] = 7.8, range 19–60 years old). The cLBP group consisted of 47 participants (25 males and 22 females) with a mean age of 37.3 years (SD = 12.2, range 19–60 years old; Supplementary Fig. 1). The mean pain duration for patients with cLBP was 8.55 years (SD = 7.81). Depression inventories (Beck Depression Inventory or Hospital Anxiety and Depression Scale) were collected for the 47 participants with cLBP, and 42 reported normal to mild levels. Forty-two patients reported bilateral pain and 5 reported primarily lateralized pain in the lumbar region. No significant group differences were found between total GM volume (cLBP = 754.7 mL, SD 94.7, controls = 750.0 mL, SD 76.5; P = 0.79).
Classifier Performance
A true positive (TP) indicates correct classification as cLBP, whereas a false positive (FP) indicates incorrect classification as cLBP. True and false negatives (TN/FN) indicate correct and incorrect classifications of the absence of cLBP, respectively. Accuracy represents the number of correct classifications divided by the total number of patients (i.e., [TP + TN]/[TP + FP + TN + FN]). Sensitivity and specificity represent the percentage of cLBP and non-cLBP patients correctly classified, respectively (i.e., TP/[TP + FN] and TN/[TN + FP]). Positive and negative predictive values represent the probability of a cLBP and non-cLBP prediction being correct, respectively (i.e., TP/[TP + FP] and TN/[TN + FN]).
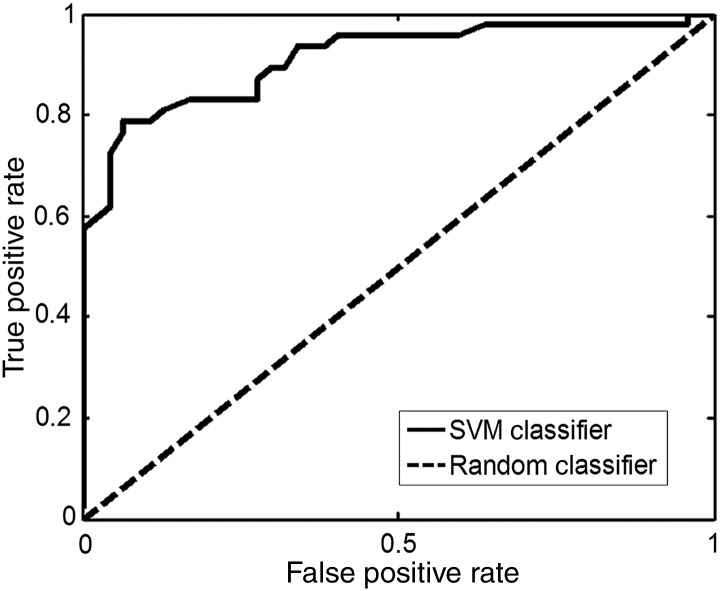
The SVM classifier obtained an average accuracy of 76%. Sensitivity and specificity were 76% and 75%, respectively. Positive and negative predictive values were 75% and 76%, respectively (Table 1). All values are significant to P < 0.001 as determined by a permutation test. A receiver operating characteristic (ROC) curve shows that the classifier performs better than random chance, with an area under curve of 0.91 (Fig. 1). This graph provides another visualization of the performance of the classifier. Whereas a random classifier results in one FP for every TP, the SVM classifier provides greater increases in the TP rate with less of an increase in the FP rate.
Table 1.
Prediction results
| Measurement | Result (%) |
|---|---|
| Accuracy | 76 |
| Sensitivity | 76 |
| Specificity | 75 |
| PPV | 75 |
| NPV | 76 |
Sensitivity = TP/(TP + FN), specificity = TN/(TN + FP), positive predictive value (PPV) = TP/(TP + FP), negative predictive value (NPV) = TN/(TN + FN), where TP = true positive (cLBP group), FP = false positive, TN = true negative, FN = false negative. All values significant to P < 0.001.
Figure 1.
ROC curve. ROC curve comparing cLBP SVM classifier with a random classifier. Area under curve for the SVM classifier is 0.82.
