Abstract
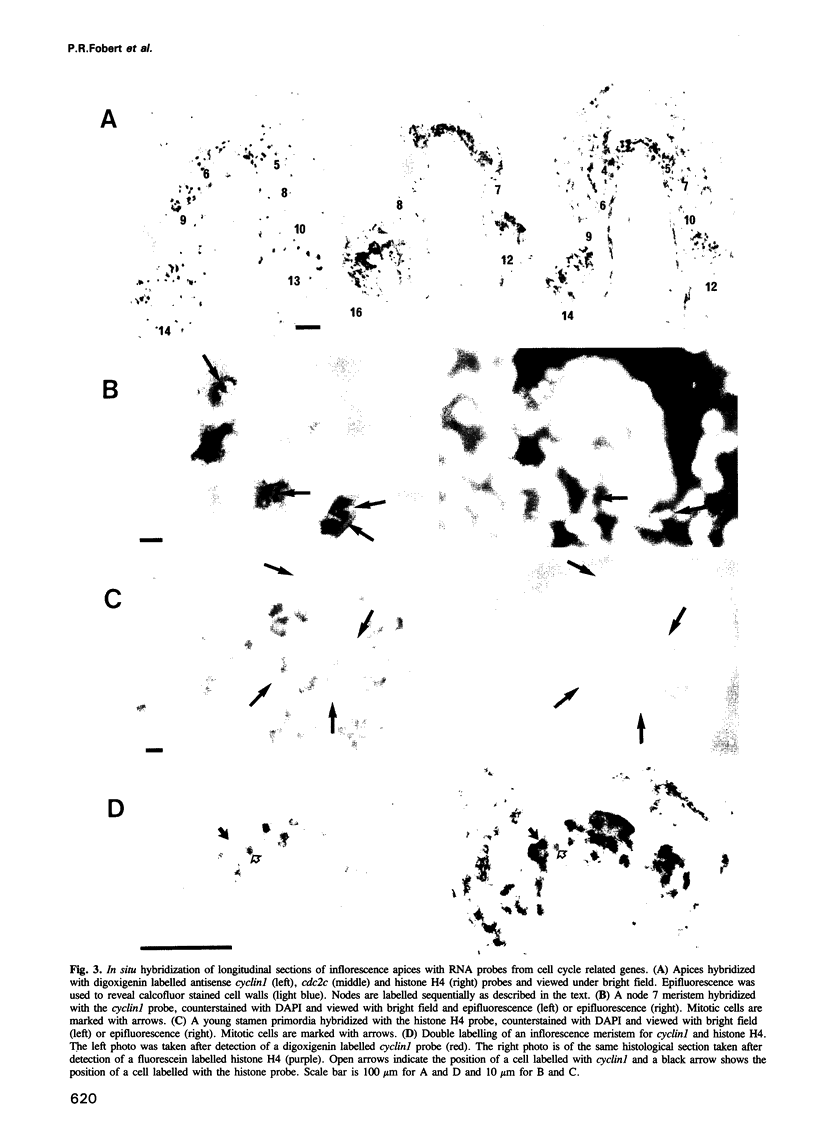
Transcripts from five cell cycle related genes accumulate in isolated cells dispersed throughout the actively dividing regions of plant meristems. We propose that this pattern reflects gene expression during particular phases of the cell division cycle. The high proportion of isolated cells suggests that synchrony between daughter cells is rapidly lost following mitosis. This is the first time that such a cell specific expression pattern has been described in a higher organism. Counterstaining with a DNA specific dye revealed that transcripts from three genes (two mitotic cyclins and a cdc2-like gene) accumulate during part of interphase and early mitosis whereas transcripts from a histone H4 gene are preferentially detected only in interphase cells. Double labelling for cyclin and histone H4 transcripts confirms that these genes are expressed in different cells, and therefore at different phases of the cell cycle. The results suggest that transcriptional regulation of cell cycle related genes may be important in controlling cell division in plants, and that these genes are useful markers for identifying cells at specific phases of the cell cycle within plant meristems.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D., Carpenter R., Sommer H., Hartley N., Coen E. Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell. 1993 Jan 15;72(1):85–95. doi: 10.1016/0092-8674(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986 Oct 24;47(2):285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Romero J. M., Doyle S., Elliott R., Murphy G., Carpenter R. floricaula: a homeotic gene required for flower development in antirrhinum majus. Cell. 1990 Dec 21;63(6):1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989 Apr 7;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990 Aug 10;62(3):469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilotter H., Lingner C., Rowley R., Young P. G. Regulation of the G2-mitosis transition. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):954–971. doi: 10.1139/o92-140. [DOI] [PubMed] [Google Scholar]
- Ferreira P. C., Hemerly A. S., Villarroel R., Van Montagu M., Inzé D. The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell. 1991 May;3(5):531–540. doi: 10.1105/tpc.3.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. J., Mandel T., Roth I., Kuhlemeier C. The patterns of gene expression in the tomato shoot apical meristem. Plant Cell. 1993 Mar;5(3):297–309. doi: 10.1105/tpc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Kouchi H., Suzuka I., Ishii T. Isolation and characterization of cDNA clones for plant cyclins. EMBO J. 1991 Sep;10(9):2681–2688. doi: 10.1002/j.1460-2075.1991.tb07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly A., Bergounioux C., Van Montagu M., Inzé D., Ferreira P. Genes regulating the plant cell cycle: isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3295–3299. doi: 10.1073/pnas.89.8.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H., Mink M., Pfosser M., Bögre L., Györgyey J., Jonak C., Gartner A., Dudits D., Heberle-Bors E. Alfalfa cyclins: differential expression during the cell cycle and in plant organs. Plant Cell. 1992 Dec;4(12):1531–1538. doi: 10.1105/tpc.4.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol. 1991 Aug;2(4):213–222. [PubMed] [Google Scholar]
