Abstract
Members of the M13 class of metalloproteases have been implicated in diseases and in reproductive fitness. Nevertheless, their physiological role remains poorly understood. To obtain a tractable model with which to analyze this protein family’s function, we characterized the gene family in Drosophila melanogaster and focused on reproductive phenotypes. The D. melanogaster genome contains 24 M13 class protease homologs, some of which are orthologs of human proteases, including neprilysin. Many are expressed in the reproductive tracts of either sex. Using RNAi we individually targeted the five Nep genes most closely related to vertebrate neprilysin, Nep1-5, to investigate their roles in reproduction. A reduction in Nep1, Nep2, or Nep4 expression in females reduced egg laying. Nep1 and Nep2 are required in the CNS and the spermathecae for wild-type fecundity. Females that are null for Nep2 also show defects as hosts of sperm competition as well as an increased rate of depletion for stored sperm. Furthermore, eggs laid by Nep2 mutant females are fertilized normally, but arrest early in embryonic development. In the male, only Nep1 was required to induce normal patterns of female egg laying. Reduction in the expression of Nep2-5 in the male did not cause any dramatic effects on reproductive fitness, which suggests that these genes are either nonessential for male fertility or perform redundant functions. Our results suggest that, consistent with the functions of neprilysins in mammals, these proteins are also required for reproduction in Drosophila, opening up this model system for further functional analysis of this protein class and their substrates.
Keywords: Drosophila, reproduction, neprilysins, proteolysis, neuropeptides
PROTEASES play key roles in diverse physiological systems. One such family of metalloproteases, the M13 class of neutral endopeptidases, consists mainly of membrane-bound zinc proteases that are involved in the processing of neuropeptides and peptide hormones (reviewed in Turner et al. 2000; Turner et al. 2001; Bland et al. 2008). In mammals, seven members of this family have been identified, of which neprilysin (NEP) and endothelin converting enzyme (ECE) are the best studied. These proteins have been implicated in various diseases including cardiovascular disease (Segura and Ruilope 2011; Wick et al. 2011), Alzheimer’s disease (Mulder et al. 2012; Klein et al. 2013), inflammation and inflammatory disorders (Wong et al. 2011), and cancer (Smollich et al. 2007; Maguer-Satta et al. 2011).
In addition to their role in disease, NEPs are essential for development and reproduction in mammals. The mammalian Neprilysin-2, called NL1 in mice, is highly expressed in the testis. NL1-deficient males sire fewer pups, even though spermatogenesis appears to be unaffected (Carpentier et al. 2004). In females, NEP expression in the uterus is modulated by estrogen treatment in rats (Pinto et al. 1999) and during the estrogen/progesterone cycle in humans (Head et al. 1993). In female rats and mice, controlled degradation of tachykinins, particularly substance-P, by NEP in the uterus is essential for controlling uterine contractions at different stages of pregnancy; an inability to degrade tachykinins in the uterus is associated with a reduction in litter size (Pinto et al. 1999; Pintado et al. 2003). In rats, tachykinins and their receptors have been implicated in the regulation of luteinizing hormone (LH) release (Sahu et al. 1987; Sahu and Kalra 1992; Bonavera et al. 1994). In humans, loss of function in either the tachykinin, Neurokinin B (which is preferentially deactivated by neprilysin) , or its receptor NK3-R correlates with a failure to enter puberty (Rance et al. 2010; Young et al. 2010). The exact ways in which neprilysins act to help regulate these aspects of reproduction are still largely unknown.
While the diverse role of Neps and their substrates in mammals has been the target of intense investigation, in other organisms the functions of neprilysins in reproduction are less clear. Consistent with their mammalian counterparts, neuropeptides and peptide hormones (including tachykinins) play important roles in regulating reproductive success in most organisms studied to date. In the sea squirt, Ciona intestinalis, tachykinins regulate oocyte growth (Aoyama et al. 2012). Between mammals and the zebrafish, Danio rerio, the estrogen-dependent features of tachykinins and their receptors appear to be conserved (Biran et al. 2012). In the insects Drosophila melanogaster and Tribolium castaneum, the tachykinin-like neuropeptide, natalisin, plays a role in regulating mating and reproductive outcomes (Jiang et al. 2013). Finally, in both the locust Locusta migratoria and the cockroach, Leucophaea maderae, the functional cleavage of tachykinins by neprilysins in the brain is conserved (Isaac and Nässel 2003).
To understand the physiological roles of neprilysins in reproduction, and by extension the neuropeptides that they regulate, we focused on this gene family in the genetically tractable model D. melanogaster. The D. melanogaster genome has 24 NEP-like genes, most of which are actively transcribed (Coates et al. 2000; Chintapalli et al. 2007; Bland et al. 2008). However, little is known about their roles in vivo. Neprilysin-like activity has been detected in extracts of larval imaginal discs and of neuronal membranes from larval and adult heads of Drosophila (Isaac et al. 2002; Wilson et al. 2002). At least two Drosophila genes, Nep2 (Bland et al. 2007) and Nep4 (Meyer et al. 2009), are active proteases with specific substrate affinities that can be inhibited with the M13-specific peptidase inhibitors thiorphan and phosphoramidon. Nep2 has been shown to cleave locustatachykinin-1 (LomTK-1) and Drosophila tachykinins in vitro (Thomas et al. 2005). Roles for Drosophila Nep2 in renal function and reproduction have been suggested based on its expression in Malpighian tubules and the reproductive organs of both sexes (Thomas et al. 2005; Chintapalli et al. 2007).
Here, we examined the phylogeny of Drosophila neprilysin proteins and analyzed the function and the expression patterns of a subfamily, containing Neprilysin1 (Nep1), Neprilysin2 (Nep2), Neprilysin3 (Nep3), Neprilysin4 (Nep4), and Neprilysin5 (Nep5), whose expression pattern and sequence homology is most similar to the canonical mammalian neprilysin. Our mutational and RNAi studies revealed that neprilysins are important in males for maximizing egg laying in their mates as well as for regulating egg production and sperm use in mated females.
Materials and Methods
Sequence comparison and tree building
Protein sequences were downloaded from Flybase (Marygold et al. 2013) and aligned using Muscle (Edgar 2004), and the alignment was checked by eye in MEGA 5.05 (Tamura et al. 2011). The program ProML, part of the Phylip 3.69 suite, was used to make the tree (Felsenstein 2005) and it was visualized for publication using FigTree v. 1.3.1 (Rambaut 2010).
In situ hybridization
Fly culture:
yw and Canton-S stocks were maintained on a standard diet (6.4% cornmeal, 5.2% molasses, 1.8% dextrose, 1.2% yeast, 1% propionic acid, 0.75% agar, 0.15% methyl-4-hydroxybenzoate in 1.5% ethanol) at 25° in plastic vials.
Egg collection:
Flies were allowed to lay eggs for 17 hr on apple juice agar plates (3% agar, 5.5% sucrose, 2.5% EtOH, 1.25% glacial acetic acid in apple juice) supplemented with yeast paste in a 25° incubator. Adult flies were then removed, and the embryos were washed off the original plates with water and transferred to a nylon mesh. To remove remainders of the yeast paste and apple juice agar, the embryos were washed with water. Embryos were dechorionated, permeabilized, and fixed as described in Sullivan et al. 2000.
In situ hybridization: DIG RNA labeling:
We used cDNA clones GH03315 (Nep1-RB), GH07643 (Nep2), RE48040 (Nep3), LD25753 (Nep4), and AT14086 (Nep5), from the Drosophila Genomics Research Center (DGRC), for preparation of probes. Overnight restriction digest at 37° was done with NotI and BstBI for antisense and sense Nep1 probe, EcoRI and XhoI for Nep2, NotI and Asp718I for Nep3, EcoRI and XhoI for Nep4, and SalI and NruI for Nep5.
Linearized template DNA was purified using QiaQuick PCR purification kit (Qiagen). RNA labeling was performed with the DIG RNA labeling kit (Roche) and 1 µg of purified DNA following the manufacturer’s protocol. Probes were hydrolyzed to a desired length of 200 bases. The RNA transcripts were analyzed for size by formaldehyde agarose gel electrophoresis and ethidium bromide staining. The labeling efficiency was tested using DIG quantification test strips and control strips (Roche).
Tissue collection, fixation and hybridization:
Third instar larval tissue:
The posterior end of third-instar larvae was removed with forceps and the larvae were inverted to expose the brain, most of the imaginal discs, and parts of the gut and fat body.
Adult tissue:
Adult abdomens were removed from the thorax and opened on the ventral side from anterior to posterior to expose all the tissues to the solutions. The thorax was separated from head and abdomen and the dorsal side of the cuticle was removed. For in situ hybridization on adult brains, the proboscis and part of the cuticle and the air sacs were removed from isolated heads.
All dissected tissues were kept in PBT (PBS, 0.1% Tween 20) on ice for maximum 1 hr before fixation. Fixation was done on a shaking platform for 60 min at room temperature in 1 ml of 4% paraformaldehyde containing 0.1% sodium deoxycholate.
Embryos:
Embryos were collected and fixed as in Sullivan et al. (2000). Before starting the proteinase K treatment, embryos were rehydrated in the following conditions for 10 min each: 25% PBT/75% MeOH; 50% PBT/50% MeOH; 75% PBT/25% MeOH; and 100% PBT.
All tissues were rinsed in 1 ml PBT and washed 5 × 5 min in 1 ml PBT after fixation or rehydration. Different tissues were incubated in a volume of 150 µl proteinase K mixture: inverted third-instar larvae, 15 µg/ml proteinase K for 2 min at 37°; adult abdomen, 15 µg/ml proteinase K for 3 min at 37°; adult thorax, 10 µg/ml proteinase K for 2 min at 37°; adult brain, 10 µg/ml proteinase K for 2 min at 37°; and whole-mount embryos, 40 µg/ml proteinase K for 3 min at RT. Prehybridization, hybridization, and detection were as described in Clements et al. (2008).
Fertility/fecundity assays and sperm competition
Fly stocks and media:
All flies were raised at room temperature (23° ± 1°) in glass bottles on standard yeast–glucose media (Gligorov et al. 2013). Females were aged 3–5 days from eclosion in groups of 5–12 in glass vials with added yeast. Male flies were aged 3–5 days from eclosion in groups of 10–20 in glass vials on standard yeast–glucose media. The RNAi lines used for Nep1, Nep2, Nep3, Nep4, and Nep5 were all obtained from the Vienna Drosophila RNAi Center (VDRC) (Dietzl et al. 2007) and are identified in Supporting Information, Table S3. Knockdown of transcripts was confirmed by RT–PCR as previously described (Ram and Wolfner 2007) and quantified using Image-J (Schneider et al. 2012). A Nep2 null allele, Nep2Δ, was generated by means of a deletion generator compound element as described in (Huet et al. 2002). The starting stock was yw;;P (Whyteside and Turner 2008) DG19304. Loss of transcript was confirmed by qRT–PCR.