SVM Regions
Discriminative regions were identified at P < 0.05 significance level (FDR corrected; Table 2). Decreased GM density adjacent to the right amygdala, in the left medial orbital gyrus and in the right cuneus (secondary visual cortex; V2), coupled with increased GM density in the right cerebellum, regions of the temporal lobe, the left primary and secondary somatosensory cortices (S1 and S2), the left primary motor cortex (M1), the right calcarine sulcus (primary visual cortex; V1), and the right DLPFC were most helpful in the prediction of cLBP (Fig. 2).
Table 2.
Discriminative regions predictive of cLBP
| MNI | Anatomy | Direction | |
|---|---|---|---|
| A | 36, 4, −34 | Right borderline amygdala | Decrease |
| B | −18, 20 −16 | Left medial orbital gyrus | Decrease |
| C | 20, −98, −4 | Right cuneus (V2) | Decrease |
| D | 52, −50, −34 | Right cerebellum | Increase |
| E | −44, −52, −14 | Left fusiform gyrus | Increase |
| F | −58, −64, −8 | Left middle temporal gyrus | Increase |
| G | 66, −38, −8 | Right middle temporal gyrus | Increase |
| H | −46, −24, 12 | Left secondary somatosensory cortex (S2) | Increase |
| I | −56, −54, 10 | Left superior temporal gyrus | Increase |
| J | 22, −56, 12 | Right calcarine sulcus (V1) | Increase |
| K | 36, 48, 18 | Right DLPFC | Increase |
| L | −46, −24, 32 | Left postcentral gyrus (S1) | Increase |
| M | −56, −12, 36 | Left precentral gyrus (M1) | Increase |
Note: discriminative regions significant to P < 0.05, FDR corrected for multiple comparisons; minimum cluster size = 10 voxels.
Figure 2.
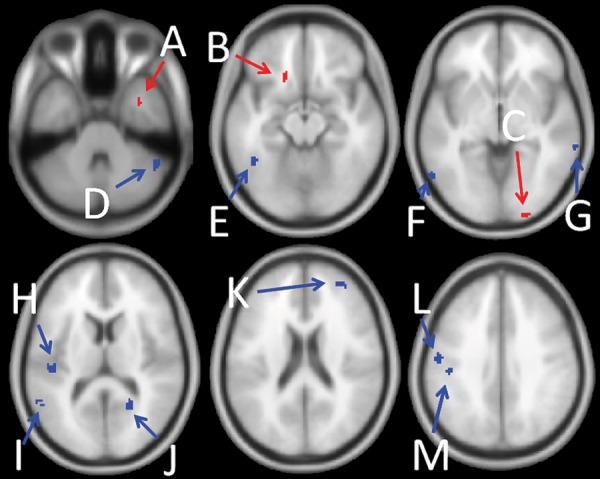
Predictive regions. Blue indicates regions where more GM density helped to predict membership in the cLBP group. Red indicates regions where less GM density helped to predict membership into the cLBP group. Region coordinates and anatomy are given in Table 2.
VBM Regions
A whole-brain VBM analysis did not reveal any areas in which GM density was significantly different between the 2 groups at the threshold of P < 0.05, FDR corrected. At the threshold of P < 0.001 uncorrected, we observed increased GM in left S1 and M1, in addition to decreased GM in the right middle occipital lobe (Fig. 3).
Figure 3.
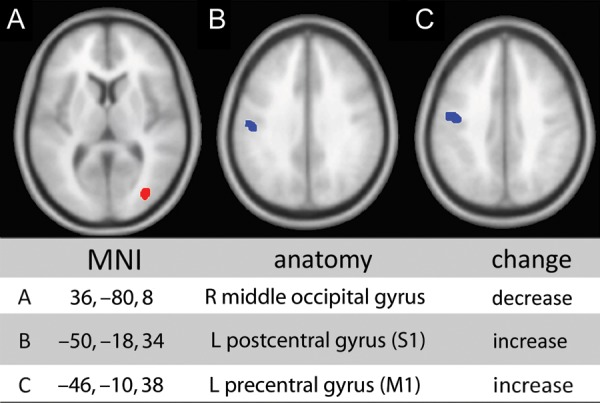
Significant GM changes in cLBP. Significant changes in the cLBP cohort versus healthy controls through VBM analysis. Blue indicates increased GM density and red indicates decreased GM density (P < 0.001 uncorrected for multiple comparisons, minimum cluster size = 10 voxels).
