Abstract
Chronic injury of alveolar lung epithelium leads to epithelial disintegrity in idiopathic pulmonary fibrosis (IPF). We had reported earlier that Grhl2, a transcriptional factor, maintains alveolar epithelial cell integrity by directly regulating components of adherens and tight junctions and thus hypothesized an important role of GRHL2 in pathogenesis of IPF. Comparison of GRHL2 distribution at different stages of human lung development showed its abundance in developing lung epithelium and in adult lung epithelium. However, GRHL2 is detected in normal human lung mesenchyme only at early fetal stage (week 9). Similar mesenchymal reexpression of GRHL2 was also observed in IPF. Immunofluorescence analysis in serial sections from three IPF patients revealed at least two subsets of alveolar epithelial cells (AEC), based on differential GRHL2 expression and the converse fluorescence intensities for epithelial vs. mesenchymal markers. Grhl2 was not detected in mesenchyme in intraperitoneal bleomycin-induced injury as well as in spontaneously occurring fibrosis in double-mutant HPS1 and HPS2 mice, whereas in contrast in a radiation-induced fibrosis model, with forced Forkhead box M1 (Foxm1) expression, an overlap of Grhl2 with a mesenchymal marker was observed in fibrotic regions. Grhl2's role in alveolar epithelial cell plasticity was confirmed by altered Grhl2 gene expression analysis in IPF and further validated by in vitro manipulation of its expression in alveolar epithelial cell lines. Our findings reveal important pathophysiological differences between human IPF and specific mouse models of fibrosis and support a crucial role of GRHL2 in epithelial activation in lung fibrosis and perhaps also in epithelial plasticity.
Keywords: GRHL2, idiopathic pulmonary fibrosis, epithelial integrity, cell flattening, epithelial plasticity
idiopathic pulmonary fibrosis (IPF) is an age-related chronic lung disease of unknown cause that has a poor prognosis and few treatment options (10, 17, 20, 26, 29, 33). IPF was once thought to be the result of a chronic inflammatory process, but current evidence indicates that the fibrotic response in this disease is driven by chronic injury and activation of type II alveolar epithelial cells (AEC) (30, 31). Thus injured and activated AECs produce a variety of mediators that may induce the expansion of fibroblast populations and their differentiation to myofibroblasts, which organize into characteristic clusters called fibroblast/myofibroblast foci. The origin of fibroblasts in IPF is still under vigorous debate but may include the migration and proliferation of resident mesenchymal cells and/or recruitment of bone marrow-derived cells such as circulating fibrocytes. A growing body of evidence also indicates a role of epithelial-mesenchymal plasticity, which has been termed “epithelial cell-flattening events” (31, 34). Histological analyses have suggested that AEC can undergo cell flattening and cell elongation, events that may precede a transition into a fibroblastic and eventually a myofibroblastic phenotype (31).
Lung fibrosis has been studied extensively in mouse models such as intratracheal (IT) (11, 12) or intraperitoneal (IP) administration of bleomycin (9). Administration of bleomycin results in marked but eventually transient lung fibrosis, prominent alveolar epithelial cell hyperplasia, with a pattern of spatial and temporal heterogeneity, and persistence of aberrant remodeling well after the bleomycin treatment. But there are marked differences in lung injury pattern between the IT vs. IP routes of administration of bleomycin. Whereas in the IT model an extensive inflammation response precedes the fibrotic response, IP bleomycin elicits mainly an injury to alveolar epithelium and endothelium with less marked inflammatory responses. In contrast, spontaneous pulmonary fibrosis occurs in Hermansky-Pudlak syndrome (HPS mutant) mice (23, 24). HPS is a rare inherited disease affecting intracellular biogenesis of lysosome-related organelles (13). Individual mutations in HPS1 or HPS2 make these mice highly susceptible to bleomycin-induced injury-induced lung fibrosis, whereas double-mutant HPS1 and HPS2 (HPS1/2) mice develop spontaneous pulmonary fibrosis (14). Recently a radiation-induced injury model, in which Foxm1 was overexpressed, showed spontaneous pneumonitis and pulmonary fibrosis with increased expression of both interleukins and epithelial-to-mesenchymal (EMT) marker transcriptional factors such as ZEB1 (4).
The Grainyhead-like (GRHL) gene family comprises GRHL1, GRHL2, and GRHL3, which function as wound healing and injury repair genes in cutaneous tissue in Drosophila and mouse (14, 36). However, their functional role in injury repair in the lung is not yet fully understood. Among the Grainyhead family members, Grhl2 is the most abundant and universally expressed in mouse lung epithelium during lung development (2, 40, 41). We recently characterized the expression pattern of Grhl2 during lung development and showed that this gene is highly expressed very early during lung development and also in adult mouse alveolar and bronchial lung epithelium (40, 41). We also found that Grhl2 colocalizes with Nkx2.1 in lung epithelium and that these genes interact in a positive feedback loop to maintain lung epithelial cell integrity, cell identity, collective cell migration, and cell-cell interactions (40, 41). We further showed that Grhl2 knockdown in the alveolar type II-like MLE15 cell line resulted in cell-flattening events, with alteration in distribution of F-actin filaments. Moreover, we also suggested that the expression of Tert, which is an important injury repair gene, may be regulated by Grhl2 in alveolar epithelium, which corroborates another study showing that GRHL2 mediates epigenetic regulation of TERT in human keratinocytes (5, 6, 19). The fact that mutation in TERT is known to predispose to familial IPF (1, 22, 38) suggests a possible functional role of GRHL2 in the pathogenesis of IPF.
Herein, we have compared the distribution of GRHL2 immunofluorescence signal in normal human vs. IPF lungs with that observed in three mouse models of lung fibrosis: 1) IP bleomycin-induced lung injury and fibrosis, 2) spontaneously occurring pulmonary fibrosis in HPS1/2 mutants, and 3) radiation-induced lung injury in which Foxm1 is misexpressed. We observed that GRHL2 is ectopically expressed in IPF lung mesenchyme, although at a relatively low level compared with lung epithelium. This distribution of GRHL2 was similar to that found in human lung at embryonic week 9. In alveolar epithelium of IPF lung we also observed at least two subsets of AEC, one showing bright GRHL2 and ABCA3 fluorescence and the other showing a dimmer fluorescence level. On the basis of double staining in serial sections with EMT markers and GRHL2, we showed that the second dimmer set of AEC appeared to be undergoing cell flattening. Furthermore, overall GRHL2 expression was low in fibrotic areas of IPF lung compared with control donors, whereas in vitro knockdown vs. overexpression of Grhl2 in lung epithelial cells confirmed its potential role in epithelial to mesenchymal plasticity. We also observed similar cell-flattening events in alveolar epithelium in the radiation induced fibrosis model with Foxm1 misexpression, but neither in IP bleomycin-induced nor in spontaneously occurring pulmonary fibrosis in HPS1/2 mutant mice, thus revealing novel and important contrasting pathophysiological phenotypic differences between human IPF and these specific mouse models of pulmonary fibrosis.
MATERIALS AND METHODS
Lung tissue sections from human and mice.
Sections of deidentified histologically normal lung tissue (n = 2) and from IPF lungs (n = 3) were kindly provided in collaboration by Dr. Moises Selman. IPF lung samples were obtained from patients undergoing open lung biopsy for diagnosis purposes (63 ± 3 yr; two former smokers and one a lifetime nonsmoker), and the diagnosis was established according the American Thoracic Society/European Respiratory Society criteria including characteristic histological features of usual interstitial pneumonia (27). IPF patients had never been treated with corticosteroids or immunosuppressive drugs at the time of the biopsy. This study was approved by the Science and Bioethical Committee at the National Institute of Respiratory Diseases. For gene expression studies primary reverse transcription stage samples from IPF patients (n = 11) and normal donor (n = 8) were also supplied in collaboration with Dr. Andreas Guenther and Dr. Moises Selman. Normal adult lung tissue sections were obtained from Biochain (no. T2234152) as well as lung sections from a 38-wk human fetus. Human early fetal lung tissue was obtained from maternal donors undergoing elective termination at 8–11 wk of pregnancy in collaboration with Dr. Daniela Riccardi and colleagues at Cardiff. Gestational age was assessed by ultrasound measurements and confirmed by using fetal morphometric parameters after medical termination of pregnancy. Following dissections, lungs were fixed in 4% paraformaldehyde overnight and paraffin embedded, and 8-mm sections were used for immunofluorescence. Lung sections from the following mouse models were processed as follows: HPS1/2 mouse (14-mo-old) and BL/6J (12-mo-old) lung sections were processed as described earlier by Mahavadi et al. (23, 24). Bleomycin-induced injury lung sections were processed as described earlier by Cushing et al. (9). For radiation-induced lung injury model, epithelial-specific Foxm1-overexpressing mice (Spc-rtTAtg/−/tetO-Foxm1-ΔNtg/−-epiFoxm1-ΔN) were described previously (3, 4). For radiation-induced fibrosis studies, thoracic contents of epiFoxm1-ΔN and littermate control mice were irradiated with a single dose of 12 Gy by use of a 137Cs Mark I-68A irradiator (J. L. Shepard and Associates, San Fernando, CA) at a dose rate of 0.725 Gy per min. Mice were anesthetized and placed in lead shielding to protect all parts of the body except the thoracic region. Lungs were isolated, fixed, and embedded into paraffin blocks or were used for total lung RNA with RNA-stat-60 (Tel-Test “B”) at 6 mo postradiation.