Each RNAi line was crossed to tubulin-GAL4/TM3, Sb; the balancer siblings from each cross (UAS–Nep/TM3, Sb) were used as controls to minimize rearing effects. Controls for the other drivers, n-syb–GAL4, slbo–GAL4, and Send1–GAL4 were generated by crossing the VDRC background line w1118 to the indicated driver line. In the case of Nep1 and Nep4, whose knockdown was lethal with tubulin-GAL4, a tubulin-GAL80[ts];tubulin–GAL4/TM3, Sb line was used instead to drive knockdown. Flies were raised at room temperature and shifted to 30° 3 days prior to eclosion. Adult flies were collected at room temperature and returned to 30° where they were aged for 3 days.
For Nep1 and Nep4, initial experiments were performed using hsp70–GAL4 (HS–GAL4) to drive knockdown. HS–GAL4;UASNep1(or Nep4)RNAi (or control) males and females were aged for 3 days prior to heat shock as previously described. For heat shock, flies were moved to vials without food that contained a wet piece of Whatman paper, after which they were placed in a water bath at 37° for 1 hr. The heat-shocked flies were allowed to recover at room temperature in vials containing fresh food and were then mated 12 hr later for all assays in which they were used.
Fertility/fecundity assays:
In all assays involving male fertility, we used 3- to 5-day-old Canton-S virgin females. Females were placed singly in glass vials with food and allowed access to an RNAi (or mutant) male or a control male. Pairs were watched to confirm that mating had occurred. The male was removed upon dismounting. Assays for the effects of the Neps on female fertility were performed the same way using 3- to 5-day-old Canton-S males as mates for either RNAi (or mutant) or control females.
After mating, individual females were housed on yeast glucose media for 24 hr after which each female was transferred to a fresh vial, and the eggs laid in the previous vial were counted as described in Gligorov et al. (2013) except that Nep1 and Nep4 RNAi and control females were housed at 30° for the entire experiment to ensure adequate suppression of GAL80. The progeny present in each vial were counted after eclosion. Hatchability was calculated as (#progeny/#eggs) except in the case of assays involving Nep1 (line 1) and Nep4 (line 1). For these two lines the UAS–RNAi construct is inserted on the second chromosome, allowing for the inheritance of both the UAS–RNAi construct and the tubulin–GAL4 driver together in the absence of the GAL80 suppressor. This occurs in 25% of the progeny, resulting in lethality. To calculate differences in hatching rate for all viable progeny the number of observed progeny was compared to the number of expected progeny. The number of expected progeny was calculated by day as ((# eggs) × (0.75, expected survival) × (average hatchability of eggs laid by control females (#progeny/#eggs))). Comparisons of single day and total egg, progeny production, expected progeny, and hatchability between control and experimental females were performed using a Wilcoxon nonparametric test and statistics comparing the overall 10-day trends were performed using a repeated measures ANOVA. All statistical analysis was performed with the Jmp9 software
Sperm competition
After mating, Nep2Δ or control females were individually housed for 3 days on yeast–glucose media after which each female was allowed access to a single bwD male for 12 hr. After the bwD male was removed, the females were transferred individually to fresh vials and allowed to lay eggs for 4 days before being transferred to fresh food vials and allowed to lay eggs for an additional 4 days. Because the Nep2Δ stock is in a y w background and the dominant bwD eye color phenotype (brown) requires the presence of a w+ allele to be scored, only female progeny who carried the w+ allele from the male were scored for the presence of bwD (provided by the second male) or red eyes (provided by the first male). P1 was calculated as (# progeny sired by the first male)/(total progeny). Comparisons between the P1 of control and experimental females were performed using a one-way ANOVA and by Wilcoxon nonparametric tests.
Embryo collection and staining for development and sperm tails
For assaying the ability of eggs laid by Nep2Δ females to develop into embryos, we collected 1.5- to 3.5-hr-old eggs, fixed them using methanol/heptane, and stained them with DAPI, as described in Krauchunas et al. (2012). For DAPI staining, fixed embryos were incubated in PBS containing 1 μg/ml DAPI for 5 min and were washed 5 × 15 min in PBST. To assess the presence of sperm tails in eggs laid by Nep2Δ and control females we collected eggs laid in a 1-hr window at room temperature and prepared them as previously described except that rat anti-sperm tail antibody was used at a dilution of 1:800 instead (Karr 1991; T. Karr, ASU, personal communication). Images were collected using a Leica CTR5000 microscope (DAPI) (courtesy of Dan Barbash) or a Leica TCS SP2 confocal microscope (sperm tail).
Sperm counts
Nep2Δ or control mated females were frozen in liquid nitrogen at 2 hr ASM or kept in glass vials on yeast–glucose media for 4 days and then frozen. Frozen females were stored at −80° for <2 weeks before counting. Reproductive tracts were dissected and then stained with orcein (Neubaum and Wolfner 1999; Mueller et al. 2008; Avila et al. 2010). A transillumination microscope was used at 1000× magnification to visualize sperm. Comparisons between the number of sperm present in control and experimental females were performed using Wilcoxon nonparametric tests.
Results
Sequence and phylogenetic analysis of neprilysins
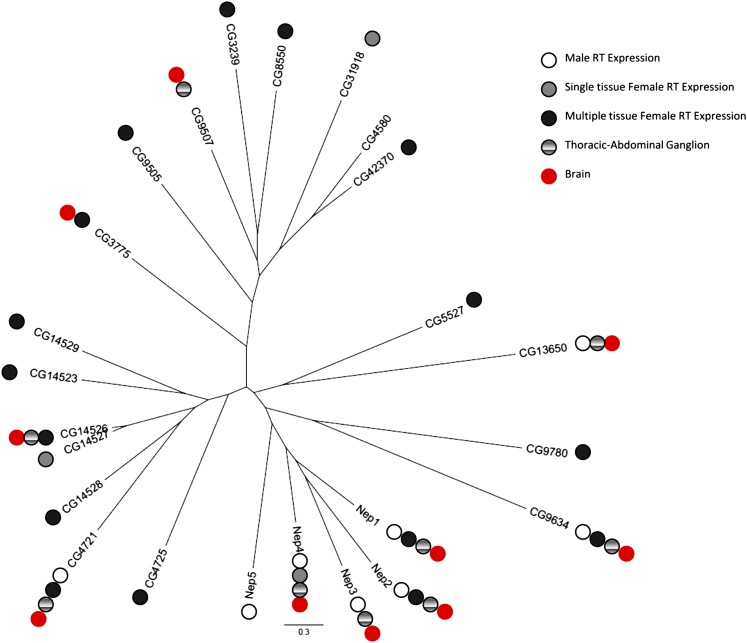
Twenty-four peptidase sequences encoded in the D. melanogaster genome are classified as M13 metallopeptidases based on gene prediction, sequence homology, and searches for known active site regions using the MEROPS database (Rawlings et al. 2012). We created a tree of all 24 M13 class proteins by comparing their protein sequence similarity (see Materials and Methods). Using this analysis, the M13 class proteins fall into three related groups, one of which contains Nep1, Nep3, Nep4, and Nep5, which have been previously shown to be the most closely related to mammalian Neps (Turner et al. 2001), and Nep2.
CNS and reproductive tract expression in both sexes is characteristic of the canonical mammalian neprilysin (Li et al. 1995; Ouimet et al. 2000). Thus, we mapped the known expression patterns (Fly Atlas; Chintapalli et al. 2007) for either the reproductive tract (RT) (as indicated by genome-wide microarray data determined in females for the ovaries and the spermathecae, and in males for the testes and the accessory glands) or the CNS onto the gene tree (Figure 1). All but two of the 24 genes (CG9507 and CG4580) have some expression in the RT of either sex. Most genes (19/24) show some expression in female reproductive tracts; only 8/24 are detectably expressed in male reproductive tract tissues. Fourteen genes show female RT expression only, 3/24 show male RT expression only, and 5/24 are expressed in both (Table S1). The high frequency of female RT expressed genes in this family suggests that the function of M13 class proteins is likely important in these tissues, but also suggests the possibility of functional redundancy, which could complicate genetic analysis. We decided to focus on the clade containing the candidate genes with the closest homology to mammalian neprilysins. Further, consistent with what is observed in mammals, this clade is also enriched for the somewhat rarer pattern of male RT expression, as well as expression in the CNS.
Figure 1.
Phylogeny and expression of Drosophila neprilysins. A phylogenetic tree of the 24 known D. melanogaster M13 class proteases based on protein sequence similarity. The proteins fall into three distinct clades. Mapping the reproductive tract expression of each gene onto the tree reveals broad expression in the female RT (shaded and solid) and enrichment of male expression (open) in the clade that contains Nep1–5. The same clade also demonstrates enrichment for brain (red) and abdominal-thoracic ganglion expression (hatched).
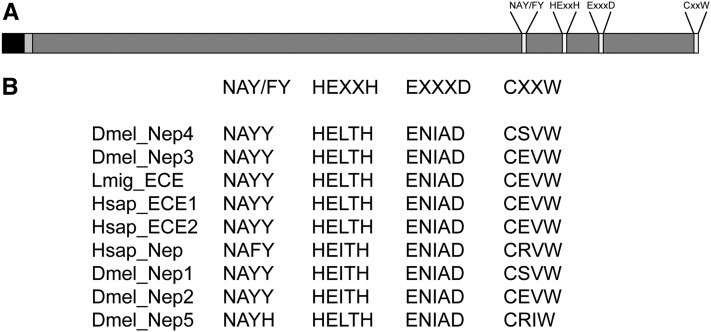
We characterized the five genes in this clade: Nep1 (CG5905), Nep2 (CG9761), Nep3 (CG9565), Nep4 (CG5894), and Nep5 (CG6265). Figure 2 shows a schematic representation of neprilysin and a sequence alignment of the different functional motifs of D. melanogaster Nep1–Nep5, the ECE homolog of L. migratoria (LomECE), and Homo sapiens ECE-1, ECE-2, and neprilysin. A full sequence alignment and phylogenetic analysis can be found in Figure S1 and Figure S2.
Figure 2.
Conserved binding motifs in Drosophila, human, and locust neprilysins. (A) Schematic representation of neprilysin. Solid, cytoplasmic domain; light shading, transmembrane domain; dark shading, extracellular domain. NAYY/F, important for substrate binding; HExxH, zinc-binding domain; ExxxD, zinc-binding domain; CxxW: sequence critical for protein folding and maturation of the enzyme. (B) Alignment of NAY/FY, HExxH, ExxxD, and CxxW sequences of D. melanogaster Nep1-5, L. migratoria ECE, and H. sapiens ECE1-2.
Expression patterns of Nep1–5
FlyAtlas (Chintapalli et al. 2007) and RNAseq (Celniker et al. 2009) data suggested that Neps1–5 are expressed throughout development in a variety of tissues. To gain a more precise understanding of the locations and timing of these genes’ expression patterns, we performed in situ hybridization to look for the expression of each NEP gene in embryos, larvae, and adult flies. Sense-strand hybridizations were used as controls (Figure S3). A summary of the expression data can be found in Table S2.