Discussion
In this study, we have compared patterns of GM density between participants with cLBP and matched healthy controls. We used SVM analyses of MRI brain scans to show that healthy controls and patients with cLBP exhibit different distributed patterns of GM density throughout the brain, despite having similar total GM volume. Moreover, we demonstrate that brain structure may assist in the diagnosis of cLBP. Along with a univariate VBM analysis, we found a set of distributed key regions that characterize cLBP, supporting the hypothesis of a complex pattern of abnormal brain morphology compared with few localized changes. The specific brain regions exhibiting GM differences include those believed to be involved in the pain experience, in addition to novel areas that may be associated with chronic pain. This work is an early step toward the diagnostic use of anatomical brain structure in cLBP.
Classification and Prediction Results
In support of the hypothesis that cLBP pathology is associated with a distributed pattern of abnormal brain morphology, the SVM classifier characterized a pattern of regional GM density that distinguished cLBP patients from healthy controls with 76% accuracy, through a cross-validation scheme that has been shown to provide representative measurements of accuracy (Kohavi 1995; Airola et al. 2011). This pattern includes atypical GM density both in areas of the brain typically associated with acute and chronic pain and in areas less commonly reported and understood in studies of acute and chronic pain. These regions are discussed below.
Areas of Altered GM
The most notable findings relate to increased GM in and near the left S2. SVM analysis identified an increase in GM in S2, and both SVM and uncorrected VBM analyses identified a cluster of GM increase in the left M1 and left somatosensory cortices (S1/S2). While the clusters were not identical between SVM and VBM, there is a significant overlap even with different methodological techniques. These findings in S1 and S2 likely reflect ascending thalamocortical projections and sensory-discriminative nociceptive processing that are involved in acute pain (Flor et al. 1997; Coghill et al. 1999; Peyron et al. 2000; Price 2000; Treede et al. 2000; Hofbauer et al. 2001; Apkarian et al. 2005; May 2008).
The association of M1 and pain is well established (Dettmers et al. 2001; Maihofner et al. 2007; Kim et al. 2008; Wasan et al. 2011). There is substantial evidence that stimulation of the motor cortex leads to reduced pain. A recent meta-analysis of 33 studies investigating the efficacy of motor cortex stimulation for chronic pain showed a 72.6% weighted response rate (95% confidence interval 67.7–77.4) for reductions in pain versus sham treatment (Lima and Fregni 2008). In addition to M1 findings, we observed an increased GM in the premotor cortex, although these regions did not survive cluster correction. As M1 receives strong input from S1, a GM increase in both may suggest an alteration or dysfunction of proprioception and motor planning resulting from cLBP. This is supported by recent studies that suggest reorganization of the motor cortex in cLBP patients due to the region's involvement in postural control and stability (Brumagne, Janssens, Janssens, et al. 2008; Brumagne, Janssens, Knapen, et al. 2008; Tsao et al. 2008). The changes in the motor cortex may thus not only be associated with the perception of chronic pain, but also the behavioral changes that result. Somatotopically, the S1 and M1 regions observed are more lateral and deeper than expected on the homunculus, corresponding to the head, neck, and face regions instead of the lower trunk and extremities. Flor et al. (1997) showed through magnetoencephalography that cLBP patients had a medially expanded S1 somatotopic representation of the back that correlated with chronicity of pain (Flor 2003). Our structural findings may be a result of similar cortical reorganization. However, the left laterality of our somatotopic findings was not expected, as our patients presented with axial pain. As the left and right sides of S2 are also deeply interconnected, it was expected that bilateral changes would be observed. The left laterality of S2 may reflect the lateralization of function, similar to the lateralization of function of the neighboring insula (Craig 2005). These findings as a whole suggest involvement of a sensory-motor network involved in proprioception that may be altered in those with chronic pain.