RNA purification and qRT-PCR.
Total RNA was isolated from mouse lung tissue, sorted cells and cell lines using an RNeasy kit (Qiagen) and treated with DNase1 (Qiagen). Isolated RNA was reverse transcribed by using TaqMan Reverse Transcription Reagents (Applied Biosystems) or Omniscript RT kit (Qiagen) following the manufacturer's protocols. A StepOnePlus system (Applied Biosystems) using TaqMan gene expression assays (Applied Biosystems) was used for quantitative RT-PCR (qRT-PCR) analyses (data available on request). Reactions were performed with TaqMan PCR master mix or Fast TaqMan PCR master mix (Applied Biosystems) or with SYBR green PCR master mix. Relative expression levels were determined by the comparative 2−ΔΔCT method, normalized to Gapdh.
Plasmid construction.
Full-length Grhl2 cDNA was subcloned from the pGADT7-HA-Grhl2 vector provided by Dr. Bogi Anderson (University of California, Irvine, CA). Cloning is described in our previous publication (40). Briefly, the Grhl2 cDNA was amplified by PCR using primers 5′-CAAGCGGCCGCCATGTCACAAGAGTCGGAC-3′ and 5′CGCTGATGGAGATCTGAGGATCCATTC-3′, which contain Not1 and BamH1 sites, respectively. This fragment was then inserted in place of the DsRed gene in the dual promoter-reporter lentiviral plasmid, pCMV-dsred-UBC-GFP (40), to generate pCMV-Grhl2-UBC-GFP. The construct was verified by restriction enzyme digestion and by sequencing. This Grhl2 vector was used to overexpress Grhl2 in flat E10, an adult lung epithelial cell line that expresses type I cell genes, provided by Dr. A. Malkinson (University of Colorado, Denver, CO) and Dr. Randy Ruch (Medical College of Ohio).
Lentivirus production and transduction.
For Grhl2 knockdown studies, mission shRNA transduction particles and nontargeting control shRNA (SHC002V) or Grhl2shRNA (TRCN0000084224) targeting Grhl2 were purchased from Sigma Aldrich. MLE15 epithelial cell transductions with lentiviral vectors were performed for 16 h in the presence of polybrene (5 μg/ml) at a multiplicity of infection of 50. Stable cell lines were generated by puromycin dihydrochloride (5 μg/ml, Sigma) selection for 6 days. Cells were harvested 48 h after infection and analyzed for transduction efficiency and viability by flow cytometry analysis of GFP expression and exclusion of propidium iodide. Knockdown and overexpression of Grhl2 in MLE15 and E10 cells, respectively, were quantified at the mRNA level by qRT-PCR and at the protein level by immunostaining and Western blot as described previously in our earlier publication (40).
Immunocytochemistry.
E10 cells that were transduced with lentiviruses packaged with CMV-GRHL2-UBC-GFP plasmid as described previously (40) were grown on glass coverslips to reach 70–80% confluence, fixed with 4% formaldehyde in 1× PBS, and processed for immunostaining. For GRHL2 nuclear staining, cells were treated with 0.01% Triton X-100 (Fisher Scientific) in 1× PBS, blocked with 1× PBS containing 2% FBS for 30 min, and incubated overnight at 4°C with rabbit GRHL2 antibody (HPA00482, Sigma; 1:500 dilution). After three washes with 1× PBS, cells were incubated with secondary anti-rabbit CY3-conjugated antibody (Invitrogen, 1:2,000 dilution) for 1 h at room temperature. Images were taken on a Leica inverted microscope. Images were processed with Image J analysis software.
Immunofluorescence.
Human and mouse lung paraffin sections were processed as described in Ref. 40. Briefly, the sections were deparaffinized at 55°C for 1 h, cleared in HistoChoice (H2904 Sigma-Aldrich), and hydrated by incubation for 3 min each with 100, 95, and 70% ethanol dilutions and water. Antigen retrieval of the tissue sections was performed in antigen unmasking solution (H-3300, Vector Laboratories) and heating them in a microwave oven. The sections were allowed to cool down to room temperature for 1 h and subsequently permeabilized by incubating them in 1× TN buffer (20 mM Tris·HCl, pH 7.4, 150 mM NaCl) containing 0.5% Triton X-100 (TNT) at room temperature for 1 h. They were blocked in 1× TN buffer containing 3% BSA and 0.5% Tween-20 at room temperature for 30 min. The primary antibodies were then applied to sections and incubated in a moist chamber at 4°C overnight. The following day, the sections were washed in 1× TNT for 5 min and then incubated for 1 h with Alexa Fluor-labeled secondary antibodies (Invitrogen) at a dilution of 1:200. The sections were again washed in 1× TNT for 5 min and mounted with Prolong Gold with DAPI (Invitrogen). The sections were imaged via a confocal microscope (Zeiss 710) and processed with ZEN 2009 software (Carl Zeiss). Primary antibodies and IgG controls used for this study are shown in Table 1. The following secondary antibodies were employed: donkey anti-rabbit Alexa Fluor 647, donkey anti-mouse Alexa Fluor 647, donkey anti-rabbit Alexa Fluor 488, donkey anti-mouse Alexa Fluor 488 (Invitrogen).
Table 1.
List of primary antibodies
| Name of Antibody (anti-) | Host | Catalog Number, Source | Dilution |
|---|---|---|---|
| Grainyhead-like 2 (GRHL2) | Rabbit | HPA004820, Sigma-Aldrich | 1:300 |
| E-cadherin (CDH1) | Mouse | 610182, BD Transduction Laboratories | 1:500 |
| Vimentin (clone V-9) | Mouse | V6630, Sigma-Aldrich | 1:200 |
| ATP-binding cassette subfamily A member 3 (ABCA3) (clone 17-H5-24) | Mouse | WMAB-ABCA3-13, Seven Hills Bioreagents | 1:500 |
| p63 (4A4) | Mouse | sc-8431, Santa Cruz Biotechnology | 1:100 |
| β-Tubulin4 | Mouse | MU178-UC, Biogenex | 1:100 |
| α-Smooth muscle actin (α-SMA) (CLONE 1A4) Cy3 conjugated | Mouse | C6198, Sigma-Aldrich | 1:100 |
| NK2 homeobox 1 (NKX2-1), also known as thyroid transcription factor 1 (TTF-1) | Mouse | MAB5460, Millipore | 1:1000 |
| Forkhead box protein M1 FOXM1 (K-19) | Rabbit | sc-500, Santa Cruz Biotechnology | 1:50 |
| Zinc finger E-box binding homeobox1 (ZEB1) | Mouse | AMAb90510, Atlas Antibodies, Sweden | |
| CD68 (clone KP1) | Mouse | 08-0125, Life Technologies | Predilute, ready to use |
| Normal Rabbit IgG | sc-2027, Santa Cruz Biotechnology | Concentration similar to the respective primary antibody used | |
| Normal mouse IgG | sc-2025, Santa Cruz Biotechnology | Concentration similar to the respective primary antibody used |
Confocal imaging.
Images were acquired via an LSM 710 confocal system mounted on an AxioObserver.Z1 microscope equipped with a ×20/0.8 Plan-APOCHROMAT lens and controlled by ZEN 2010 software (Carl Zeiss Microimaging, Thornwood, NY). Fluorescence excitation lasers were 405 nm for DAPI, 488 nm for Alexa 488, 561 nm for Cy3, and 633 nm for Alexa 647 dyes. For all dyes the confocal pinhole was set to 1 Airy unit of Alexa 647. Fluorescence emission was detected with a spectral array detector and dye fluorescence was separated from tissue autofluorescence by linear unmixing. To increase the contiguous area that was imaged, the optical zoom factor was set to 0.6 and adjacent fields of view were tiled together with no overlap.
Quantitation of signal intensity and surface area of cells from lung sections.