Embryonic expression of Nep1–5
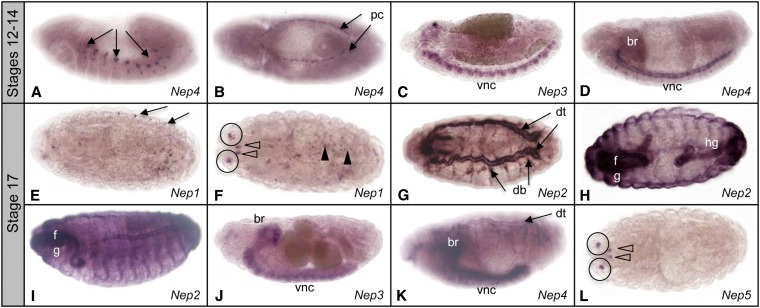
Two of the genes (Nep3 and Nep4) were expressed before embryonic stage 17 (Figure 3). Nep4 RNA was detected as early as stage 12 in two patches of cells per hemisegment (Figure 3A). These have been reported independently to correspond to muscle founder cells (Meyer et al. 2009). In stage 13, Nep4 is expressed in two rows of cells that border the amnioserosa (Figure 3B), which we identify as the pericardial cells. These cells flank the aligned cardioblast cells of the dorsal vessel. This staining is visible from stage 13 until stage 16 when dorsal closure is finalized and the cardioblasts of each side fuse. At stage 14 Nep3 is expressed generally in the central nervous system (Figure 3C). This staining is visible until stage 17. Nep4 expression can also be detected in the brain and ventral nerve cord of stage 14 to stage 17 embryos (Figure 3, D and K). The staining is localized in cells along the longitudinal connectives and transversal commissures of the ventral nerve cord.
Figure 3.
Embryonic expression pattern of neprilysin genes. (A and B) Embryonic stage 12 Nep4 expression in muscle founder cells (arrows in A) and in pericardial cells (pc). (C) Embryonic stage 13 Nep3 expression in the ventral nerve cord (vnc). (D) Embryonic stage 14 Nep4 expression in brain (br) and ventral nerve cord (vnc). (E and F) Embryonic stage 17 Nep1 expression in peripheral nervous system (arrows), antenno-maxillary complex (circled), and cells in the pharynx (open arrowheads) and midgut (solid arrowheads). (G–I) Embryonic stage 17 Nep2 expression in dorsal trunk (dt), dorsal branches (db), foregut (fg), and hindgut (hg). (J) Embryonic stage 17 Nep3 expression in brain (br) and ventral nerve cord (vnc). (K) Embryonic stage 17 Nep4 expression in brain (br), ventral nerve cord (vnc), and tracheal dorsal trunk (dt). (L) Embryonic stage 17 Nep5 expression in antenno-maxillary complex (circled) and in the pharynx (open arrowheads).
All five of these Nep genes are expressed in stage 17 embryos. Nep1 is expressed in neurons of the peripheral nervous system on the left and right side of the embryo (Figure 3, E and F), and in the antenno-maxillary complex, which is part of the peripheral nervous system and located at the anterior side of the embryo, in front of the first thoracic segment. Nep1 RNA was also detected in the anterior of the pharynx and in cells of the embryonic midgut. Nep2 is strongly expressed in the tracheal system including in the dorsal trunk and the dorsal branches (Figure 3G). In the intestinal tract Nep2 is expressed in the foregut (Figure 3, H and I). Nep2 expression can also be detected in the hindgut (Figure 3H) and epidermis (Figure 3I). Nep3 expression remains in the CNS where it becomes more intense in the brain hemispheres (Figure 3J). Nep4 expression is detectable in the dorsal trunk and dorsal branches of the tracheal system and continues to be detected in the brain and ventral nerve cord (Figure 3K). Expression of Nep5 is restricted to four small groups of cells at the anterior of stage 17 embryos (Figure 3L).
Larval expression patterns of Nep1–5
Nep1–4 are expressed in the nervous system of third instar larvae (Figure 4). Nep1 is expressed strongly in the mushroom bodies of the brain, neurons in the pars intercerebralis, and neurons in the ventral ganglia (Figure 4A). Nep2 is expressed in three neurons of both hemispheres of the larval brain and a limited number of six neurons in the ventral ganglia (Figure 4B). Similar to the expression of Nep3 in embryos, a strong general staining of Nep3 is detected in the larval brain hemispheres and ventral ganglia. In the hemispheres, the staining is more intense in the central part compared to that in the optic neuropils (Figure 4C). The expression of Nep4 in third instar larvae is restricted to the central nervous system. Based on the size of the cells that are stained in the brain and ventral ganglia we identify the Nep4-expressing cells as glia (Figure 4D), consistent with a previous report of colocalization of Nep4 with the glial marker Repo (Meyer et al. 2009).
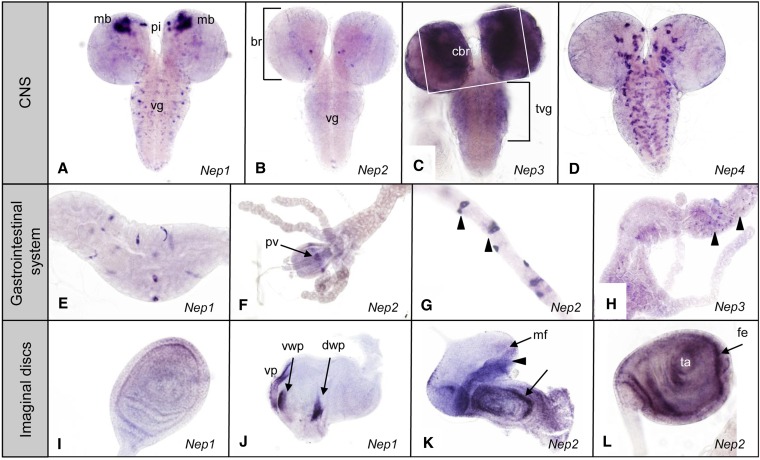
Figure 4.
Larval expression pattern of neprilysin genes. (A–D) Expression of Nep1–4 in larval CNS. (A) Nep1 in mushroom bodies (mb), pars intercerebralis (pi), and ventral ganglia (vg). (B) Nep2 in few cells in the brain (br) and in the ventral ganglia (vg). (C) Nep3 in central brain (cbr) and thoracic ventral ganglia (tvg). (D) Nep4 in glia of the larval CNS. (E–H) Expression of Nep1–3 in the larval gastrointestinal system. (E) Nep1 in cells in the midgut. (F–G) Nep2 in the proventriculus (pv) and in the stellate cells of the Malpighian tubules (arrowheads). (H) Nep3 in scattered cells in the larval midgut (arrowheads). (I–L) Expression of Nep1 and Nep2 in larval imaginal discs. (I) Nep1 in leg disc. (J) Nep1 in dorsal and ventral wing pouch (dwp–vwp) and ventral pleura (vp) of the wing disc. (K) Nep2 in the eye disc anterior (arrowhead) to the morphogenetic furrow (mf) and in the second antennal segment (arrow). (L) Nep2 in the leg disc femur (fe) and tarsus (ta).
Outside the CNS, Neps are expressed in the gut and the Malpighian tubules, as well as in developing wing, leg, and eye-antennal discs. More specifically Nep1 expression is detected in cells of the midgut (Figure 4E), wing disc (Figure 4I), and leg disc (Figure 4J). Nep2 remains expressed in the foregut, but only in a limited number of cells of the proventriculus (Figure 4F). In the eye-antennal disc Nep2 is expressed anterior to the morphogenetic furrow in the undifferentiated precursor cells of the eye disc and more generally in the antennal part (Figure 4K). In the leg discs Nep2 is expressed in the outer concentric ring, giving rise to the first two segments of the fly leg, and in the central part of the disc (Figure 4L). Nep2 is also expressed in the stellate cells of the larval Malpighian tubules (Figure 4G), which perform excretory and osmoregulatory functions analogous to vertebrate renal tubules (Dow and Romero 2010). Nep3 expression is detected in a small number of cells in the larval midgut (Figure 4H). We did not detect expression of Nep5 above background level in third-instar larval tissues.
Adult expression patterns for Nep1-5
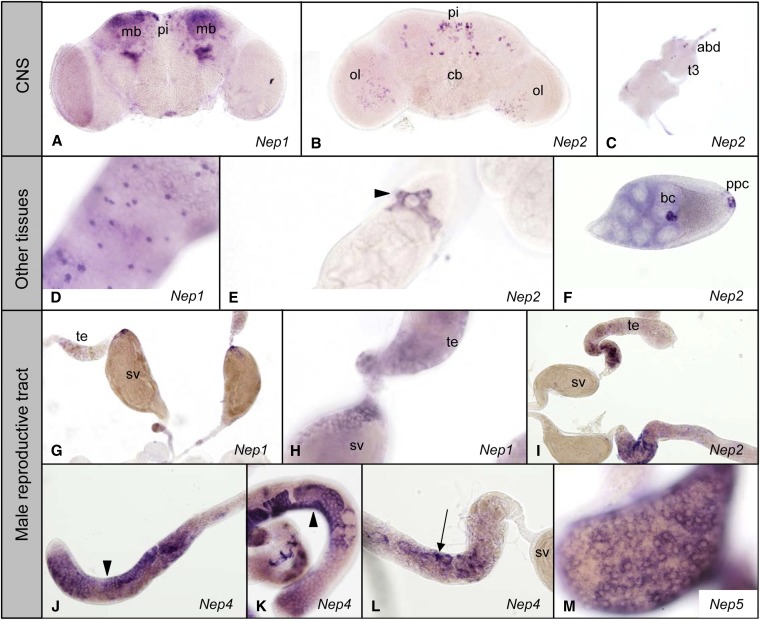
Consistent with its larval tissue expression pattern, Nep1 is expressed in the mushroom bodies and neurons of the pars intercerebralis of the adult brain (Figure 5A) and in cells of the adult midgut (Figure 5D). In the male reproductive organs Nep1 is expressed at the end of the testicular tube near and in the seminal vesicles (Figure 5, G and H). Nep2 is detected in neurons of the pars intercerebralis and in a limited number of cells in the optic lobes of the brain (Figure 5B). In the ventral ganglion a few neurons also show expression of Nep2 (Figure 5C). In the male reproductive organs Nep2 is expressed in cells at the end of the testicular tube where it meets the seminal vesicle (Figure 5I). In the female gonad, strong staining was detected in posterior polar cells and in border cells of stage 8, 9, and 10 follicles (Figure 5F). By means of immunohistochemistry we also detected Nep2 expression in the spermatheca (See Figure S4). As in larvae, Nep2 is expressed in the adult Malpighian tubules and more specifically in the stellate cells, which are located between the principal cells of the Malpighian tubules (Figure 5E). No expression of Nep3 above background level was detected in adult tissues despite previous reports of broad expression (Chintapalli et al. 2007; Celniker et al. 2009). The expression of Nep4 in adult flies is restricted to the male gonads. Expression of Nep4 is detected in different parts of the testicular tubes (Figure 5, J–L). In the apex of the testis the localization of the staining corresponds to the somatic cyst cells that surround the spermatocytes in this part of the tube. Nep4 is also expressed at the end of the testis close to the contact with the seminal vesicle, in cells other than the somatic cyst cells. As is true for Nep4, the expression of Nep5 in adult tissues is also restricted to the male gonads, more specifically in the membrane of the seminal vesicles where mature spermatids are stored after transport from the testicular tubes (Figure 5M).