Our SVM analysis identified regions previously implicated in pain, including an area bordering the amygdala and the right DLPFC. The amygdala, part of the limbic system, plays a key role in emotion and affective disorders. There is evidence to suggest parallel processing networks, one involving the somatosensory areas in the sensory-discriminative dimension, and another involving the amygdala in the affective dimension (Giesecke et al. 2005). It is believed, through circuits to the brainstem (periaquaductal gray) and spinal cord, that the amygdala plays a role in descending inhibitory pain control (Neugebauer et al. 2004). Interestingly, the amygdala may also play a role in the enhancement of pain responses following a stressor (Rhudy and Meagher 2003). Decreased GM near the amygdala in those with cLBP may reflect alterations of these pathways.
The DLPFC is a region that has been shown functionally to be involved in the experience, localization, and modulation of pain (Coghill et al. 1999; Lorenz et al. 2003). Specifically, the DLPFC may limit the magnitude of perceived pain through a "top-down" mechanism by modulating cortical–subcortical and cortical–cortical pathways, particularly by disrupting midbrain-medial thalamic connectivity (Lorenz et al. 2003). The DLPFC may also serve to direct attention away from pain, as shown by its involvement in working memory and the placebo analgesia effect (MacDonald et al. 2000; Wager et al. 2004). The changes observed in the DLPFC may be reflective of a lack of inhibitory control over nociceptive input.
Changes in several brain regions not previously associated with pain, such as the visual system, were found with the SVM analysis. While each of these findings may individually be difficult to interpret, the totality of these changes may be understood in the full context of cLBP. An increase in GM in the right calcarine sulcus (V1) and left fusiform gyrus was observed, as well as a GM decrease in the right cuneus (V2). The right cuneus has previously been found to activate in response to acute cold pain, which, accompanied by the calcarine sulcus, plays a role in intensity encoding of pain (Fulbright et al. 2001; Kong et al. 2010). High-intensity noxious stimuli evoked significantly greater activity in these regions when compared with low-intensity stimuli. It is also possible that cLBP patients may be more dependent on vision for balance and postural control, which could partially explain both the visual findings and the disruption in M1/S1 proprioceptive networks (Maribo et al. 2012).
Finally, we noted GM differences within several areas of the temporal lobe. The increased GM was observed both in the bilateral middle temporal gyrus and in the left occipitaltemporal lobe (Table 2). These regions are potentially associated with a secondary component of cLBP. Schmidt-Wilcke et al. (2006) and Schmidt-Wilcke et al. (2007) found GM changes in the left occipitaltemporal lobe in patients with cLBP and in the right temporal lobe in those with fibromyalgia. Several of these occipital–temporal regions also overlap with previous findings that have been associated with unpleasant emotions and may further represent an affective dimension of pain (Lane et al. 1997). While the function of these areas in relation to pain remains speculative, these findings support the idea that chronic pain is associated with a complex, distributed pattern of changes in the central nervous system. Further work is necessary to more completely characterize these patients and to better understand systemic brain changes.
One intriguing question is the implication of the directionality of GM change. In a recent VBM study, healthy individuals had transient increased GM in pain modulatory regions, including the cingulate and somatosensory cortex, following repetitive noxious stimuli (Teutsch et al. 2008). It is suggested that increased GM in these regions, particularly in the somatosensory cortex, represents the engagement of a normal antinociceptive system. The GM increases within these areas in the chronic pain group may be a result of a sustained but faulty engagement or disruption of this response mechanism. It may also indicate that chronic pain is no longer dependent on peripheral afferent input, but is maintained by central processing.