The immunofluorescence was quantified and signal area was calculated by using Fiji Image J application (downloaded from http://fiji.sc/). The unprocessed Zeiss confocal images were opened in Fiji Image J and individual red, green, and blue channels were split. Subsequently, by use of a drawing toolbar, the desired signal regions were demarcated and added to ROI Manager in the software. In each case, the signal intensity and the area were measured for about four to seven cells from insets. The mean and standard deviation of the values were calculated and the graphs were plotted with MS-Excel.
RESULTS
GRHL2 expression pattern in embryonic, fetal, and adult human lung.
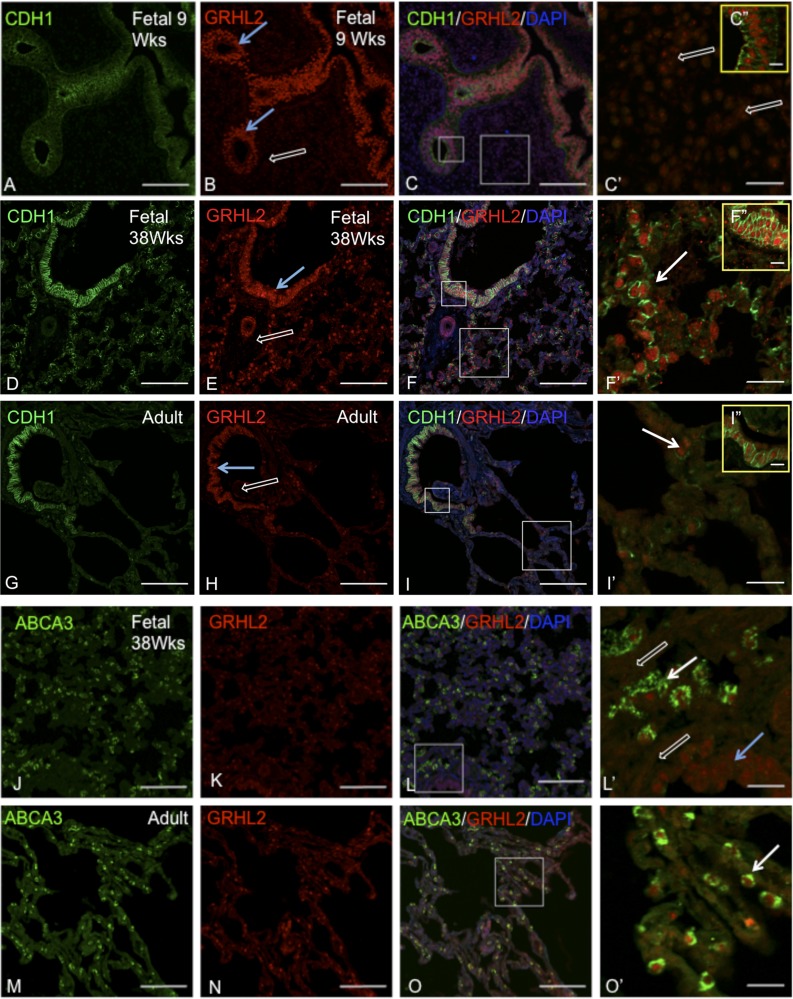
We have previously characterized GRHL2 expression patterns at different stages of mouse lung development, namely at E9.5, E15.5, and E18.5 and in adult lung, by immunohistochemistry and immunofluorescence (40). GRHL2 is exclusively expressed in mouse lung epithelium at all stages of normal mouse lung development and is not detected in mouse lung mesenchyme. Here, in Fig. 1, we show the expression patterns of GRHL2 in human early fetal (9 wk), late fetal (week 38), and normal adult lungs (58-yr-old male). In the early fetal lung (Fig. 1, A–C, C′, and C″) double staining of GRHL2 and CDH1 showed that GRHL2 is expressed at high levels in developing epithelium (indicated by blue arrows in B). We also observed relatively lower GRHL2 expression signal in the mesenchyme (indicated by open white arrows in C′). However, double staining of GRHL2 with CDH1 in normal late fetal (38 week) and adult lung tissues (Fig. 1, D–I, F′, and I′) showed that GRHL2 is exclusively expressed in both airway (blue arrow) and alveolar epithelium (white arrows) but is not detected in mesenchyme (white open arrows) at these stages. These results suggest a dynamic change in the expression pattern of GRHL2 during normal human lung development. By double staining of GRHL2 with a type II AEC surface marker, ATP-binding cassette subfamily A member 3 (ABCA3) in fetal (Fig. 1, J–L and L′), and normal adult human lung tissues (Fig. 1, M–O and O′), we observed that GRHL2 is expressed in developing alveolar epithelium both at the fetal stage and also in differentiated alveolar type II cells in adult lung. It can be noted that, although GRHL2 is expressed in both airway and alveolar epithelium, ABCA3 is exclusively expressed only in developing alveolar epithelium (L and L′, white arrow) and differentiated type II alveolar epithelium (O and O′, white arrow) but not in airway epithelium (L and L′, blue arrow). The expression pattern of GRHL2 in human fetal and adult lung is therefore consistent with our previous findings in mouse lung. The expression pattern of ABCA3 is also consistent with previous studies (35).
Fig. 1.
GRHL2 is abundantly expressed in the developing lung epithelium but only appears in lung mesenchyme at week 9. Double staining of Grhl2 and its target E-cadherin (CDH1) in embryonic lung tissue (A–C), fetal lung tissue (D–F), and normal adult lung tissue (G and H). C′, F′, and I′ are insets. C″, F″, and I″ are enlarged regions of the small box shown in C, F, and I, respectively. Blue arrows show airway epithelium. Open white arrows show mesenchyme and white arrows show epithelium. Double staining of GRHL2 and alveolar type II epithelial cell marker ABCA3 in fetal lung tissue (J–L) and normal adult lung tissue (M–O). L′ and O′ are insets. Insets do not show DAPI for clarity. Scale is 100 μm for A–O, 20 μm for C′, F′, I′, L′, and O′, and 10 μm for C″, F″, and I″.
Differential expression pattern of GRHL2 in IPF vs. normal human lung.
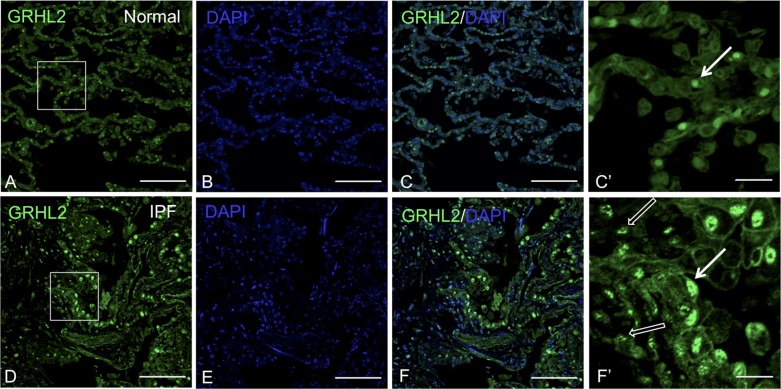
We and other researchers have shown that Grhl2 is the predominant member of the Grhl family expressed in mouse lung epithelium (2, 40). Grhl family members are epidermal wound healing genes, reported to play an important role in injury repair (36). We performed immunofluorescence studies to determine the expression levels and distribution of GRHL2 in normal human adult vs. IPF lungs. In normal adult lung (Fig. 2, A–C and C′) GRHL2 signal was observed only in epithelium but not in mesenchyme. However, to our surprise we found that in IPF lungs GRHL2 fluorescent signal not only is intensified in alveolar epithelium (white arrow) but also appeared ectopically in lung mesenchyme (open white arrow in F′). Consistent with earlier findings we also observed hyperplastic AEC in IPF lung (F′), which were distinct from the normal cuboidal AEC (C and C′). (Control staining with isotype IgG in normal and IPF lung data available on request.)
Fig. 2.
GRHL2 expression is observed in both lung epithelium and mesenchyme of idiopathic pulmonary fibrosis (IPF) lung. GRHL2 Immunofluorescence staining in normal (A–C) and IPF lung (D–F). C′ and F′ are insets of their respective images that lack DAPI for clarity. White arrows in C′ and F′ indicate epithelial cells whereas open white arrows in F′ indicate mesenchyme. Scale bar for A–F is 100 μm and for C′ and F′ it is 20 μm.
Differential expression pattern of GRHL2 in IPF distinguishes at least two subsets of type II alveolar epithelial cells.