Figure 5.
Adult expression pattern of neprilysin genes. (A–C) Expression of Nep1–2 in the adult CNS. (A) Nep1 in the adult brain mushroom bodies (mb) and pars intercerebralis (pi). (B) Nep2 in cells in the pars intercerebralis (pi), central brain (cb), and optic lobes (ol). (C) Nep2 in the third thoracic (t3) and abdominal (abd) neuromere. (D) Nep1 in adult midgut cells. (E) Nep2 in adult stellate cells of the Malpighian tubules (arrowhead). (F) Nep2 in border cells (bc) and posterior polar cells (ppc) of a stage 10 ovarian follicle. (G–M) Expression of neprilysin genes in the male reproductive tract. (G–H) Nep1 in the testicular tube (te) and the seminal vesicles (sv). (I) Nep 2 in the part of the testis (te) close to the seminal vesicle (sv). (J–L) Nep4 in the somatic cyst cells (arrowheads) and in other cells (arrows) in the part of the testes close to the seminal vesicle (sv). (M) Nep5 in the seminal vesicle.
Nep1 plays a role in regulating male fertility
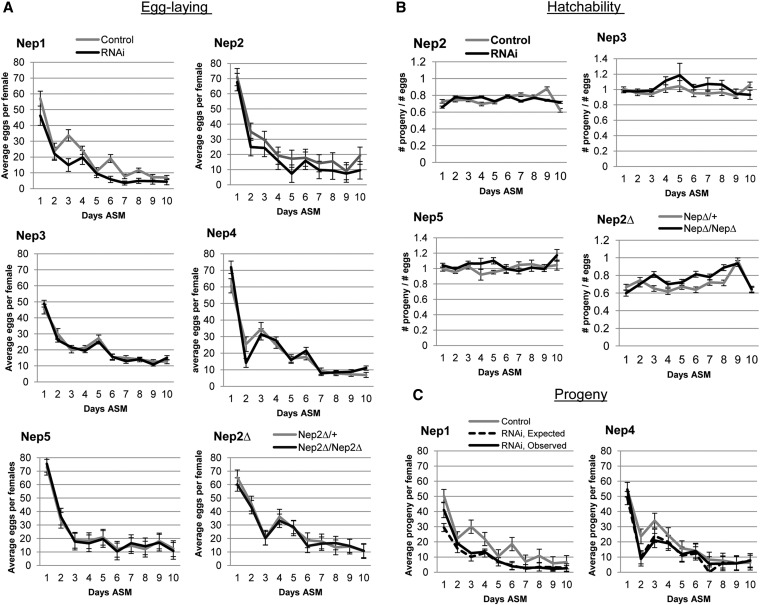
Combined with the previously reported expression patterns from Fly Atlas and modENCODE, our data showed that in adult flies, Nep4 and Nep5 are expressed predominantly or exclusively in the male reproductive tract. Conversely Nep1 and Nep2 are expressed at high levels in the reproductive tracts of both sexes as well as in the CNS (Table S1). We confirmed Nep3 expression in the male by RT–PCR (Figure S5). To test if any of these genes are essential for male fertility we generated RNAi-mediated knockdown males. For Nep2, Nep3, and Nep5 this was done by crossing the appropriate VDRC line to the ubiquitous driver tubulin–GAL4. Since knockdown of Nep1 and Nep4 was lethal for both sexes using tubulin–GAL4, we used tubulin–GAL80[ts] to suppress GAL4 function during development (Elliott and Brand 2008; Southall et al. 2008). We confirmed knockdown using RT–PCR (Figure S5 and Table S3). Control or knockdown males, in parallel, were mated to virgin females. Egg production and fertility was measured daily over a 10-day period for each female. Mates of Nep1 knockdown males laid significantly fewer eggs than mates of control males (Figure 6A). No differences were seen in the total number of eggs produced by females mated to Nep2, Nep3, Nep4, or Nep5 knockdown males compared to controls (Figure 6A). Mates of Nep2 knockdown males showed a trend toward reduced fertility. We obtained and tested males from a Nep2 deletion line to clarify the trend observed in mates of Nep2 knockdown males. Mates of Nep2 null males showed no difference in egg laying from controls, suggesting that Nep2 from the male is not essential for stimulating egg laying in females.
Figure 6.
Egg laying in mates of Nep RNAi males. (A) The mean number of eggs laid per female mated to either control males (gray line) or RNAi/null males (black line) over a 10-day period. Only mates of Nep1 RNAi males laid fewer eggs than mates of control males (Nep1: rmANOVA P = 0.0041, control N = 16, Nep1 RNAi N = 17). Mates of Nep2–5 RNAi laid comparable numbers of eggs as control mated females (Nep2—rmANOVA P = 0.095, control N = 11, Nep2 RNAi N = 14; Nep3—rmANOVA P = 0.7556, control N = 17, Nep3 RNAi N = 21; Nep4—rmANOVA P = 0.6972, control N = 16, Nep4 RNAi N = 16; Nep5—rmANOVA P = 0.8986, control N = 22, Nep5 RNAi N = 25; Nep2 null—rmANOVA P = 0.3448, control N = 18, Nep2 null N = 21). (B) The mean hatchability (#progeny/#eggs) per female for mates of control or RNAi/null males for the egg-laying assays in A. None of the Neps had a significant effect on hatching rate (Nep2—rmANOVA P = 0.4326 ; Nep3—rmANOVA P = 0.1494; Nep5—rmANOVA P = 0.1909; Nep2 null—rmANOVA P = 0.3673). (C) The mean observed (black line) and expected (dashed line) progeny ((# eggs) × (expected survival rate) × (average hatchability of eggs laid by control females)) for mates of Nep1 and Nep4 males. No difference in observed vs. expected progeny was seen for mates of either Nep1 or Nep4 RNAi males (Nep1, rmANOVA P = 0.3714; Nep4, rmANOVA P = 0.2874).
While a Nep1 mutant line exists (Spradling et al. 1995; Spradling et al. 1999; Bellen et al. 2004), it is not a null allele, so we used an alternative RNAi line to verify the egg-laying defects seen in mates of Nep1 knockdown males (Figure S6A). Similar results for mates of Nep1RNAi males were obtained using the conditional ubiquitous driver hsp70–GAL4 (HS–GAL4) (Brand and Perrimon 1993) (data not shown). An alternative line for Nep4 was also tested (Figure S6A). Alternative lines obtained for Nep5 did not knock down and alternative Nep3 lines were not available at the time of this study.
The proportion of progeny that eclosed from eggs laid by females (hatchability) mated to Nep2, Nep3, and Nep5 knockdown males was comparable to those of controls (Figure 6B). Some small differences in egg laying were observed for Nep3 knockdown males within the first 24 hr after mating, but these differences were not consistently reproducible.
To determine whether Nep1 or Nep4 is essential for hatchability, we used the tubulin–GAL80[ts];tubulin–GAL4/TM3,Sb driver used to produce viable Nep1 and Nep4 (see Materials and Methods for details) and compared the expected progeny ((#eggs laid) × (expected maximum hatch rate (0.75)) × (average hatching rate for controls per day)) to the observed progeny. In both cases, no difference was seen between the expected progeny and those observed (Figure 7C) suggesting that Nep1 and Nep4 are not required for hatchability in mates of RNAi males. We used an alternative Nep4 line to verify these results (Figure S6B). Mates of males knocked down using the alternative Nep1 RNAi line (Nep1–39759) lay few (most <10) eggs, so we calculated total hatchability over the entire 10-day period as (# total progeny/#total eggs). There was a significant reduction in hatchability for mates of these males (Figure S6B); however, we believe this is due to the insertion site on the third chromosome (see legend to Figure S6).
Figure 7.
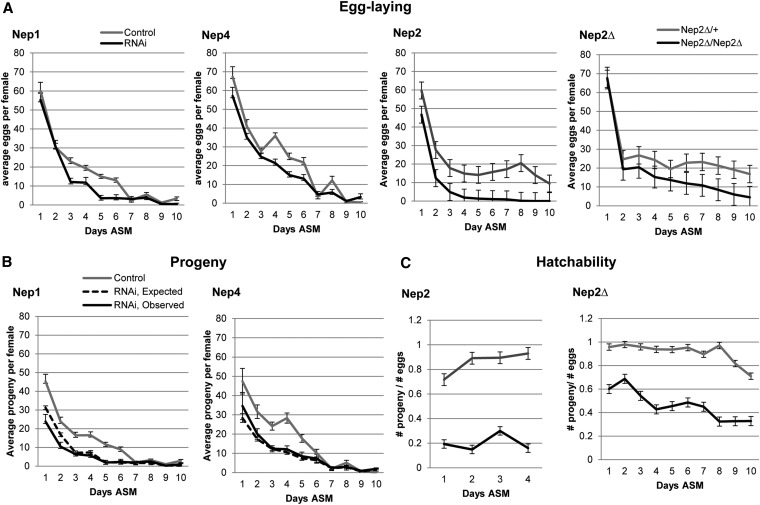
Egg laying in Nep RNAi females. (A) The mean number of eggs laid by control (gray line) or RNAi/null females (black line) mated to WT males over a 10-day period. Nep1, Nep2, and Nep4 RNAi and Nep2 null females lay fewer eggs than controls (Nep1—rmANOVA P = 0.0015, control N = 20, Nep1 RNAi N = 18; Nep2—rmANOVA P = <0.0001*, control N = 12, Nep2 RNAi N = 12; Nep4: rmANOVA P = 0.0207*, control N = 16, Nep4 RNAi N = 16; Nep2 null—rmANOVA P = <0.0001*, control N = 18, Nep2 null N = 15). (B) The mean observed (black line) and expected (dashed line) progeny ((# eggs) × (expected survival rate) × (average hatchability of eggs laid by control females)) for Nep1 and Nep4 females. No difference in overall observed vs. expected progeny was seen for either RNAi female (Nep1, rmANOVA P = 0.2853; Nep4, rmANOVA P = 0.4325). Nep1 RNAi females showed a trend for reduced observed progeny on day 1 (P = 0.0686) and a significant reduction on day 2 (P = 0.0253) after mating. (C) The mean hatchability (#progeny/#eggs) per female for Nep2. Both Nep2 RNAi and the Nep2 null females show drastically reduced hatchability (Nep2, rmANOVA P = <0.0001*; Nep2 null, rmANOVA P = <0.0001*) suggesting that Nep2 plays an essential role in this process.
In summary, Nep2, Nep3, Nep4, and Nep5 do not appear to be uniquely essential for male fertility. They may not be essential for the specific traits measured here, or they may have redundant functions. Reduced Nep1 expression in males does consistently affect egg laying in their mates suggesting a role for neprilysins in the reproductive performance of Drosophila males.