Past VBM Findings
At least 2 studies have previously investigated GM changes as a result of cLBP using classical VBM analyses. Apkarian et al. (2004) showed, through a region-of-interest analysis, decreased GM in the right thalamus and bilateral DLPFC. Schmidt-Wilcke et al. (2006) showed, on a whole-brain level, decreased GM in the brainstem, somatosensory cortex, DLPFC, occipital–temporal lobe, and temporal lobe along with an increase in GM in the basal ganglia and left thalamus using a statistical threshold of P < 0.001 uncorrected for multiple comparisons. In our study, increased GM in the DLPFC helped to drive our SVM accuracy, although the precise localization is inconsistent between all 3 studies. However, in the present study, no changes in either direction were observed in the thalamus. The increased GM in the basal ganglia (putamen) was observed, but did not survive cluster correction (data not shown). Finally, we also found that cLBP is associated with the increased GM in the left occipital–temporal lobe, localized near the region reported by Schmidt-Wilcke. This may reinforce the involvement of vision systems in cLBP.
The overall lack of consistency in these findings are difficult to explain and may be due to variability between each study's patient population, including differences in the number of participants, study demographics, neuropathology, and state of medication. In this study, we investigated a total of 47 participants with cLBP, in comparison with 18 and 26 participants from prior studies (Apkarian et al. 2004; Schmidt-Wilcke et al. 2006). We also excluded participants with neuropathic pain from diagnosed radiculopathy, while the study by Apkarian et al. included those with neuropathic pain. Finally, it is possible that differences in analgesic use between studies may explain some of the differences in results, as brain structure may be affected by prescription analgesics (Younger et al. 2011) and prescription analgesic use was an exclusionary criteria in our study. Further investigation, however, is necessary to demonstrate conclusively the effects of these potential confounds.
Limitations
An objective pain measurement would have significant ethical, legal, and medical implications; however, we note that this study is preliminary, and further work is needed before any real-world applications can be considered. Our patients were carefully screened to eliminate confounds from other conditions; however, these patients may not be fully representative of the broad spectrum of cLBP patients. By testing larger populations over broader age ranges and including medications, comorbid disorders, different subtypes of cLBP, and/or other types of chronic pain, we can determine the specificity of our findings and of our classifier to cLBP as well as its ultimate generalizability. Furthermore, this study is meant to show that neuroimaging may provide an objective physiological marker of cLBP that augments self-report. Given the complex combination of sensory, cognitive, emotional, and motivational factors that make up the chronic pain experience, it is possible that no objective marker of pain will ever replace a patient's self-report. The authors all strongly discourage inappropriate and premature extrapolation of these findings to suggest that patient self-report of pain can be replaced.
The physiological mechanisms that underlie directional changes in GM density, as measured by MRI, remain unclear. Current MRI technology does not readily allow for the observation of cellular- or molecular-level changes that underlie GM changes. Some of these changes may be attributed to degeneration, restructuring, or apoptosis of neuronal or glial cell populations. As both GM increases and decreases were important in identifying pain patients, the structural abnormalities we identified may also represent neural adaptations to aberrant peripheral nociceptive inputs. Moreover, the presence of GM increases suggests that the neural changes seen in chronic pain cannot be globally described as neurodegenerative. It is also important to note that these are results from the characterization of GM only. The incorporation of other tissue classes or imaging modalities such as white matter and functional imaging may improve characterization and performance (Brown et al. 2011).
Although subjects were homogenous with respect to diagnosis and medication state, structural differences may result from physical and psychosocial factors interrelated with pain, such as reduced physical activity and depression (Gatchel et al. 1995, 2007; Colcombe et al. 2003; Frodl et al. 2008). A recent study investigating GM volume changes in patients with fibromyalgia explained observed differences in the left anterior insula with depression severity (Hsu et al. 2009). As our healthy volunteers were excluded for a known history of any major psychiatric disorder and the majority of the cLBP group had normal or mild levels of depressive symptoms as determined by self-report inventories, our results were unlikely to be driven by affective disorder. To confirm, a separate, identical SVM analysis was performed without 5 patients who self-reported moderate or severe levels depression/anxiety and their matched controls. The resulting discriminative regions driving the classification remain unchanged and indicate that the original model is unlikely to be differentiating between affective disorder (data not shown).
From a machine learning perspective, the performance, generalizability, and significance of the SVM findings benefit from a large sample size and better feature selection/reduction methods. Incorporating more individuals may allow for improved characterization of the underlying conditions, and thus better classification. Expanding feature selection to include other tissue classes, multispectral data, behavioral, and genomic information may offer better discriminative information for predicting cLBP (Diatchenko et al. 2005).