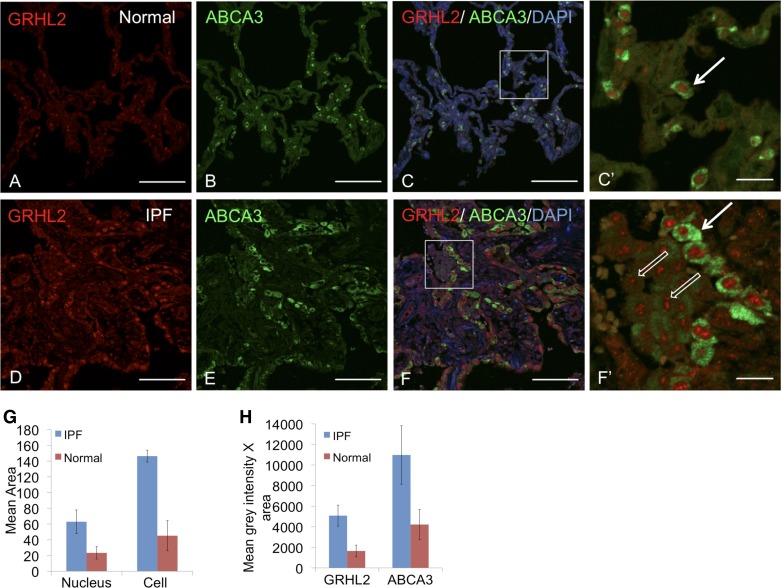
We further characterized the expression levels of GRHL2 in normal and IPF lung by double staining with AEC surface marker, ABCA3. We observed at least two different subtypes of AEC in IPF lung sections (Fig. 3, D–F and F′): 1) cells with bright GRHL2 and ABCA3 fluorescence (shown by white arrow in Fig. 3, F′), and 2) cells with relatively dim GRHL2 and ABCA3 fluorescence (shown in open white arrow in F′). However, in normal adult lung section, the fluorescence of GRHL2 and ABCA3 appeared uniform in normal adult lung and we did not observe any alternative GRHL2 and ABCA3 fluorescence patterns (Fig. 3, A–C and C′). We also found cells in the lumen of IPF lung with speckled fluorescence for GRHL2 but dim ABCA3. The total pixel intensity for GRHL2 fluorescence in the speckled cells was substantially lower than in cells lining the lumen, which showed strikingly bright GRHL2 fluorescence intensity. We calculated the average surface area of the nucleus and the cell based on fluorescence boundaries of GRHL2 and ABCA3, respectively, which are shown in Fig. 3H. We found substantial differences between normal and IPF alveolar cell and nucleus surface areas. This difference correlated well with increased fluorescence levels in alveolar cells in IPF vs. normal alveolar cells, suggesting an increase in detectable levels of both ABCA3 and GRHL2 in this subset of AEC. We postulated that these two AEC subtypes in IPF lung may reflect cell-flattening events, as were observed in our previous studies after knockdown of GRHL2 in lung epithelial MLE15 cells. Such cell-flattening events, which are related to epithelial plasticity, have been reported before in IPF (10, 30, 31).
Fig. 3.
GRHL2 and ABCA3 localization shows 2 subtypes of alveolar type II cells. Double staining of GRHL2 and ABCA3 in normal lung (A–C) and in IPF lung (D–F). C′ and F′ are insets of their respective images. In C′ and F′ white arrow indicates a subtype of alveolar type II cells that show bright GRHL2 and ABCA3 signals, whereas open white arrows in F′ indicate another subtype with lower fluorescence intensity for GRHL2 and ABCA3 signals. Insets do not show DAPI for clarity. Scale for A–F is 100 μm and for C′ and F′ it is 20 μm. Mean area of cells (4–7 in number) from inset F and F′ was calculated in G. Mean gray intensity × area was also calculated from same inset as shown in H. To calculate the intensity we compared cell with high fluorescence intensity for GRHL2 and ABCA3 in IPF with alveolar cells in normal type II cells.
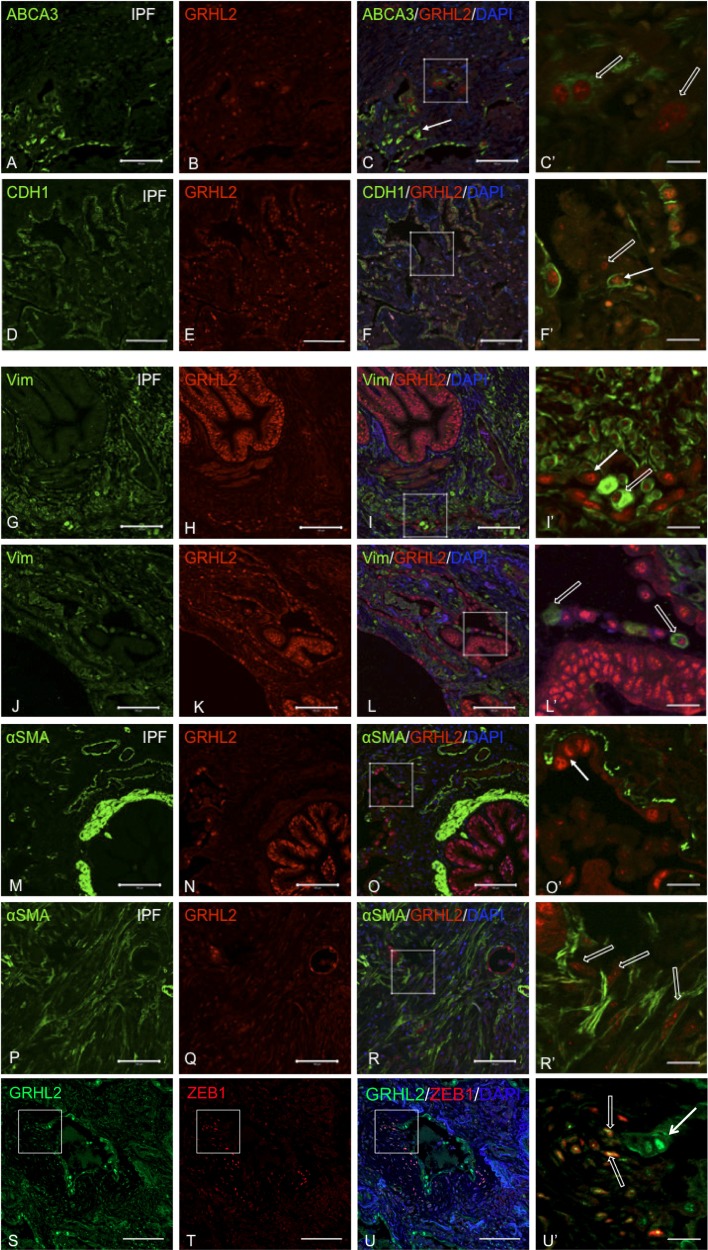
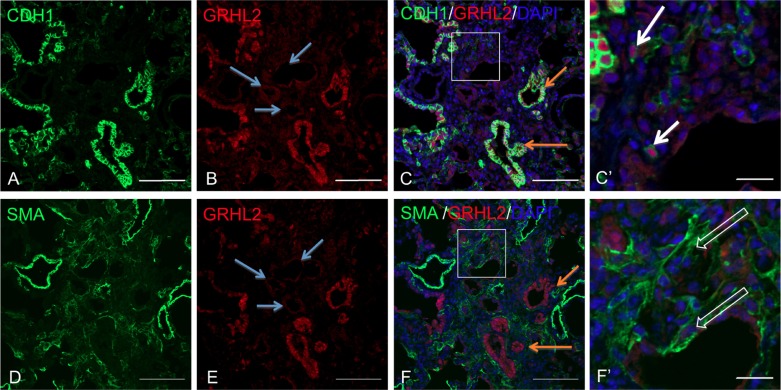
We further explored the distribution of GRHL2 along with the AEC marker, ABCA3 (Fig. 4, A–C and C′). We observed cell plasticity events (open white arrow in C′) relating to AEC flattening at the luminal surface as well as within dense fibrotic regions of IPF lungs (Fig. 4, D–F and F′), suggesting that these cell-flattening events are not restricted to epithelial cells that may be shedding into the lumen. We have previously reported that, as mouse alveolar type II cells from Sftpc GFP mice flatten in vitro, levels of GRHL2 and CDH1 drop substantially (40). By knocking down GRHL2 in lung epithelial MLE15 cells, epithelial markers such as Cldn4 and Cdh1 were also downregulated (40), as also shown in Fig. 9B. In the present study, by double staining GRHL2 with CDH1 (Fig. 4, G–I and I′), we confirmed cell-flattening events (shown in open white arrows) in IPF lung. We also observed AEC, which were low for GRHL2 fluorescence in the lumen and lacked CDH1 signal or showed very low levels of CDH1 fluorescence. This expression pattern of CDH1 correlated with the expression pattern of ABCA3. To further evaluate whether these cells were undergoing cell-flattening events, we also double stained GRHL2 with mesenchymal markers such as Vimentin (VIM) (Fig. 4, J–L and L′), α-smooth muscle actin (α-SMA) (Fig. 4, M–R and O′ and R′), and ZEB1 (Fig. 4, S–U and U′) in IPF lungs. In the epithelial layer surrounding distal lumen or in collapsing alveolar epithelium we observed both GRHL2- and VIM-positive cells but never detected GRHL2 and α-SMA-positive cells. GRHL2- and α-SMA-positive cells were only found within certain densely fibrotic regions (Fig. 4, P–R and R′). It should also be noted that we observed several cell-flattening events in alveolar epithelium as shown by open arrows in Fig. 4, C′, F′, I′, and L′, but rarely in airway epithelium (Fig. 4, L and O). Double staining of GRHL2 and ZEB1, an activator of EMT (Fig. 4 S–U and U′) also revealed several fibroblast-like cells positive for both GRHL2 and ZEB1 (open white arrows in U′) as well as AEC, which showed only Grhl2 stain but not ZEB1.