Nep1, Nep2, and Nep4 are essential for female fertility
Nep1, Nep2, and Nep4 are expressed in the CNS and in the female reproductive tract, based on our expression data (Figure 5) and Fly Atlas (Chintapalli et al. 2007). To assess the role of these Neps in female fertility, we knocked down Nep1 and Nep4 ubiquitously, using tubulin–GAL80[ts];tubulin;GAL4 and Nep2 using tubulin–GAL4. We then mated control and knockdown females, in parallel, to Canton-S males and tracked their egg laying over a 10-day period. Knockdown was confirmed via RT–PCR (Figure S5 and Table S3). Nep1, Nep2, and Nep4 RNAi females laid significantly fewer eggs than controls laid over the entire 10-day period (Figure 7A). Similar results were obtained using alternate RNAi lines for Nep1 and Nep4 (Figure S6A), as well as the Nep2 null mutant line (Figure 7A). Nep1 results were also confirmed using the conditional ubiquitous driver hsp70–GAL4 (HS–GAL4) (data not shown).
To assess whether Nep1 or Nep4 are essential for hatchability we compared numbers of expected to observed progeny. In both cases there was no difference in the overall number of progeny across the 10-day period (Figure 7B). However, fewer progeny than expected were observed for Nep1 RNAi females in the first 2 days after mating (Figure 7B). Similar phenotypes for Nep1 RNAi females in early hatchability were seen using the hsp70–GAL4 driver (data not shown). Similar to our observations in males, the alternative line Nep1–39759 RNAi females showed a more severe phenotype, with a reduction in hatchability across all 4 days measured (Figure S6B). Females knocked down for Nep4 using the alternative RNAi line Nep4–16668 also showed significant decreases in hatchability (Figure S6B) possibly due to increased knockdown (Table S3) or background effects (see legend to Figure S6).
The proportion of eggs laid by Nep2 RNAi females that become adult progeny is greatly reduced compared to that of control females (20% in RNAi females vs. 80–90% in controls) (Figure 7C). It was not possible to calculate hatchability for the entire 10-day period, since egg laying reached zero for all Nep2 RNAi females by day 5. The reduction in hatchability could reflect roles for Nep2 in the polar and/or border cells of the follicular epithelium during oogenesis (Figure 5F) and/or roles during embryogenesis where it is also expressed (Bland et al. 2007). Similar results were seen using females that were homozygous for a null mutation of Nep2 (Figure 7C). Together these results indicate that Nep1, Nep2, and Nep4 play essential roles in regulating female fertility and fecundity.
Characterization of the hatchability defects in Nep2 null mutants
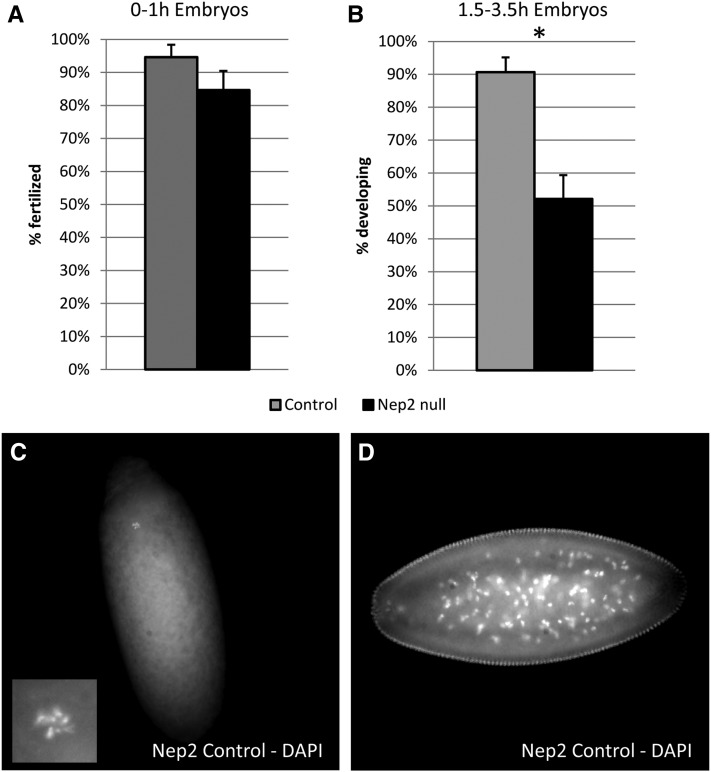
To test whether the hatchability defect observed for Nep2 mutant females was due to a failure of the eggs to be fertilized, we examined early embryos (0–1 hr) laid by these females for the presence of a sperm tail (Karr 1991). There was no difference in the percentage of fertilized embryos laid by Nep2 null females compared to controls (Figure 8A). We therefore tested whether Nep2 is important for early embryogenesis by staining 1.5- to 3.5-hr-old embryos laid by Nep2 null females or controls with DAPI and scoring for their stage of embryonic development. While nearly all of the eggs laid by control females contain developing embryos, significantly more of the eggs laid by Nep2 nulls (close to 50%) contained only a clear polar body rosette (Figure 8B). The presence of a polar body rosette is typical for activated but unfertilized eggs (Figure 8C) whereas the typical developing embryo at this time range was observed to be at stage 4 (Figure 8D). Since there is no difference in fertilization rate between eggs laid by Nep2 null females and controls, our data suggest that some of the eggs laid by Nep2 null females are able to activate and complete meiosis but fail to develop further. This fraction of arrested eggs observed for Nep2 mutant females is consistent with the magnitude of the hatching defects seen above (Figure 7) and suggests that Nep2 plays a role in egg laying and that loss of Nep2 in the female has an effect on very early embryogenesis.
Figure 8.
Eggs laid by Nep2 null females arrest during early embryogenesis. (A) Eggs laid by Nep2 null females are fertilized at the same rate as eggs laid by control females (WRST P = 0.1593, control N = 37, Nep2 null N= 39) based on sperm tail staining. (B) DAPI staining of 1.5- to 3.5-hr-old eggs laid by Nep2+ control or Nep2 null females were sorted into two categories: developing or nondeveloping. All nondeveloping embryos contained a polar body rosette (C), whereas developing embryos were all at stage 4+ (D) of development consistent with the time point chosen. Eggs laid by Nep2 null females are significantly more likely to fall into the nondeveloping category than eggs laid by control females (WRST P < 0.0001*, control N= 43, Nep2 null N = 48). Since the fertilization rate between Nep2 null and control females is not different this result suggests that Nep2 may be critical for early embryogenesis.
Tissue-specific contribution to egg laying and hatchability effects for Nep1 and Nep2 females
The egg-laying defects in Nep1 and Nep2 RNAi females suggested that one or more of the tissues in which we detected Nep expression must be essential for egg laying. Both Nep1 and Nep2 are highly expressed in the CNS and the spermathecae, two tissues that are known to influence egg laying (Yang et al. 2009; Schnakenberg et al. 2011; Sun and Spradling 2013). Nep2 is also expressed in the border cells of the follicular epithelium, which are important for micropyle development and for anterior–posterior polarity in the egg (Furriols et al. 2007). To test whether expression of Neps in these tissues are required for normal egg laying or hatchability we individually used nsyb–GAL4 (Pauli et al. 2008), Send1–GAL4 (Schnakenberg et al. 2011), and slbo–GAL4 (Rorth et al. 1998) drivers to locally drive RNAi in the CNS, the spermathecae, and the border cells, respectively.
Females knocked down for either Nep1 or Nep2 in the CNS laid fewer eggs than control females (Figure S7 and Figure S8). Similarly, knockdown of Nep1 or Nep2 in the spermathecae also reduced egg laying (Figure S7 and Figure S8). However, knockdown in either tissue did not fully recapitulate the egg-laying phenotypes seen in the ubiquitous knockdown of Nep1 or Nep2 in females. Contrary to expectations, reduction of Nep2 expression in the border cells slightly increased egg laying. However, none of the targeted tissues for Nep2 resulted in a decrease in hatchability. These results suggest that Neps are important in both the CNS and the spermathecae for normal egg laying but not hatchability and that either a combination of both sources or an as-yet untested source of Nep expression may be responsible for the majority of the reduction in egg laying observed. One possible source of expression is the seminal receptacle, where Nep1 and Nep2 transcripts have both been detected (Prokupek et al. 2010). However, we cannot test this, as currently no GAL4 drivers target only this organ. Additionally in the case of Nep2, which encodes a secreted protein (Thomas et al. 2005), it is possible that secreted Nep2 from surrounding tissues may partially compensate for loss of Nep2 expression in our target tissues.
Sperm storage and depletion are abnormal in Nep2 null females
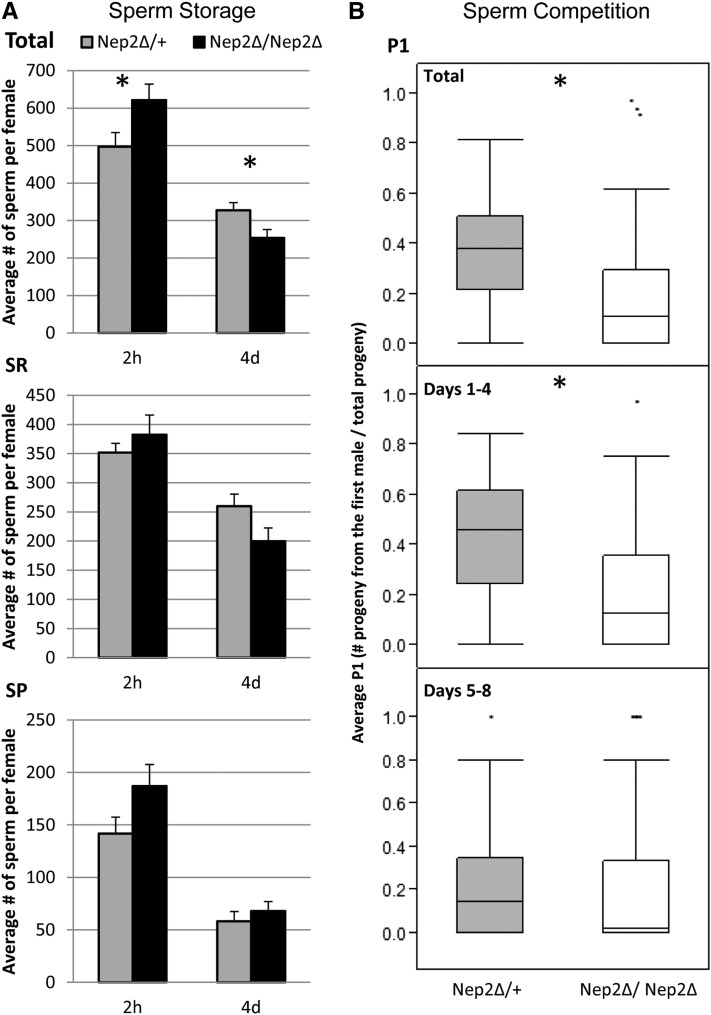
Neprilysins have been implicated in the regulation of muscle contraction in the mammalian uterus (Pinto et al. 1999; Pintado et al. 2003) and their substrates, the tachykinins, have been shown to induce muscle contraction in the oviduct of locusts (Kwok et al. 1999). Muscle contractions are also important in the Drosophila uterus, which goes through conformational changes after mating that facilitate sperm storage (Adams and Wolfner 2007; Avila and Wolfner 2009). The spermathecae and the seminal receptacle, which store these sperm, also experience contractions (Middleton et al. 2006), the importance of which is unknown but may facilitate the release or storage of sperm. To determine whether Nep2 is essential for sperm storage or release, we counted the number of sperm stored at 2 hr and 4 days ASM in Nep2 null females compared to controls.