- Imajuku Y., Hirayama T., Endoh H., Oka A. Exon-intron organization of the Arabidopsis thaliana protein kinase genes CDC2a and CDC2b. FEBS Lett. 1992 Jun 8;304(1):73–77. doi: 10.1016/0014-5793(92)80592-5. [DOI] [PubMed] [Google Scholar]
- Koning A. J., Tanimoto E. Y., Kiehne K., Rost T., Comai L. Cell-specific expression of plant histone H2A genes. Plant Cell. 1991 Jul;3(7):657–665. doi: 10.1105/tpc.3.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. C., Jørgensen J. E., Lawton M. A., Lamb C. J., Doerner P. W. Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7360–7364. doi: 10.1073/pnas.89.16.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Pandey N. B. Multiple regulatory steps control histone mRNA concentrations. Trends Biochem Sci. 1988 Feb;13(2):49–52. doi: 10.1016/0968-0004(88)90027-8. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Heintz N. Transcriptional regulation in the eukaryotic cell cycle. Trends Biochem Sci. 1991 Nov;16(11):430–435. doi: 10.1016/0968-0004(91)90170-z. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., May G. S., Morris N. R. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J Cell Biol. 1987 Jun;104(6):1495–1504. doi: 10.1083/jcb.104.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993 Jun;18(6):195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Reed S. I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Hue I., Huijser P., Flor P. J., Hansen R., Tetens F., Lönnig W. E., Saedler H., Sommer H. Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 1992 Jan;11(1):251–263. doi: 10.1002/j.1460-2075.1992.tb05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Huijser P., Nacken W., Saedler H., Sommer H. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science. 1990 Nov 16;250(4983):931–936. doi: 10.1126/science.250.4983.931. [DOI] [PubMed] [Google Scholar]
- Schwob E., Nasmyth K. CLB5 and CLB6, a new pair of B cyclins involved in DNA replication in Saccharomyces cerevisiae. Genes Dev. 1993 Jul;7(7A):1160–1175. doi: 10.1101/gad.7.7a.1160. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Staiger C., Doonan J. Cell division in plants. Curr Opin Cell Biol. 1993 Apr;5(2):226–231. doi: 10.1016/0955-0674(93)90107-2. [DOI] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Futcher B. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 1993 May;12(5):1955–1968. doi: 10.1002/j.1460-2075.1993.tb05845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3093–3097. doi: 10.1073/pnas.89.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]