Fig. 4.
Colocalization of GRHL2 with epithelial and mesenchymal markers in IPF lung suggests cell-flattening events. Double staining of GRHL2 and ABCA3 (A–F) or CDH1 (G–I) or Vimentin (VIM; J–I) or α-smooth muscle actin (α-SMA; M–R) or ZEB1 (S–U). C′, F′, I′, L′, O′, R′, and U′ are insets of their respective images. White arrow in F, I′, L′, and O′ indicates epithelial nature of cells whereas open arrow in C′, F′, and I′ indicates cell flattening in collapsing alveolar regions. In R′, open arrows show myofibroblasts distant from collapsing alveoli. Scale bar for A–U is 100 μm and for C′, F′, I′, L′, O′, R′, and U′ it is 20 μm.
Fig. 9.
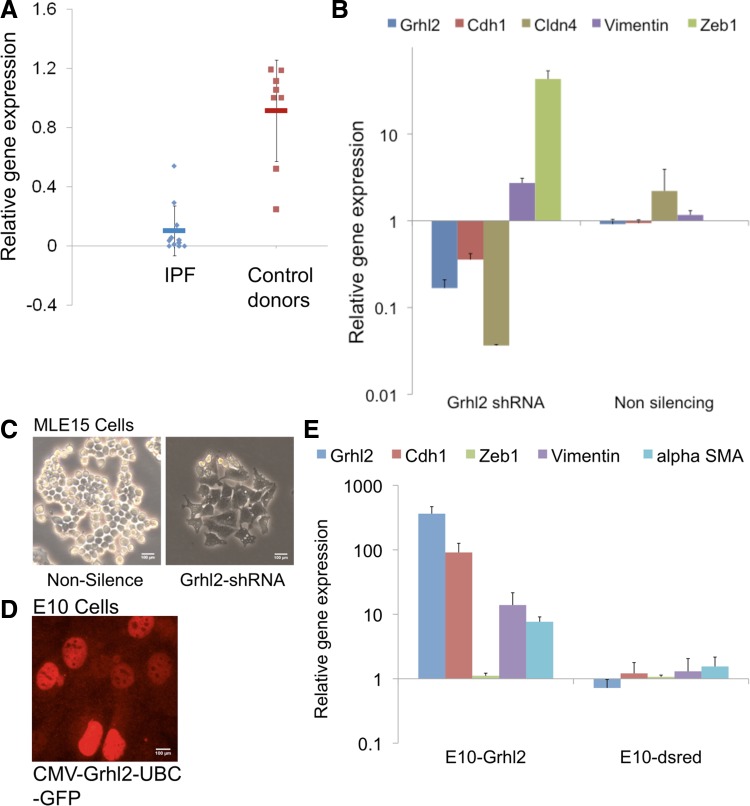
Altered expression level of GRHL2 in human IPF in vitro manipulation of Grhl2 in mouse epithelial cells lines highlights the functional role of GRHL2 in IPF. A: GRHL2 expression level in IPF patients showed substantial decrease compared with non-IPF donors. GAPDH was used as endogenous control for normalization. B: epithelial and mesenchymal expression level in Grhl2 knockdown studies in MLE15 cells showed decrease in epithelial genes such as Cdh1 and Cldn4 but showed increased expression of Vim and Zeb1. C: cell morphology of MLE15 cells transduced with nonsilencing (Nonsilence) and Grhl2 shRNA. Grhl2 knockdown in MLE15 shows cell flattening of MLE15 after 92 h of incubation. D: Grhl2 staining in E10 cells transduced with CMV-GRHL2-UBC-GFP to show that stable transfections and expression of Grhl2. E: overexpression of Grhl2 in E10 cells showed upregulation of Grhl2 and Cdh1 with modest increase in mesenchymal genes. E10 cells were either transduced with lentivirus containing CMV-Grhl2-UBC-GFP (E10-Grhl2) or with empty vector (CMV-dsred-UBC-GFP). GRHL2 expression studies were performed with 11 IPF patients and 8 non-IPF donors. Grhl2 knockdown and overexpression studies were performed in triplicate.
There have been recent reports that basal cells may be involved during lung injury repair and regeneration (28), so we double stained GRHL2 with β-tubulin-4 to observe the distribution of ciliated cells in IPF lung and with p63 (images available on request) to observe any changes in the distribution of basal cells. Tiling with confocal imaging of the entire section was performed. In Fig. 6, we show a representative image from one section. We did not observe any changes in distribution of ciliated cells in airway epithelium, as indicated by the red arrow, nor did we observe any expansion of basal cells as indicated by red arrows. We also did not observe any p63-positive cells in any other distal areas of the IPF sections and observed only limited cell-flattening events in more proximal airways, corroborating previous histological studies.
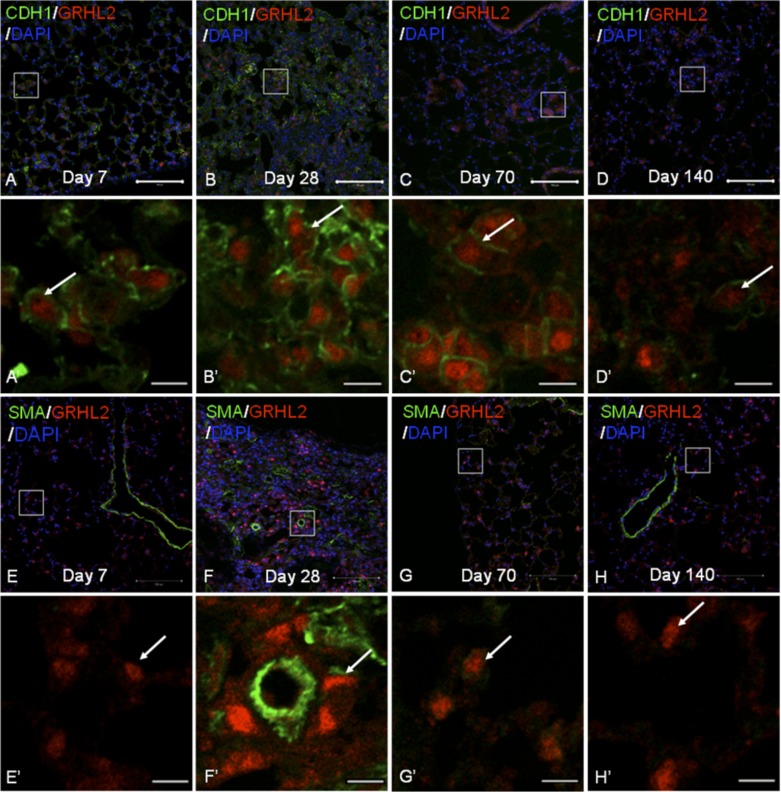
Fig. 6.
Grhl2 distribution in bleomycin-induced injury model does not show an overlap with α-SMA. A: double staining of Grhl2 and its target E-cadherin (Cdh1) at different time points during bleomycin injury model (A–D) and with Grhl2 and α-SMA (E–H). A′-H′ are insets of A–H, respectively. Scale bar for A–H is 100 μm, and for A′ to H′ it is 10 μm.