After mating to wild-type males, females that were null for Nep2 stored more sperm overall at 2 hr post-mating (a time when sperm storage has just completed; Bloch Qazi et al. 2003) than control females stored (Figure 9A) and had marginally more sperm in the spermathecae. By 4 days ASM, however, Nep2 null females retained fewer sperm than controls and had marginally fewer sperm stored in their seminal receptacles. This shift from surplus to deficit illustrates that Nep2 null females are defective in sperm retention. These results suggest that Nep2 plays a role not only in the initial storage of sperm but also in controlling the release of sperm from the sperm storage organs. Although Nep2 plays a role in sperm storage and release, the number of sperm stored in the sperm storage organs at the 4-day time point is too high to suggest that these sperm storage differences alone underlie the egg-laying defects seen in Nep2 null females.
Figure 9.
(A) Counts of sperm stored in both sets of sperm storage organs (Total), the seminal receptacle (SR), and the paired spermatheca (SP), of Nep2 null (solid) vs. control females (shaded) at 2 hr and 4 days after the start of mating (ASM). Overall Nep2 null females store more sperm at 2 hr ASM (ANOVA, F= 4.8029, P = 0.0398, control N = 13, Nep2 null N = 10) and fewer sperm at 4 days ASM (ANOVA, F = 6.0175 P = 0.0215*, control N = 13, Nep2 null N = 14) than control females. Within the SR, Nep2 null females store the same number of sperm at 2 hr ASM (ANOVA, F = 0.71 P = 0.4061, control N = 17, Nep2 null N = 15) and marginally fewer sperm at 4 days ASM (ANOVA, F = 3.920 P = 0.0580, control N = 14, Nep2 null N = 15) than controls. Within the SP, Nep2 null females store the same number of sperm at both 2 hr ASM (ANOVA, F = 3.1304. P = 0.0901, control N = 13, Nep2 null N = 12) and 4 days ASM (ANOVA, F = 0.5584 P = 0.4614, control N = 14, Nep2 null N = 15). (B) For sperm competition assays Nep2 null or control females were first mated to a Canton-S male and then allowed to mate a second time with a bwD male. The proportion of female progeny sired by the first male (Canton-S) referred to as P1 (# progeny from first male/total progeny) was significantly reduced in Nep2 null females compared to control females (WRST P = <0.0001*, control N = 76, Nep2 null N = 72). This difference is most apparent in the first 4 days ASM (WRST P = <0.0001*) compared to days 5–8 (WRST P = 0.1886)
To confirm the role of Nep2 in regulating sperm release or depletion we performed a sperm competition assay in which we mated Nep2 null and control females to a Canton-S male and then subsequently to a bwD male. Loss of Nep2 function dramatically decreases P1 (the proportion of progeny sired by the first male) (Figure 9B) suggesting that Nep2 aids in sperm retention and works to help sperm resist displacement by rival ejaculates. This is consistent with the observation that sperm deplete faster in singly mated Nep2 null females. Together these results indicate a role for Nep2 in female regulated sperm use.
Discussion
Drosophila neprilysin genes
We investigated a group of genes encoding M13 class proteases in D. melanogaster with expression patterns suggesting that they may play roles in reproduction or the CNS. Sequence analysis of protein sequences of Nep1–Nep5 with the sequences of human family members ECE-1, ECE-2, and neprilysin and locust LomECE and phylogenetic analysis revealed distinct similarities for Drosophila neprilysins 1–5. Drosophila Nep1 and Nep4 are most closely related to a group of vertebrate neprilysin homologs. Nep3 is most similar to LomECE and vertebrate ECE and Nep5 clusters in a group with Kell homologs. Nep2 is an invertebrate-specific protein. Overall, our analysis indicates that Nep1–5 are evolutionarily closely related yet representative of the functional divergence that seems to have occurred in this gene family.
Implications of neprilysins 1–5 expression patterns
The strong conservation of domains important for correct protein folding and activity in Nep1–Nep5 suggests that the functional specificity of the enzymes may at least in part depend on their specific spatiotemporal expression patterns, an aspect that has previously also been observed in Caenorhabditis elegans (Turner et al. 2001). Analysis of the expression patterns of Nep1–Nep5 by in situ hybridization supports this hypothesis. Overall our expression analysis suggests that these five neprilysins may be involved in a range of developmental and physiological processes that in turn may be mediated by numerous bioactive (neuro)peptides.
Additionally, the observed expression patterns for Nep1 and Nep2 suggest that neprilysins may affect reproductive behavior at multiple levels. Nep1 and Nep2 are expressed in the pars intercerebralis. Prominent among the peptidergic neurons in the pars intercerebralis are the insulin-producing cells (IPCs). Drosophila insulin-like peptides (DILPs) are necessary for vitellogenesis (Richard et al. 2005). DILPs regulate stem-cell division in the ovary (LaFever and Drummond-Barbosa 2005) and mediate sexual attractiveness (Kuo et al. 2012). Another neuropeptide expressed in the pars intercerebralis is SIFamide that modulates courtship (Terhzaz et al. 2007). The expression of Nep1 in the mushroom bodies may be significant in several ways. sNPF is expressed in the mushroom bodies and has been implicated in regulating IPCs (Nassel et al. 2013) while it also acts as a neuromodulator in olfactory memory (Knapek et al. 2013). Mushroom bodies also play important roles in courtship behavior and courtship learning or conditioning (Sakai and Kitamoto 2006; Keleman et al. 2012; Zhou et al. 2012). Finally, the expression of Nep1 in abdominal ganglia suggests a possible role in modulating sperm transfer and copulation duration that is regulated by corazonin expressed in the abdominal ganglia (Tayler et al. 2012).
Drosophila neprilysins are important for fertility
Nep1, Nep2, and Nep4 are essential for normal female reproductive fitness. Nep1 and Nep4 are essential for egg laying. Part of the effects of Nep1 expression on egg laying can be traced to its role in the spermathecae and the CNS. Nep2 is also essential for both the post-mating increase in female egg laying and the hatchability of laid eggs. The hatching defects seen in Nep2 RNAi and null females are not due to a failure in fertilization but instead manifest in an early embryonic arrest, suggesting that Nep2 in the female is essential for the development of her progeny. Expression of Nep2 in both the CNS and the spermathecae contributes to the egg-laying defect but not to the hatchability defect. Surprisingly, even though Nep2 is present in the border cells of the follicular epithelium, the expression of Nep2 in these cells is not essential for fertility.
In addition to egg production, Nep2 influences sperm storage and depletion in females. Loss of Nep2 in the female also negatively affects retention of sperm from the first mating when a second mating occurs. This suggests that Nep2 may play a role in sperm retention, helping to insulate stored sperm from displacement by rival ejaculates. Whether this reduction in the presence of the first male’s sperm is detrimental to the female is unclear. Together our data paint a broad role for neprilysins, and particularly for insect-specific Neps like Nep2, in regulating female reproductive success.
Nep1 is also important in male reproductive fitness. Knockdown of Nep1 in males decreased egg laying in their mates. This finding is consistent with experiments in mice, where loss of NL1 in males caused reduced litter sizes (Carpentier et al. 2004). In contrast Nep2–5 do not appear to have nonredundant, essential roles in male fertility.
The Neps we tested represent only a fraction of the neprilysin-like homologs identified to date in Drosophila. The similarity between Nep1 and other vertebrate Neps makes it a good potential model for finding substrates for neprilysins that are conserved throughout female reproduction. Nep2 offers insight into the insect specific lineages of neprilysin-like genes. Further research on the substrates of Nep2 may reveal divergent or species-specific mechanisms for neprilysins in reproduction. Substrates of Nep2, or Nep2 itself, could also prove to be useful targets for controlling pests and insect disease vectors by reducing fertility.
Distribution and potential targets of neprilysins in D. melanogaster
In this study, we focused on the importance of neprilysins in Drosophila fertility. Among the important functions of these proteases, neprilysins are largely responsible for the degradation of neuropeptides (Turner et al. 2000; Turner et al. 2001). Reduced expression of individual neprilysins should result in an overabundance of their cleavage targets, which could be the ultimate cause of phenotypes such as those that we described here. The cleavage specificities of mammalian neprilysins are well documented: they have a preference for polar residues in the P1 position (G > P > R > S) and hydrophobic residues (F > L > Y > I > V) at the P1’ position (summarized from MEROPS; Rawlings et al. 2012). In mammals, individual Neps tend to have very specific substrate affinities despite sharing conserved active site residues, suggesting that this specificity is based on other sequence features (Johnson et al. 2002; Rose et al. 2002; Bland et al. 2008; Whyteside and Turner 2008). Although the cleavage specificities of Drosophila neprilysins are not as well known, there is evidence that Drosophila Nep2 is capable of cleaving locust, human, and fly tachykinins in vitro. However, while Nep2 prefers similar polar, hydrophobic residue pairs it does not cleave at the same site used by mammalian neprilysin (Thomas et al. 2005; Bland et al. 2007). This observation, and the sheer number of Nep-like proteins in Drosophila, makes predicting protein-specific neprilysin cleavage sites in Drosophila neuropeptides problematic. To date, only Nep2 and Nep4 have been demonstrated to be proteolytically active (Thomas et al. 2005; Bland et al. 2007; Meyer et al. 2009) but their in vivo targets are as yet unknown. Neprilysin-like proteins have been shown to be involved in the degradation of the insect neuropeptide pigment dispersing factor (PDF), which has been implicated in mating behavior (Isaac et al. 2007); which Nep is responsible for the cleavage of PDF is unclear.
To identify other potential substrates of neprilysins in Drosophila that might contribute to the phenotypes we report here, we looked for potential cleavage sites in known Drosophila neuropeptides that have been associated with the regulation of physiology and behavior (reviewed in Nässel and Winther 2010) as well as in the newly identified group classified as natalisins (Jiang et al. 2013). Using the known cleavage site preferences of mammalian neprilysins and observed Drosophila Nep cleavage patterns, we identified potential Nep target sites in 56 of these 68 neuropeptides (Table S4). Of these, several stand out as notable targets for further study in reproduction.
