Abstract
Cell death is a prominent feature of B cell development. For example, a large population of B cells dies at the pre-B cell stage presumably due to the failure to express a functional immunoglobulin receptor. In addition, developing B cells expressing antigen receptors for self are selectively eliminated at the immature B cell stage. The molecular signals that control B cell survival are largely unknown. The product of the bcl-2 proto-oncogene may be involved as its overexpression inhibits apoptotic cell death in a variety of biological systems. However, the physiological role of the endogenous Bcl-2 protein during B cell development is undetermined. Here we show a striking developmental regulation of the Bcl-2 protein in B lymphocytes. Bcl-2 is highly expressed in CD43+ B cell precursors (pro-B cells) and mature B cells but downregulated at the pre-B and immature B cell stages of development. We found that Bcl-2 expressed by B cells is a long-lived protein with a half-life of approximately 10 h. Importantly, susceptibility to apoptosis mediated by the glucocorticoid hormone dexamethasone is stage-dependent in developing B cells and correlates with the levels of Bcl-2 protein. Furthermore, expression of a bcl-2 transgene rescued pre-B and immature B cells from dexamethasone-induced cell death, indicating that Bcl-2 can inhibit the apoptotic cell death of progenitors and early B cells. Taken together, these findings argue that Bcl-2 is a physiological signal controlling cell death during B cell development.
Full text
PDF
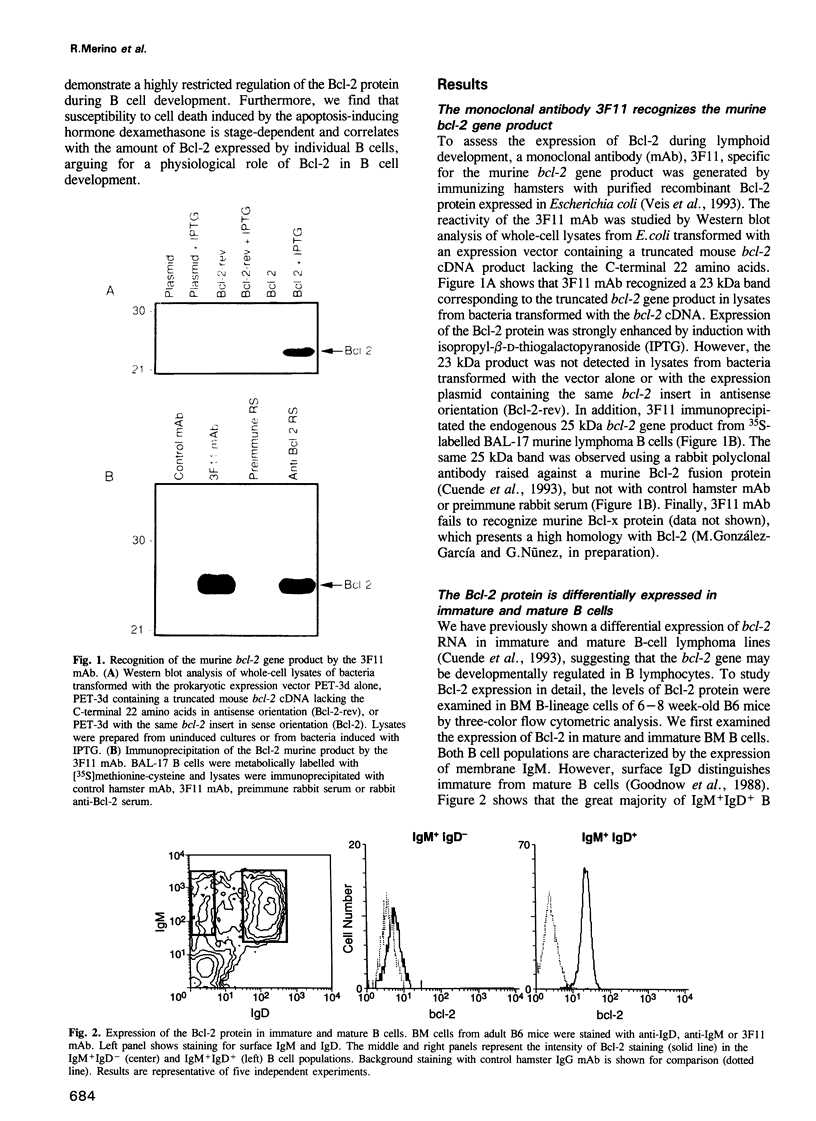
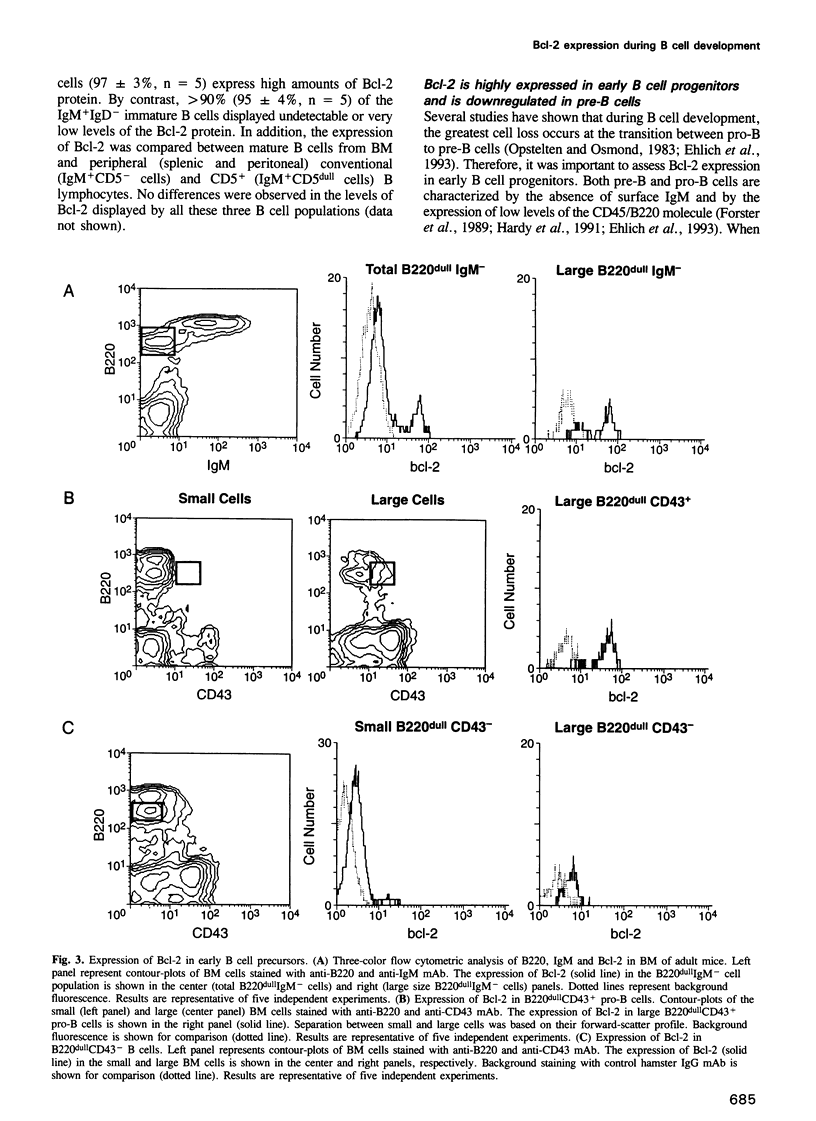
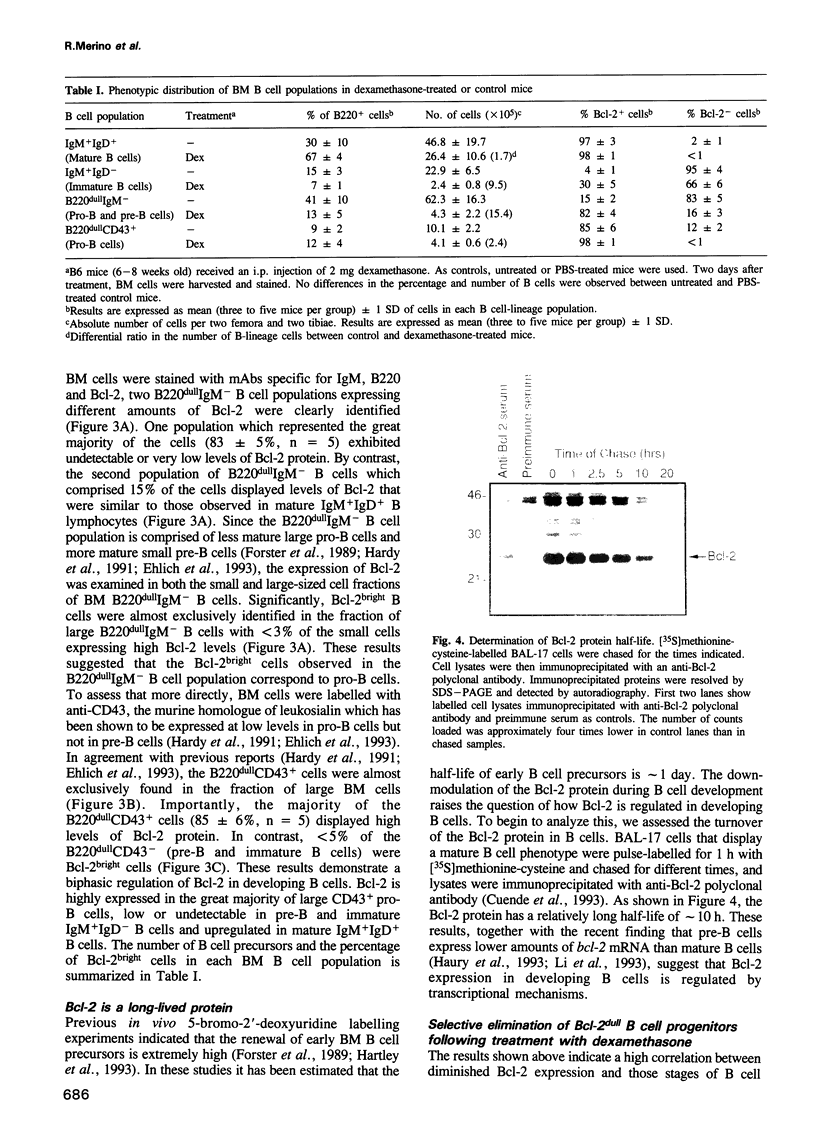
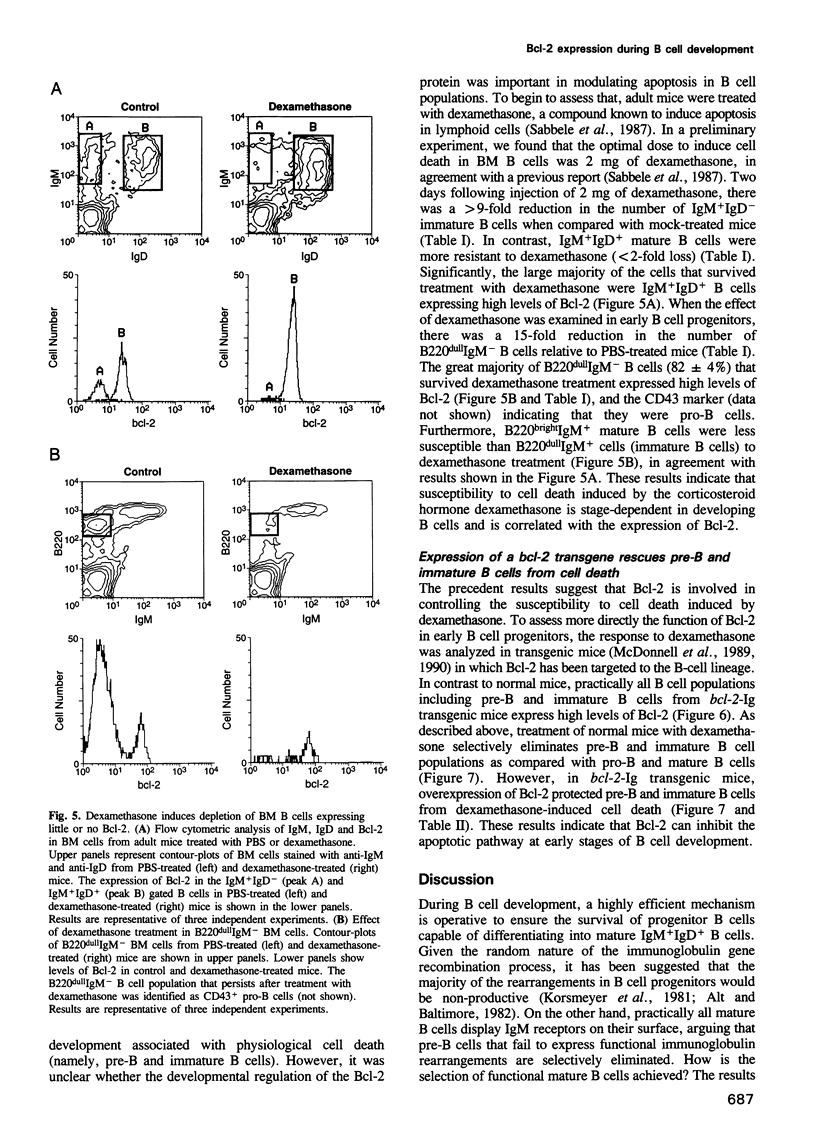
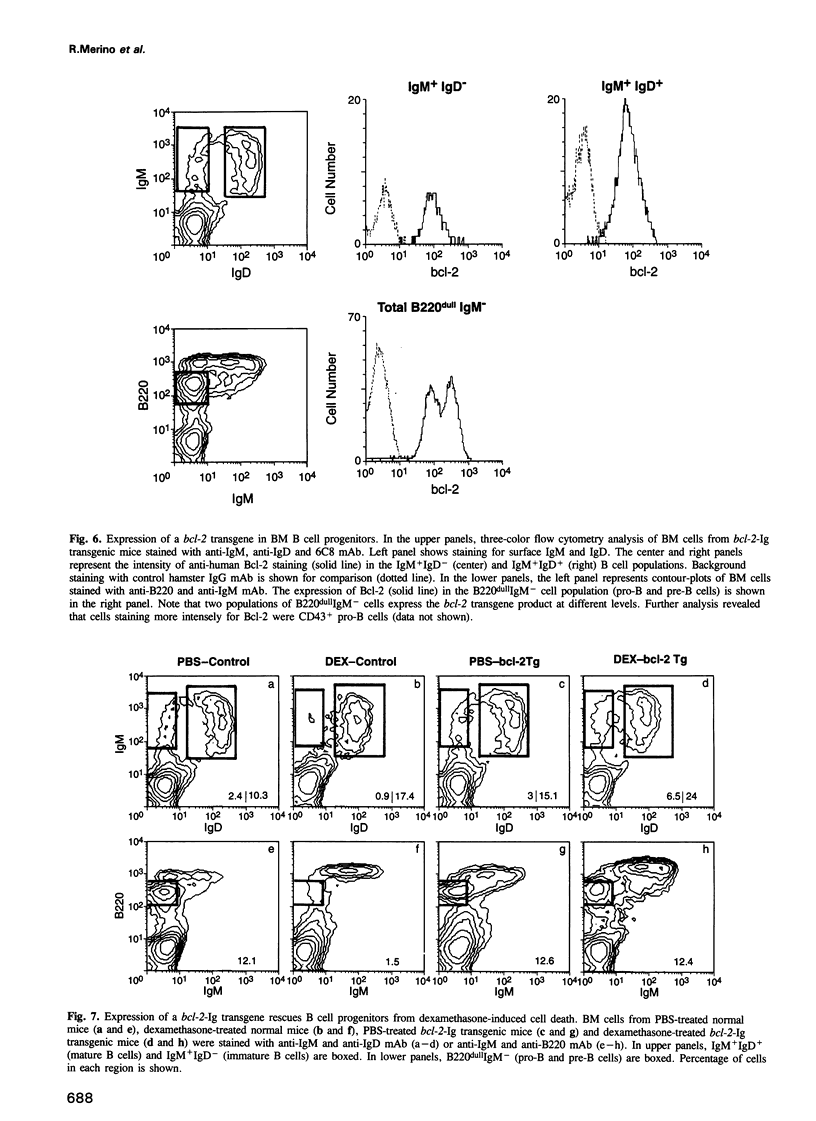
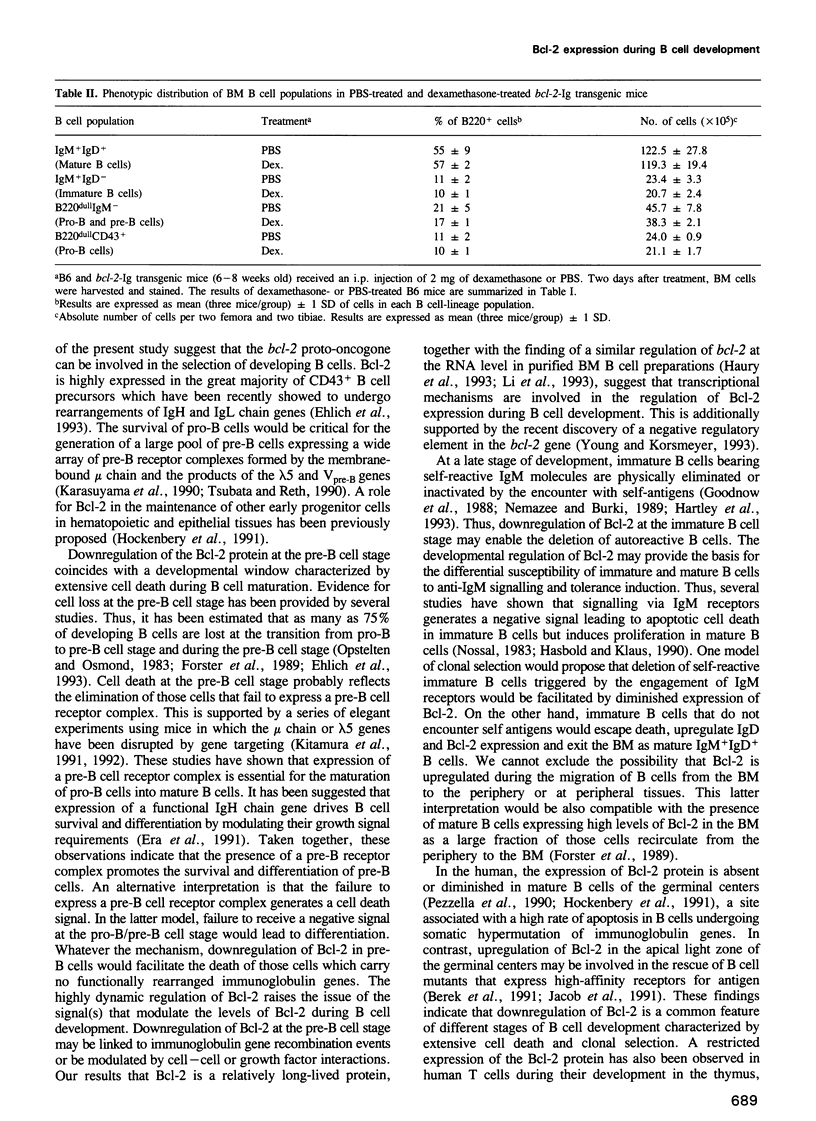


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Fernandes T. F., Haldar S., Croce C. M., Litwack G. Involvement of BCL-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992 Jan 15;52(2):491–495. [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins M. K., Marvel J., Malde P., Lopez-Rivas A. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J Exp Med. 1992 Oct 1;176(4):1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuende E., Alés-Martínez J. E., Ding L., Gónzalez-García M., Martínez C., Nunez G. Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO J. 1993 Apr;12(4):1555–1560. doi: 10.1002/j.1460-2075.1993.tb05799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Era T., Ogawa M., Nishikawa S., Okamoto M., Honjo T., Akagi K., Miyazaki J., Yamamura K. Differentiation of growth signal requirement of B lymphocyte precursor is directed by expression of immunoglobulin. EMBO J. 1991 Feb;10(2):337–342. doi: 10.1002/j.1460-2075.1991.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster I., Vieira P., Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1(4):321–331. doi: 10.1093/intimm/1.4.321. [DOI] [PubMed] [Google Scholar]
- Garcia I., Martinou I., Tsujimoto Y., Martinou J. C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992 Oct 9;258(5080):302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gratiot-Deans J., Ding L., Turka L. A., Nuñez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. J Immunol. 1993 Jul 1;151(1):83–91. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S. B., Cooke M. P., Fulcher D. A., Harris A. W., Cory S., Basten A., Goodnow C. C. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993 Feb 12;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Hasbold J., Klaus G. G. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990 Aug;20(8):1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Haury M., Freitas A., Hermitte V., Coutinho A., Hibner U. The physiology of bcl-2 expression in murine B lymphocytes. Oncogene. 1993 May;8(5):1257–1262. [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Kagan R. J., Bratescu A., Jonasson O., Matsuda T., Teodorescu M. The relationship between the percentage of circulating B cells, corticosteroid levels, and other immunologic parameters in thermally injured patients. J Trauma. 1989 Feb;29(2):208–213. doi: 10.1097/00005373-198902000-00010. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. S., Hayakawa K., Hardy R. R. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993 Sep 1;178(3):951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992 Oct 1;52(19):5407–5411. [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Olins P. O., Devine C. S., Rangwala S. H., Kavka K. S. The T7 phage gene 10 leader RNA, a ribosome-binding site that dramatically enhances the expression of foreign genes in Escherichia coli. Gene. 1988 Dec 15;73(1):227–235. doi: 10.1016/0378-1119(88)90329-0. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Opstelten D., Osmond D. G. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu-chain-bearing cells in normal mice. J Immunol. 1983 Dec;131(6):2635–2640. [PubMed] [Google Scholar]
- Osmond D. G. B cell development in the bone marrow. Semin Immunol. 1990 May;2(3):173–180. [PubMed] [Google Scholar]
- Osmond D. G., Kim N., Manoukian R., Phillips R. A., Rico-Vargas S. A., Jacobsen K. Dynamics and localization of early B-lymphocyte precursor cells (pro-B cells) in the bone marrow of scid mice. Blood. 1992 Apr 1;79(7):1695–1703. [PubMed] [Google Scholar]
- Osmond D. G., Priddle S., Rico-Vargas S. Proliferation of B cell precursors in bone marrow of pristane-conditioned and malaria-infected mice: implications for B cell oncogenesis. Curr Top Microbiol Immunol. 1990;166:149–157. doi: 10.1007/978-3-642-75889-8_19. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sabbele N. R., Van Oudenaren A., Hooijkaas H., Benner R. The effect of corticosteroids upon murine B cells in vivo and in vitro as determined in the LPS-culture system. Immunology. 1987 Oct;62(2):285–290. [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D. J., Sentman C. L., Bach E. A., Korsmeyer S. J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993 Sep 1;151(5):2546–2554. [PubMed] [Google Scholar]
- Young R. L., Korsmeyer S. J. A negative regulatory element in the bcl-2 5'-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993 Jun;13(6):3686–3697. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]