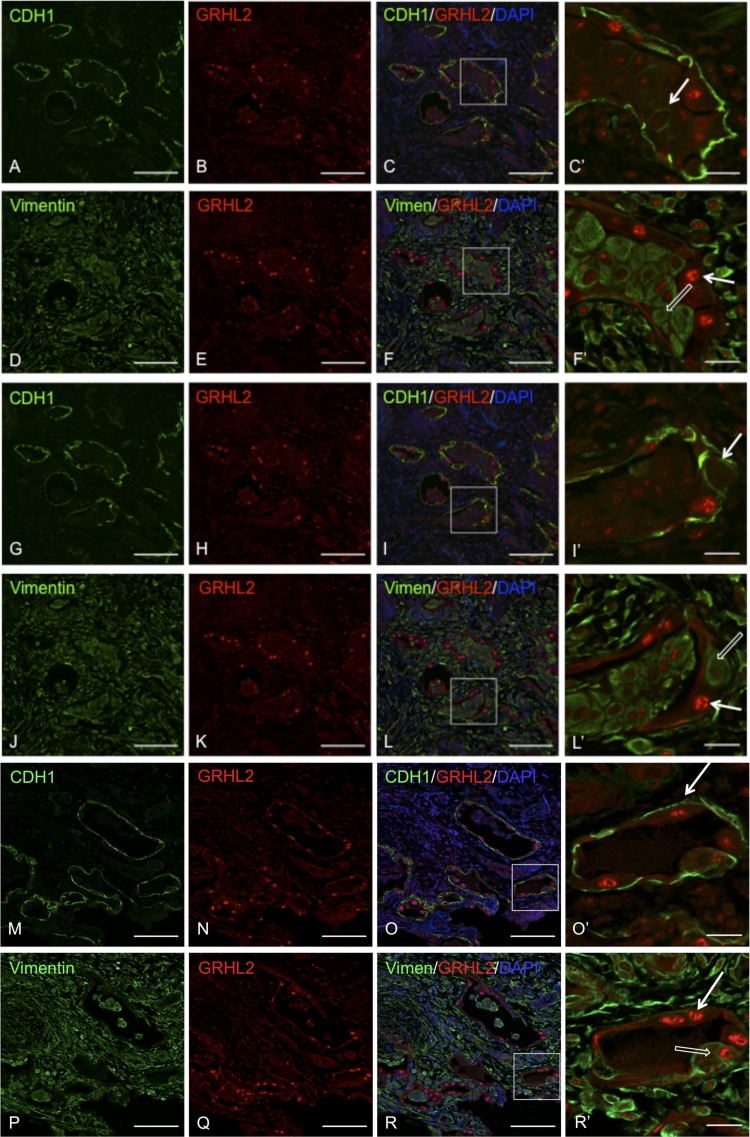
To ascertain that cell-flattening events occurred within the same cell, we performed immunofluorescence of GRHL2 with CDH1 or with VIM in serial sections. We performed tiling of the entire IPF lung sections and found several interesting pathophysiological features. Figure 5 shows hyperplastic AEC lining honeycomb-like structures in IPF lung. We show two sets of regions, A–I and J–R, in which GRHL2-CDH1 and GRHL2-VIM double staining have been performed in IPF lung sections. The first two sets of panels, A–F and G–L, are from the same region but show different insets to illustrate cells in the lumen that express both CDH1 (white arrows) and VIM (open white arrow), suggesting cell plasticity events related to cell flattening. In M–R we show another region where cells in the AEC layer show both epithelial (white) and mesenchymal (open white arrows) markers, suggesting that cell plasticity events also occur in the epithelial layer. Another important feature was altered distribution of CDH1 in hyperplastic AEC, with excessive distribution of CDH1 in the basal region (white arrow in Fig. 5O′) rather than the lateral region. This was suggestive of alteration of apical-to-basolateral polarity and disruption of adherens junctions between AEC. These results collectively show cell-flattening events within hyperplastic AEC in IPF.
Fig. 5.
Colocalizaton of GRHL2 with epithelial and mesenchymal markers in serial sections shows cell-flattening events. Double staining of GRHL2 and CDH1 (A–C, G–I, and M–O). Double staining of GRHL2 and VIM in IPF lung is shown in (D–F, J–L, and P–R. C′, F′, L′, O′, and R′ are insets of their respective images. In C′, I′, and O′ white arrow indicates a subtype of epithelial cells that show bright GRHL2 and CDH1 signals, whereas in F′, L′, and R′ white open arrow indicates another subtype that has both CDH1 and VIM signals. Insets do not show DAPI for clarity. Scale bar for A–R is 100 μm and for C′, F′, I′, O′, and R′ it is 20 μm.
GRHL2 expression pattern in mouse models of fibrosis reveals distinct differences from IPF.
Three mouse models of pulmonary fibrosis were compared for Grhl2 expression pattern. These were 1) IP bleomycin-induced lung injury (9), 2) spontaneously occurring pulmonary fibrosis in the HPS1/2 model (24), and 3) overexpression of activated form of Foxm1 followed by irradiation, which leads to pulmonary fibrosis (4). As shown in Fig. 6, in the IP bleomycin model we examined the expression of Grhl2 along with Cdh1 (A–D) or with α-SMA (E–H) during the course of injury at days 7, 28, 70, and 140. At day 28, when the fibrotic response was at its peak, we observed a higher number of AEC showing more Grhl2 fluorescence (white arrows in insets) and also for its target Cdh1. The increased fluorescence intensity for Grhl2 was also observed at day 70 when the fibrotic response starts to resolve, whereas by day 140, when there is no longer fibrosis, the fluorescence intensity of Grhl2 appeared to be back at basal levels. Grhl2 and α-SMA double staining during the course of bleomycin injury (Fig. 6, E–H) showed no overlap of Grhl2 expression pattern with α-SMA expression. At day 28, when the fibrotic response was at its peak, we were unable to observe two distinct populations of AEC based on fluorescence intensity of Grhl2. AEC cell shape appeared altered within fibrotic regions. Gene expression analysis of lung from various time points of injury and repair vs. controls (treated with PBS) did not show significant changes in Grhl2 levels (data not shown).
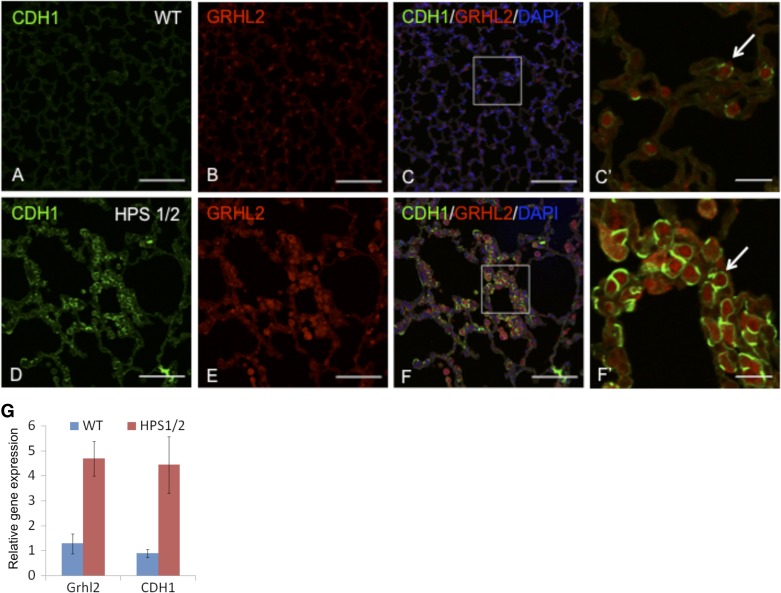
In HPS1/2 mutants, we double stained Grhl2 with its target Cdh1 and observed changes in expression level and their pattern compared with wild-type lung (Fig. 7). We observed that the AEC (shown by white arrow) appear larger with altered morphology consistent with previous observations (24). We also observed substantial increases in Grhl2 and Cdh1 fluorescence levels in HPS1/2 mice (C′) compared with wild-type (Fig. 7F). Gene expression analysis from the same paraffin sections showed increased expression of Grhl2 and Cdh1 (Fig. 7G), thus validating the immunofluorescence signals.
Fig. 7.
Grhl2 expression pattern in double-mutant HPS1 and HPS2 (HPS1/2) lung tissue is altered. Double staining of Grhl2 and its target (Cdh1) in normal adult lung tissue (A–C) and HPS1/2 lung tissue (D–F). C′ and F′ are insets. White arrow in C′ and F′ indicate epithelial cells. Insets do not show DAPI for clarity. Scale bar for A–F is 100 μm and for C′ and F′ it is 20 μm. G: gene expression analysis of Grhl2 and Cdh1 was performed in triplicates from the same paraffin sections after recovery of RNA as described in materials and methods. Gapdh was used as endogenous controls.