The first candidate, neuropeptide F (NPF), is associated with the regulation of male courtship behavior in Drosophila (Lee et al. 2006) and with ovarian development in the locust (Cerstiaens et al. 1999). NPF has also been implicated in regulating feeding behavior in Drosophila (Lee et al. 2004), where increases in food intake are correlated with but not completely dependent on post-mating increases in female egg laying (Carvalho et al. 2006; Apger-Mcglaughon and Wolfner 2013). Another candidate, allatostatin A (AstA), has been implicated in regulating oviduct muscle contraction in other arthropods (Donini and Lange 2002; Garside et al. 2002; Woodhead et al. 2003). Further, allatostatins are essential for inhibiting juvenile hormone (JH) biosynthesis (Altaratz et al. 1991; Stay and Tobe 2007). JH has been implicated in a wide array of reproductive events including vitellogenesis and egg production (Gavin and Williamson 1976; Soller et al. 1999; Dubrovsky et al. 2002; Raushenbach et al. 2004) and is regulated by mating (Moshitzky et al. 1996). If AstA is a neprilysin candidate, then knockdown of that neprilysin in females could result in persistence or more allatostatin, which in turn could decrease egg production in a way similar to what we observed here for females knocked down for Nep1, Nep2, and Nep4. A third candidate, the sex peptide (SP), is a male-derived seminal fluid protein that is transferred to the female during mating and regulates female post-mating behaviors (Kubli 2003). Not only does SP regulate long-term post-mating increases in egg laying (Liu and Kubli 2003), it too has been implicated in changes in feeding and excretion post-mating (Carvalho et al. 2006; Cognigni et al. 2011; Apger-Mcglaughon and Wolfner 2013). In addition, SP is able to activate JH–B3 biosynthesis (Moshitzky et al. 1996). Further, myoinhibitory peptide-1 (MIP-1), part of a family of neuropeptides that are able to bind the sex peptide receptor (SPR) in addition to SP (Kim et al. 2010), is a potential target of neprilysin degradation. These latter two examples suggest that Neps could play a role in determining the stability and balance of the mix of neuropeptides that are available to bind SPR after mating and through this might be important in controlling the extent of post-mating changes in behaviors and egg production. Further, SP is known to be cleaved in the hemolymph of females and some of the detected fragments are consistent with a cleavage at one of our predicted neprilysin cleavage sites (R,L) (Pilpel et al. 2008).
Based on Fly Atlas (Chintapalli et al. 2007) and modENCODE (McQuilton et al. 2012; Young et al. 2012) data (Table S5) one of these neuropeptides, AstA, has a precursor sequence that is expressed in the female spermathecae, a site where our data suggest that the expression of Nep1 and Nep2 is needed for normal female egg laying. However, it is important to note that Nep4 and Nep2 encode secreted proteins (Thomas et al. 2005; Meyer et al. 2009) so their site of action is likely not limited by their expression patterns. Similarly, there is evidence that neuropeptides, particularly tachykinins, from one area of the body can activate receptors elsewhere in insects (Winther and Nassel 2001).
In conclusion, we have shown that Drosophila neprilysins play an important role in regulating reproductive success of both males and females. It is likely that they do this by regulating the degradation of one or several neuropeptides substrates in both the female reproductive tract and the CNS. Future identification of the in vivo targets of Drosophila Neps will be important for identifying modulators of insect reproduction, as well as serving as a model for the effects of neprilysin action in all organisms.
Supplementary Material
Acknowledgments
We thank Daniel Barbash for the use of his Leica CTR5000 microscope, Amber Krauchunas and Norene Buehner for their advice and assistance in embryo staining, Geoff Findlay for comments on this manuscript and for his help with MUSCLE, and the members of the Cornell Superfly Club, David Deitcher, and anonymous reviewers, for helpful suggestions and comments on this manuscript. We also acknowledge the support of KULeuven and VIB to P.C. and grants RO1-038921 to M.F.W. and RO1-HD059060 to M.F.W. and Andrew Clark for support of this work.
Footnotes
Communicating editor: S. E Bickel
Literature Cited
- Adams E. M., Wolfner M. F., 2007. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 53: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaratz M., Applebaum S. W., Richard D. S., Gilbert L. I., Segal D., 1991. Regulation of juvenile hormone synthesis in wild-type and apterous mutant Drosophila. Mol. Cell. Endocrinol. 81: 205–216. [DOI] [PubMed] [Google Scholar]
- Aoyama M., Kawada T., Satake H., 2012. Localization and enzymatic activity profiles of the proteases responsible for tachykinin-directed oocyte growth in the protochordate, Ciona intestinalis. Peptides 34: 186–192. [DOI] [PubMed] [Google Scholar]
- Apger-McGlaughon J., Wolfner M. F., 2013. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J. Insect Physiol. 59: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Wolfner M. F., 2009. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc. Natl. Acad. Sci. USA 106: 15796–15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Ravi Ram K., Bloch Qazi M. C., Wolfner M. F., 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., et al. , 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran J., Palevitch O., Ben-Dor S., Levavi-Sivan B., 2012. Neurokinin Bs and neurokinin B receptors in zebrafish-potential role in controlling fish reproduction. Proc. Natl. Acad. Sci. USA 109: 10269–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland N. D., Thomas J. E., Audsley N., Shirras A. D., Turner A. J., et al. , 2007. Expression of NEP2, a soluble neprilysin-like endopeptidase, during embryogenesis in Drosophila melanogaster. Peptides 28: 127–135. [DOI] [PubMed] [Google Scholar]
- Bland N. D., Pinney J. W., Thomas J. E., Turner A. J., Isaac R. E., 2008. Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol. Biol. 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi M. C., Heifetz Y., Wolfner M. F., 2003. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 256: 195–211. [DOI] [PubMed] [Google Scholar]
- Bonavera J. J., Sahu A., Kalra S. P., Kalra P. S., 1994. The hypothalamic peptides, beta-endorphin, neuropeptide K and interleukin-1 beta, and the opiate morphine, enhance the excitatory amino acid-induced LH release under the influence of gonadal steroids. J. Neuroendocrinol. 6: 557–564. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Carpentier M., Guillemette C., Bailey J. L., Boileau G., Jeannotte L., et al. , 2004. Reduced fertility in male mice deficient in the zinc metallopeptidase NL1. Mol. Cell. Biol. 24: 4428–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho G. B., Kapahi P., Anderson D. J., Benzer S., 2006. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol. 16: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerstiaens A., Benfekih L., Zouiten H., Verhaert P., De Loof A., et al. , 1999. Led-NPF-1 stimulates ovarian development in locusts. Peptides 20: 39–44. [DOI] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Clements J., Hens K., Francis C., Schellens A., Callaerts P., 2008. Conserved role for the Drosophila Pax6 homolog Eyeless in differentiation and function of insulin-producing neurons. Proc. Natl. Acad. Sci. USA 105: 16183–16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates D., Siviter R., Isaac R. E., 2000. Exploring the Caenorhabditis elegans and Drosophila melanogaster genomes to understand neuropeptide and peptidase function. Biochem. Soc. Trans. 28: 464–469. [PubMed] [Google Scholar]
- Cognigni P., Bailey A. P., Miguel-Aliaga I., 2011. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Donini A., Lange A. B., 2002. The effects of crustacean cardioactive peptide on locust oviducts are calcium-dependent. Peptides 23: 683–691. [DOI] [PubMed] [Google Scholar]
- Dow J. A., Romero M. F., 2010. Drosophila provides rapid modeling of renal development, function, and disease. Am. J. Physiol. Renal Physiol. 299: F1237–F1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky E. B., Dubrovskaya V. A., Berger E. M., 2002. Juvenile hormone signaling during oogenesis in Drosophila melanogaster. Insect Biochem. Mol. Biol. 32: 1555–1565. [DOI] [PubMed] [Google Scholar]
- Edgar R. C., 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. A., Brand A. H., 2008. The GAL4 system: a versatile system for the expression of genes. Methods Mol. Biol. 420: 79–95. [DOI] [PubMed] [Google Scholar]
- Felsenstein J., 2005. PHYLIP, Phylogeny Inference Package, version 3.6. Department of Genome Sciences, University of Washington, Seattle. [Google Scholar]
- Furriols M., Ventura G., Casanova J., 2007. Two distinct but convergent groups of cells trigger Torso receptor tyrosine kinase activation by independently expressing torso-like. Proc. Natl. Acad. Sci. USA 104: 11660–11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside C. S., Koladich P. M., Bendena W. G., Tobe S. S., 2002. Expression of allatostatin in the oviducts of the cockroach Diploptera punctata. Insect Biochem. Mol. Biol. 32: 1089–1099. [DOI] [PubMed] [Google Scholar]
- Gavin J. A., Williamson J. H., 1976. Juvenile hormone-induced vitellogenesis in apterous4, a non-vitellogenic mutant in Drosophila melanogaster. J. Insect Physiol. 22: 1737–1742. [DOI] [PubMed] [Google Scholar]
- Gligorov D., Sitnik J. L., Maeda R. K., Wolfner M. F., Karch F., 2013. A novel function for the hox gene abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS Genet. 9: e1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. R., MacDonald P. C., Casey M. L., 1993. Cellular localization of membrane metalloendopeptidase (enkephalinase) in human endometrium during the ovarian cycle. J. Clin. Endocrinol. Metab. 76: 769–776. [DOI] [PubMed] [Google Scholar]
- Huet F., Lu J. T., Myrick K. V., Baugh L. R., Crosby M. A., et al. , 2002. A deletion-generator compound element allows deletion saturation analysis for genomewide phenotypic annotation. Proc. Natl. Acad. Sci. USA 99: 9948–9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R. E., Nassel D. R., 2003. Identification and localization of a neprilysin-like activity that degrades tachykinin-related peptides in the brain of the cockroach, Leucophaea maderae, and locust, Locusta migratoria. J. Comp. Neurol. 457: 57–66. [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Parkin E. T., Keen J. N., Nassel D. R., Siviter R. J., et al. , 2002. Inactivation of a tachykinin-related peptide: identification of four neuropeptide-degrading enzymes in neuronal membranes of insects from four different orders. Peptides 23: 725–733. [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Johnson E. C., Audsley N., Shirras A. D., 2007. Metabolic inactivation of the circadian transmitter, pigment dispersing factor (PDF), by neprilysin-like peptidases in Drosophila. J. Exp. Biol. 210: 4465–4470. [DOI] [PubMed] [Google Scholar]
- Jiang H., Lkhagva A., Daubnerova I., Chae H. S., Simo L., et al. , 2013. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl. Acad. Sci. USA 110: E3526–E3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Swenson H. R., Ramage R., Ahn K., 2002. Mapping the active site of endothelin-converting enzyme-1 through subsite specificity and mutagenesis studies: a comparison with neprilysin. Arch. Biochem. Biophys. 398: 240–248. [DOI] [PubMed] [Google Scholar]
- Karr T. L., 1991. Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. Mech. Dev. 34: 101–111. [DOI] [PubMed] [Google Scholar]
- Keleman K., Vrontou E., Kruttner S., Yu J. Y., Kurtovic-Kozaric A., et al. , 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489: 145–149. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Bartalska K., Audsley N., Yamanaka N., Yapici N., et al. , 2010. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 107: 6520–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Patte-Mensah C., Taleb O., Bourguignon J. J., Schmitt M., et al. , 2013. The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 70: 254–260. [DOI] [PubMed] [Google Scholar]