Conclusions
Our investigation of cLBP using SVM learning and VBM suggests that the pathology of cLBP involves changes in GM that are present throughout a distributed system of pain-processing and pain-associated areas within the brain. The significant accuracy of our classifier, in addition to the relevance of discriminative regions identified, represent a promising advance in both our understanding of brain's role in cLBP and our ability to objectively classify the disease. Future studies should investigate the brain regions identified here to determine functional significance of the structural pathology that we have detected. In addition, structural studies should further investigate these areas for potential use as biomarkers in diagnosis and the prediction of treatment response.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health (DA026092 and DA029262 to S.M. and K99 DA023609 to J.Y.), IASP International Collaborative Research grant to J.H. and S.M., and the Redlich Pain Research Endowment to S.M.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Airola A, Pahikkala T, Waegeman W, De Baets B, Salakoski T. An experimental comparison of cross-validation techniques for estimating the area under the ROC curve. Comput Stat Data Anal. 2011;55:1828–1844. doi:10.1016/j.csda.2010.11.018. [Google Scholar]
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. doi:10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Apkarian AV. Functional imaging of pain—new insights regarding the role of the cerebral-cortex in human pain perception. Semin Neurosci. 1995;7:279–293. doi:10.1006/smns.1995.0031. [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. doi:10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. doi:10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. doi:10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Borsook D, Sava S, Becerra L. The pain imaging revolution: advancing pain into the 21st century. Neuroscientist. 2010;16:171–185. doi: 10.1177/1073858409349902. doi:10.1177/1073858409349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS ONE. 2011;6:e24124. doi: 10.1371/journal.pone.0024124. doi:10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumagne S, Janssens L, Janssens E, Goddyn L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait Posture. 2008;28:657–662. doi: 10.1016/j.gaitpost.2008.04.015. doi:10.1016/j.gaitpost.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Janssens L, Knapen S, Claeys K, Suuden-Johanson E. Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur Spine J. 2008;17:1177–1184. doi: 10.1007/s00586-008-0709-7. doi:10.1007/s00586-008-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burges CJC. A tutorial on support vector machines for pattern recognition. Data Min Knowl Discov. 1998;2:121–167. doi:10.1023/A:1009715923555. [Google Scholar]
- Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Int Syst Technol. 2011;2:1–27. [Google Scholar]
- Chou R, Qaseem A, Snow V, Casey D, Cross JT, Jr, Shekelle P, Owens DK. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Argoff CE, Carragee EJ. Management of low back pain. BMJ. 2008;337:a2718. doi: 10.1136/bmj.a2718. doi:10.1136/bmj.a2718. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. doi:10.1093/gerona/58.2.M176. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. doi:10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cristianini N, Shawe-Taylor J. An introduction to support vector machines and other kernel-based learning methods. Cambridge, NY: Cambridge University Press; 2000. [Google Scholar]
- Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153–154. doi: 10.1212/01.wnl.0000295509.30630.10. doi:10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Adler T, Rzanny R, van Schayck R, Gaser C, Weiss T, Miltner WH, Bruckner L, Weiller C. Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci Lett. 2001;307:109–112. doi: 10.1016/s0304-3940(01)01953-x. doi:10.1016/S0304-3940(01)01953-X. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. doi:10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41:66–72. doi: 10.1080/16501960310010179. doi:10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224:5–8. doi: 10.1016/s0304-3940(97)13441-3. doi:10.1016/S0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. doi:10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Moller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. doi:10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Troche CJ, Skudlarski P, Gore JC, Wexler BE. Functional MR imaging of regional brain activation associated with the affective experience of pain. AJR Am J Roentgenol. 2001;177:1205–1210. doi: 10.2214/ajr.177.5.1771205. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. doi:10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Polatin PB, Mayer TG. The dominant role of psychosocial risk factors in the development of chronic low back pain disability. Spine. 1995;20:2702–2709. doi: 10.1097/00007632-199512150-00011. doi:10.1097/00007632-199512150-00011. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. doi:10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. doi:10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. doi:10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Howley T, Madden MG, O'Connell ML, Ryder AG. The effect of principal component analysis on machine learning accuracy with high-dimensional spectral data. Knowledge-Based Systems. 2006;19:363–370. doi:10.1016/j.knosys.2005.11.014. [Google Scholar]
- Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–267. doi: 10.1016/j.pain.2009.03.017. doi:10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1347–1358. doi: 10.1098/rstb.1999.0483. doi:10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academics Press; 2011. [PubMed] [Google Scholar]
- Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. doi:10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Chu C, Tan GC, Draganski B, Johnson H, Paulsen JS, Kienzle W, Tabrizi SJ, Ashburner J, Frackowiak RS. Automatic detection of preclinical neurodegeneration: presymptomatic Huntington disease. Neurology. 2009;72:426–431. doi: 10.1212/01.wnl.0000341768.28646.b6. doi:10.1212/01.wnl.0000341768.28646.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–1434. doi: 10.1136/bmj.332.7555.1430. doi:10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohavi R. A study of cross-validation and bootsrap for accuracy estimation and model selection. Proceedings of the 14th International Joint Conference on Artificial Intelligence, Montreal, Canada, August 1995; 1995. pp. 1137–1143. [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. doi:10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. doi:10.1016/S0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–2337. doi: 10.1212/01.wnl.0000314649.38527.93. doi:10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. doi:10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. doi:10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. doi:10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- Maribo T, Schiottz-Christensen B, Jensen LD, Andersen NT, Stengaard-Pedersen K. Postural balance in low back pain patients: criterion-related validity of centre of pressure assessed on a portable force platform. Eur Spine J. 2012;21:425–431. doi: 10.1007/s00586-011-1981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. doi:10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Bokde AL, Born C, Hampel H, Stetter M. Classifying brain states and determining the discriminating activation patterns: support vector machine on functional MRI data. NeuroImage. 2005;28:980–995. doi: 10.1016/j.neuroimage.2005.06.070. doi:10.1016/j.neuroimage.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. doi:10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. NeuroImage. 2009;45:S199–209. doi: 10.1016/j.neuroimage.2008.11.007. doi:10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis . Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. doi:10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Negative affect: effects on an evaluative measure of human pain. Pain. 2003;104:617–626. doi: 10.1016/S0304-3959(03)00119-2. doi:10.1016/S0304-3959(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Rubinstein SM, van Tulder M. A best-evidence review of diagnostic procedures for neck and low-back pain. Best Pract Res Clin Rheumatol. 2008;22:471–482. doi: 10.1016/j.berh.2007.12.003. doi:10.1016/j.berh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. doi:10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–S116. doi: 10.1016/j.pain.2007.05.010. doi:10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. e2 doi:10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. NeuroImage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. doi:10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87:113–119. doi: 10.1016/S0304-3959(00)00350-X. doi:10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171. doi: 10.1093/brain/awn154. doi:10.1093/brain/awn154. [DOI] [PubMed] [Google Scholar]
- van den Bosch MA, Hollingworth W, Kinmonth AL, Dixon AK. Evidence against the use of lumbar spine radiography for low back pain. Clin Radiol. 2004;59:69–76. doi: 10.1016/j.crad.2003.08.012. doi:10.1016/j.crad.2003.08.012. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427–434. doi: 10.1097/00007632-199702150-00015. doi:10.1097/00007632-199702150-00015. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Gunter JL, Senjem ML, Whitwell JL, Kantarci K, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. NeuroImage. 2008;39:1186–1197. doi: 10.1016/j.neuroimage.2007.09.073. doi:10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. doi:10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–374. doi: 10.1097/ALN.0b013e318220e880. doi:10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB. Variations in brain gray matter associated with chronic pain. Curr Rheumatol Rep. 2010;12:462–469. doi: 10.1007/s11926-010-0129-7. doi:10.1007/s11926-010-0129-7. [DOI] [PubMed] [Google Scholar]
- Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. doi:10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. doi:10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.