In radiation-induced pulmonary fibrosis with Foxm1 misexpression, we double stained Grhl2 and Cdh1 (Fig. 8, A–C and C′) and Grhl2 and α-SMA (Fig. 8, D–F and F′). In this model we observed a higher fibrotic response than that noticed following IP bleomycin. Airway epithelium, as shown in serial sections in Fig. 8, C and F, exhibited high fluorescence signals for Grhl2 and Cdh1 but no signal for α-SMA. Several collapsing alveoli were observed within fibrotic regions with differential Grh2 fluorescence intensities in AEC. Epithelial cells with intense fluorescence for Grhl2 are shown in white arrow in Fig. 8C′. AEC in which Grhl2 fluorescence intensity is very low are shown with open white arrows in Fig. 8F′). There were several collapsing alveoli, which had completely lost epithelial markers, Grhl2 and Cdh1, but were expressing myofibroblast marker, α-SMA (shown with blue arrows in Fig. 8, B and E). Gene expression analysis of lung samples did not show significant changes in levels of Grhl2 (data not shown).
Fig. 8.
Grhl2 expression pattern in serial section of lung from irradiation mouse model. Double staining of Grhl2 and Cdh1 is shown in A–C and C′. Double staining of Grhl2 and α-SMA is shown in D–F and F′. C′ and F′ are insets. White arrows in C′ and open arrows in F′ indicate alveolar epithelial cells (AEC) in same region is undergoing cell-flattening events. Blue arrows show collapsed alveolar spaces with AEC showing low Grhl2 fluorescence intensity. Orange arrows in C and F show airway epithelium with high GRHL2 and Cdh1 signal but no α-SMA signal. Scale bar for A–F is 100 μm, and for C′ and F′ it is 20 μm.
Grhl2's roles in epithelial integrity and epithelial cell plasticity.
From the immunofluorescence studies in human IPF we speculated that GRHL2 expression level must be compromised within areas of fibrotic tissue, since in IPF alveolar spaces appear to be overwhelmed with fibroblasts and myofibroblasts. Hence we compared the levels of Grhl2 in 11 IPF specimens with eight control lung donor specimens (Fig. 9A). Consistent with the above speculation, we observed significantly decreased levels of GRHL2 expression within fibrotic tissue.
To further demonstrate that Grhl2 indeed has a role in epithelial cell plasticity and to validate our fluorescence intensity and distribution data in IPF lung sections we performed knockdown vs. overexpression studies of Grhl2 in the cuboidal alveolar epithelial type II-like MLE15 cell line compared with mesenchymal-like E10 cells, respectively (Fig. 9). We had previously observed downregulation of epithelial genes after knockdown of Grhl2 in mouse alveolar MLE15 cells (40). Here, we also consistently observed downregulation of epithelial markers when Grhl2 was knocked down by using another shRNA (pKLO series) (Fig. 9A). However, EMT markers such as Vim and Zeb1 were upregulated, but α-SMA was not detected in MLE15 cells. We had previously concluded that Grhl2 knockdown in MLE15 cells results in cell flattening by showing phalloidin staining (40). In the present work, by using a different Grhl2-shRNA to knock down Grhl2, we observed even more pronounced cell-flattening events in MLE15 cells (Fig. 9C). We had previously shown that E10 cells do not express Grhl2 and also had shown that overexpression of Grhl2 in flat E10 cells resulted in a less flattened morphology (40). Here, in Fig. 9D we confirmed that GRHL2 is expressed in E10 cells transduced with CMV-Grhl2-UBC-GFP vector, by immunofluorescence staining. We also now show that overexpression of Grhl2 in E10 cells leads to increased CDH1 expression but no significant downregulation of mesenchymal genes (Fig. 9E). We did in fact observe a modest increase in mesenchymal markers, suggesting that E10 cells can express markers of both epithelial and mesenchymal lineages in the presence of high levels of Grhl2.
DISCUSSION
Herein we have discovered some interesting pathophysiological differences between human IPF lung and three mouse models of pulmonary fibrosis: 1) IP bleomycin-induced model, 2) spontaneously occurring pulmonary fibrosis in HPS1/2 mutant mice, and 3) radiation-induced fibrosis in which the activated form of Foxm1 is misexpressed. We also showed for the first time evidence of involvement of GRHL2 in IPF.
We observed that GRHL2 distribution and expression levels, based on immunofluorescence, were clearly altered in IPF lung compared with normal adult lung. Previously it has been demonstrated by gene expression studies that IPF lung is characterized by aberrant activation of developmental pathways including signaling pathways involved in epithelium and mesenchymal cross talk and epithelial plasticity (18, 32). Hence we thought it important to characterize and compare the distribution of GRHL2 during lung development as well as in adult human lung. This analysis showed interesting differences in the distribution of GRHL2 in embryonic, fetal, and adult human lung.
Although we observed high expression of GRHL2 based on fluorescence intensity in embryonic and fetal tissues, the fluorescence levels were relatively diminished in human adult lung epithelium. This developmentally dynamic expression pattern of GRHL2 was validated in human lung by gene expression analysis at the fetal stage (week 20) vs. normal adult lung as well as being confirmed at several stages of mouse lung development (data available on request).
Within IPF lung we further observed both high fluorescent intensity of GRHL2 in collapsing alveolar epithelium, together with a surprising ectopic expression in mesenchyme, rather similar to the expression pattern observed at human embryonic weeks 9–11. We speculate that increased fluorescent intensity of GRHL2 in lung epithelium and mesenchyme may reflect active tissue remodeling in IPF. Thus altered GRHL2 distribution and increased fluorescence intensity may have the future potential to be used as a possible diagnostic biomarker in combination with other putative signatures for IPF. Developmental programs are also activated in cancers during tumor progression and metastatic events. GRHL2 has been associated with breast cancer metastasis and tumor progression and also with other cancers (7, 8, 13, 19, 21, 37, 42–44). A similar systematic distribution and expression analysis of GRHL2 in lung cancer has yet to be performed.
A current model of IPF suggests that compromised epithelial integrity is a major component of pathogenesis and may in fact initiate the fibrotic response (16, 20, 29, 34, 39). This model is supported by studies demonstrating that pulmonary fibrosis can occur following the ablation of factors that normally maintain epithelial integrity without any preceding inflammation (25, 39, 45). The model suggests that chronic injury results in the activation of a subset of bronchial and AEC that become hyperplastic with enlarged cell shape, whereas another subset of alveolar type II cells that are sensitive to chronic injuries undergo cell-flattening events. Herein, and as summarized in the working model in Fig. 10, we were able to document these two subsets of AEC based on relative fluorescent intensities of GRHL2 and ABCA3. This model of IPF pathogenesis also suggests that a compromise in epithelial integrity during IPF results in cell flattening of AEC. Previously we showed that knockdown of GRHL2 leads to disruption of epithelial integrity, with cell flattening of AEC-like MLE15 cells, which suggested a functional role for Grhl2 in IPF. Now, by comparing GRHL2 fluorescence levels with epithelial marker CDH1 vs. mesenchymal markers VIM and SMA, we identified actual cell-flattening events in human AEC in IPF.
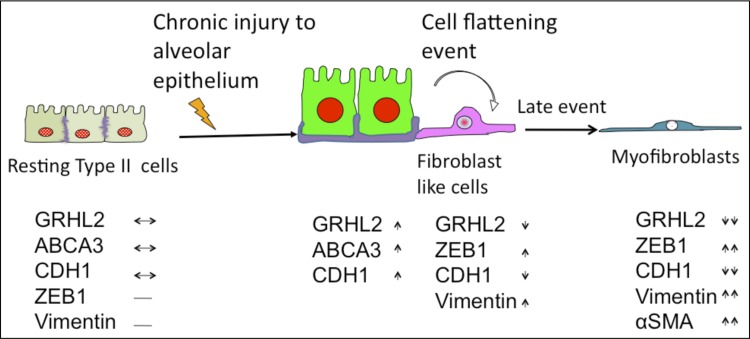
Fig. 10.