- Knapek S., Kahsai L., Winther A. M., Tanimoto H., Nassel D. R., 2013. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. J. Neurosci. 33: 5340–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas A. R., Horner V. L., Wolfner M. F., 2012. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster. Dev. Biol. 370: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E., 2003. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 60: 1689–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. H., Fedina T. Y., Hansen I., Dreisewerd K., Dierick H. A., et al. , 2012. Insulin signaling mediates sexual attractiveness in Drosophila. PLoS Genet. 8: e1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Nassel D. R., Lange A. B., Orchard I., 1999. Locustatachykinin isoforms in the locust: distribution and quantification in the central nervous system and action on the oviduct muscle. Peptides 20: 687–694. [DOI] [PubMed] [Google Scholar]
- LaFever L., Drummond-Barbosa D., 2005. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309: 1071–1073. [DOI] [PubMed] [Google Scholar]
- Lee G., Bahn J. H., Park J. H., 2006. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. USA 103: 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., You K. H., Choo J. K., Han Y. M., Yu K., 2004. Drosophila short neuropeptide F regulates food intake and body size. J. Biol. Chem. 279: 50781–50789. [DOI] [PubMed] [Google Scholar]
- Li C., Booze R. M., Hersh L. B., 1995. Tissue-specific expression of rat neutral endopeptidase (neprilysin) mRNAs. J. Biol. Chem. 270: 5723–5728. [DOI] [PubMed] [Google Scholar]
- Liu H., Kubli E., 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100: 9929–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguer-Satta V., Besancon R., Bachelard-Cascales E., 2011. Concise review: neutral endopeptidase (CD10): a multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells 29: 389–396. [DOI] [PubMed] [Google Scholar]
- Marygold S. J., Leyland P. C., Seal R. L., Goodman J. L., Thurmond J., et al. , 2013. FlyBase: improvements to the bibliography. Nucleic Acids Res. 41: D751–D757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilton P., St Pierre S. E., Thurmond J., 2012. FlyBase 101: the basics of navigating FlyBase. Nucleic Acids Res. 40: D706–D714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Panz M., Zmojdzian M., Jagla K., Paululat A., 2009. Neprilysin 4, a novel endopeptidase from Drosophila melanogaster, displays distinct substrate specificities and exceptional solubility states. J. Exp. Biol. 212: 3673–3683. [DOI] [PubMed] [Google Scholar]
- Middleton C. A., Nongthomba U., Parry K., Sweeney S. T., Sparrow J. C., et al. , 2006. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshitzky P., Fleischmann I., Chaimov N., Saudan P., Klauser S., et al. , 1996. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 32: 363–374. [DOI] [PubMed] [Google Scholar]
- Mueller J. L., Linklater J. R., Ravi Ram K., Chapman T., Wolfner M. F., 2008. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics 178: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder S. D., Veerhuis R., Blankenstein M. A., Nielsen H. M., 2012. The effect of amyloid associated proteins on the expression of genes involved in amyloid-beta clearance by adult human astrocytes. Exp. Neurol. 233: 373–379. [DOI] [PubMed] [Google Scholar]
- Nässel D. R., Winther A. M., 2010. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 92: 42–104. [DOI] [PubMed] [Google Scholar]
- Nässel D. R., Kubrak O. I., Liu Y., Luo J., Lushchak O. V., 2013. Factors that regulate insulin producing cells and their output in. Front Physiol 4: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum D. M., Wolfner M. F., 1999. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153: 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet T., Facchinetti P., Rose C., Bonhomme M. C., Gros C., et al. , 2000. Neprilysin II: a putative novel metalloprotease and its isoforms in CNS and testis. Biochem. Biophys. Res. Commun. 271: 565–570. [DOI] [PubMed] [Google Scholar]
- Pauli A., Althoff F., Oliveira R. A., Heidmann S., Schuldiner O., et al. , 2008. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev. Cell 14: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel N., Nezer I., Applebaum S. W., Heifetz Y., 2008. Mating increases trypsin in female Drosophila hemolymph. Insect Biochem. Mol. Biol. 38: 320–330. [DOI] [PubMed] [Google Scholar]
- Pintado C. O., Pinto F. M., Pennefather J. N., Hidalgo A., Baamonde A., et al. , 2003. A role for tachykinins in female mouse and rat reproductive function. Biol. Reprod. 69: 940–946. [DOI] [PubMed] [Google Scholar]
- Pinto F. M., Armesto C. P., Magraner J., Trujillo M., Martin J. D., et al. , 1999. Tachykinin receptor and neutral endopeptidase gene expression in the rat uterus: characterization and regulation in response to ovarian steroid treatment. Endocrinology 140: 2526–2532. [DOI] [PubMed] [Google Scholar]
- Prokupek A. M., Eyun S. I., Ko L., Moriyama E. N., Harshman L. G., 2010. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. J. Evol. Biol. 23: 1386–1398. [DOI] [PubMed] [Google Scholar]
- Ram K. R., Wolfner M. F., 2007. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 3: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut, A., 2010 FigTree 1.3.1 Available at: tree.bio.ed.ac.uk/software/figtree/.
- Rance N. E., Krajewski S. J., Smith M. A., Cholanian M., Dacks P. A., 2010. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 1364: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raushenbach I. Y., Gruntenko N. E., Bownes M., Adonieva N. V., Terashima J., et al. , 2004. The role of juvenile hormone in the control of reproductive function in Drosophila virilis under nutritional stress. J. Insect Physiol. 50: 323–330. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J., Bateman A., 2012. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 40: D343–D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D. S., Rybczynski R., Wilson T. G., Wang Y., Wayne M. L., et al. , 2005. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 51: 455–464. [DOI] [PubMed] [Google Scholar]
- Rorth P., Szabo K., Bailey A., Laverty T., Rehm J., et al. , 1998. Systematic gain-of-function genetics in Drosophila. Development 125: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Rose C., Voisin S., Gros C., Schwartz J. C., Ouimet T., 2002. Cell-specific activity of neprilysin 2 isoforms and enzymic specificity compared with neprilysin. Biochem. J. 363: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Kalra S. P., 1992. Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology 130: 1571–1577. [DOI] [PubMed] [Google Scholar]
- Sahu A., Crowley W. R., Tatemoto K., Balasubramaniam A., Kalra S. P., 1987. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides 8: 921–926. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kitamoto T., 2006. Differential roles of two major brain structures, mushroom bodies and central complex, for Drosophila male courtship behavior. J. Neurobiol. 66: 821–834. [DOI] [PubMed] [Google Scholar]
- Schnakenberg S. L., Matias W. R., Siegal M. L., 2011. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 9: e1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura J., Ruilope L. M., 2011. Dual-acting angiotensin receptor-neprilysin inhibition. Curr. Hypertens. Rep. 13: 74–78. [DOI] [PubMed] [Google Scholar]
- Smollich M., Gotte M., Yip G. W., Yong E. S., Kersting C., et al. , 2007. On the role of endothelin-converting enzyme-1 (ECE-1) and neprilysin in human breast cancer. Breast Cancer Res. Treat. 106: 361–369. [DOI] [PubMed] [Google Scholar]
- Soller M., Bownes M., Kubli E., 1999. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208: 337–351. [DOI] [PubMed] [Google Scholar]
- Southall, T. D., D. A. Elliott and A. H. Brand, 2008 The GAL4 System: A versatile toolkit for gene expression in Drosophila. CSH Protoc. 2008: pdb top49. [DOI] [PubMed]
- Spradling A. C., Stern D. M., Kiss I., Roote J., Laverty T., et al. , 1995. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc. Natl. Acad. Sci. USA 92: 10824–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Stern D., Beaton A., Rhem E. J., Laverty T., et al. , 1999. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153: 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stay B., Tobe S. S., 2007. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 52: 277–299. [DOI] [PubMed] [Google Scholar]
- Sullivan W., Ashburner M., Hawley R. (Editors), 2000. Drosophila Protocols. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sun J., Spradling A. C., 2013. Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. Elife 2: e00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., et al. , 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler T. D., Pacheco D. A., Hergarden A. C., Murthy M., Anderson D. J., 2012. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc. Natl. Acad. Sci. USA 109: 20697–20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S., Rosay P., Goodwin S. F., Veenstra J. A., 2007. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem. Biophys. Res. Commun. 352: 305–310. [DOI] [PubMed] [Google Scholar]
- Thomas J. E., Rylett C. M., Carhan A., Bland N. D., Bingham R. J., et al. , 2005. Drosophila melanogaster NEP2 is a new soluble member of the neprilysin family of endopeptidases with implications for reproduction and renal function. Biochem. J. 386: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Brown C. D., Carson J. A., Barnes K., 2000. The neprilysin family in health and disease. Adv. Exp. Med. Biol. 477: 229–240. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Isaac R. E., Coates D., 2001. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. BioEssays 23: 261–269. [DOI] [PubMed] [Google Scholar]
- Whyteside A. R., Turner A. J., 2008. Human neprilysin-2 (NEP2) and NEP display distinct subcellular localisations and substrate preferences. FEBS Lett. 582: 2382–2386. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Buesing E. J., Wehling C. A., Loomis Z. L., Cool C. D., et al. , 2011. Decreased neprilysin and pulmonary vascular remodeling in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 183: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. L., Shirras A. D., Isaac R. E., 2002. Extracellular peptidases of imaginal discs of Drosophila melanogaster. Peptides 23: 2007–2014. [DOI] [PubMed] [Google Scholar]
- Winther A. M., Nassel D. R., 2001. Intestinal peptides as circulating hormones: release of tachykinin-related peptide from the locust and cockroach midgut. J. Exp. Biol. 204: 1269–1280. [DOI] [PubMed] [Google Scholar]
- Wong S. S., Sun N. N., Fastje C. D., Witten M. L., Lantz R. C., et al. , 2011. Role of neprilysin in airway inflammation induced by diesel exhaust emissions. Res. Rep. Health Eff. Inst. 159: 3–40. [PMC free article] [PubMed] [Google Scholar]
- Woodhead A. P., Thompson M. E., Chan K. K., Stay B., 2003. Allatostatin in ovaries, oviducts, and young embryos in the cockroach Diploptera punctata. J. Insect Physiol. 49: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Rumpf S., Xiang Y., Gordon M. D., Song W., et al. , 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J., Bouligand J., Francou B., Raffin-Sanson M. L., Gaillez S., et al. , 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 95: 2287–2295. [DOI] [PubMed] [Google Scholar]
- Young R. S., Marques A. C., Tibbit C., Haerty W., Bassett A. R., et al. , 2012. Identification and properties of 1,119 candidate lincRNA loci in the Drosophila melanogaster genome. Genome Biol. Evol. 4: 427–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Huang H., Kim S. M., Lin H., Meng X., et al. , 2012. Molecular genetic analysis of sexual rejection: roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J. Neurosci. 32: 14281–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.