Proposed model of progression of morphological changes in AECs in human IPF. On the basis of our present findings and information from the literature, we propose the following model of progression of morphological changes in AECs in IPF. Resting AEC in normal adult express low levels of GRHL2 (light red color in the nucleus) and CDH1 (shown at the junction in purple). Chronic injury due to environmental factors such as smoking and other unknown factors activates AECs, which release excessive amounts of profibrotic mediators, become bigger in size, and express high levels of GRHL2 (shown by red nucleus) and ABCA3 (shown as green cytoplasm). Meanwhile another subset of AEC found in human IPF, which are seen both in epithelial lumen or in dense fibrotic regions, undergo cell-flattening events, characterized by expression of low levels of punctate GRHL2 (shown in diffused red color) in the nucleus with little or no detection of CDH1, but that express VIM (shown as pink color in the cytoplasm). GRHL2 and α-SMA double-positive cells are not found in epithelial lumen or regions close to collapsing alveoli. These double-positive cells, which appear myofibroblast-like, are only found in dense fibrotic regions and may be a late event in the progression of IPF. Bottom panel summarizes our observations made for expression pattern of various markers of epithelium and mesenchyme (↔ basal expression level; ↓ lower expression; ↑ high expression; ↑↑ very high expression; ↓↓ very low expression; − nondetectable or absent).
Furthermore, by analyzing the expression level of epithelial vs. mesenchymal genes in Grhl2 knockdown vs. overexpression studies, we provide further evidence of its role in cell plasticity in alveolar MLE15 epithelial cells. In addition, a decreased level of Grhl2 transcripts in fibrotic tissue from IPF patients is consistent with the notion that epithelial integrity-maintaining genes are downregulated in the fibrotic areas of IPF lung and are likely to be important in the pathogenesis of the disease. It should be further noted that the overall decreased levels of Grhl2 in fibrotic tissue may not represent the elevated expression pattern of Grhl2 specifically within collapsing epithelium. Future studies employing laser capture microscopy of activated AEC followed by single cell gene expression analysis will be required to settle this issue.
Several signaling pathways are activated in IPF and mouse models of pulmonary fibrosis, like Notch, Wnt/β catenin, and AKT/PI3 kinase signaling (32). In our previous work on knockdown studies of Grhl2 in AEC-like MLE15 cells we showed by microarray and with further validation by qRT-PCR that Notch1 levels were compromised (40). We have also performed chromatin immunoprecipitation (ChIP) DNA deep sequencing analysis in AEC-like MLE15 cells and isolated lung buds at E11.5 to show that GRHL2 may regulate the above-mentioned pathways (S. Varma, unpublished data), giving us a possible further clue to its functional role in IPF and other lung diseases. The TGF-β signaling pathway, which is crucial in pathogenesis of IPF and which also can induce AEC flattening events, is known to be associated with the GRHL2 transcriptional regulatory network (8). In fact, GRHL2 can negatively regulate the TGF-β pathway by targeting downstream factors such as ZEB1 (8). In recent work by Gao et al. (15), several direct targets of GRHL2 were determined in human mucociliary epithelium by integrating GRHL2 ChIP sequencing and differential expression analysis by RNA deep sequencing. Thus there is a considerable amount of evidence emerging both from our work presented here as well as from that of others supporting a putative functional role of GRHL2 in pathogenesis IPF and other lung injuries.
In this study both bleomycin-induced fibrosis and the spontaneous HPS1/2 fibrosis model showed similarities to IPF in terms of increased Grhl2 fluorescence levels in AEC, suggesting a common epithelial wounding response. However, there was an important difference in that detectable levels of Grhl2 were only found within the mesenchyme of IPF lungs, but not in these mouse models of pulmonary fibrosis. We also did not find double-positive cells for Grhl2 and mesenchymal markers in these mouse models, suggesting that cell-flattening events may be occurring in mouse lung injury models and that this may be an important difference with what is happening in human IPF.
Interestingly, the radiation-induced fibrosis mouse model, in which an activated form of Foxm1 is misexpressed in AEC, did show an overlap of epithelial and mesenchymal markers within the same cells, thus enforcing the idea that cell-flattening events can occur in AECs under certain specific circumstances. Moreover, our observation of two subsets of AEC based on Grhl2 fluorescence level and its presence in flattened cells suggests that this irradiation model may be a closer replica of the pathogenesis of human IPF.
In summary, this report provides evidence in favor of the epithelial initiation model of IPF and supports the concept that epithelial injury and subsequent disintegrity of the epithelium may be the most crucial early step during the pathogenesis of IPF. Our work also sheds light on the controversial subject of epithelial-mesenchymal plasticity, by showing that cell flattening of AEC does indeed exist and that this may contribute to formation of fibrotic foci in human IPF. We have also described a novel distribution pattern of GRHL2 in early embryonic lung development as well as in fetal and adult human lung. Moreover, we show important differences between most mouse and human models of lung fibrosis with regard to Grhl2 patterns of expression.
GRANTS
This study was funded by a postdoctoral fellowship granted to S. Varma by the California Institute of Regenerate Medicine (CIRM) through a training grant to D. Warburton and by R01 HL083034 to M. I. Ramirez and S. Varma.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.V., M.I.R., and D.W. conception and design of research; S.V., P.M., S.S., L.C., T.H., A.E.R., D.R., J.L., T.V.K., V.V.K., A.G., M.I.R., A.P., and M.S. performed experiments; S.V., S.S., T.H., M.I.R., and M.S. analyzed data; S.V., M.S., and D.W. interpreted results of experiments; S.V., S.S., T.H., and M.I.R. prepared figures; S.V. drafted manuscript; S.V., P.M., S.S., D.R., J.L., A.G., M.I.R., M.S., and D.W. edited and revised manuscript; S.V., P.M., and D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Esteban Fernandez in the imaging core facility at Children's Hospital Los Angeles for giving useful tips for confocal image processing. We also thank many colleagues at the Saban Research Institute for providing us with aliquots of some of the primary antibodies used in this work. We thank Dr. Denise Al Alam's laboratory for preparing sections of fetal lung. We also thank Caroline Sherf for contribution in obtaining human fetal lung tissue.
REFERENCES
- 1.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Auden A, Caddy J, Wilanowski T, Ting SB, Cunningham JM, Jane SM. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns 6: 964–970, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Balli D, Ren X, Chou FS, Cross E, Zhang Y, Kalinichenko VV, Kalin TV. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene 31: 3875–3888, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balli D, Ustiyan V, Zhang Y, Wang IC, Masino AJ, Ren X, Whitsett JA, Kalinichenko VV, Kalin TV. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J 32: 231–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Dong Q, Shin KH, Kim RH, Oh JE, Park NH, Kang MK. Grainyhead-like 2 enhances the human telomerase reverse transcriptase gene expression by inhibiting DNA methylation at the 5′-CpG island in normal human keratinocytes. J Biol Chem 285: 40852–40863, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Xiao Liu Z, Oh JE, Shin KH, Kim RH, Jiang M, Park NH, Kang MK. Grainyhead-like 2 (GRHL2) inhibits keratinocyte differentiation through epigenetic mechanism. Cell Death Dis 3: e450, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, Wang P, Yang S, Yang Y, Zhang Q, Zhang W, Xiao H, Gao H. Identification of genes with a correlation between copy number and expression in gastric cancer. BMC Med Genomics 5: 14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieply B, Riley P, 4th, Pifer PM, Widmeyer J, Addison JB, Ivanov AV, Denvir J, Frisch SM. Suppression of the epithelial-mesenchymal transition by Grainyhead-like-2. Cancer Res 72: 2440–2453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 287–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol 163: 141–172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci 341: 444–449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L442–L452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dompe N, Rivers CS, Li L, Cordes S, Schwickart M, Punnoose EA, Amler L, Seshagiri S, Tang J, Modrusan Z, Davis DP. A whole-genome RNAi screen identifies an 8q22 gene cluster that inhibits death receptor-mediated apoptosis. Proc Natl Acad Sci USA 108: E943–E951, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin S, Jane SM, Darido C. The planar cell polarity pathway in vertebrate epidermal development, homeostasis and repair. Organogenesis 7: 202–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Vockley CM, Pauli F, Newberry KM, Xue Y, Randell SH, Reddy TE, Hogan BL. Evidence for multiple roles for grainyheadlike 2 in the establishment and maintenance of human mucociliary airway epithelium. Proc Natl Acad Sci USA 110: 9356–9361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev 21: 152–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175: 3–16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie WD, Hagood JS, Dave V, Perl AK, Whitsett JA, Korfhagen TR, Glasser S. Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle 9: 2769–2776, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang X, Chen W, Kim RH, Kang MK, Park NH. Regulation of the hTERT promoter activity by MSH2, the hnRNPs K and D, and GRHL2 in human oral squamous cell carcinoma cells. Oncogene 28: 565–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Kokoszynska K, Ostrowski J, Rychlewski L, Wyrwicz LS. The fold recognition of CP2 transcription factors gives new insights into the function and evolution of tumor suppressor protein p53. Cell Cycle 7: 2907–2915, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kropski JA, Lawson WE, Young LR, Blackwell TS. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis Model Mech 6: 9–17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahavadi P, Guenther A, Gochuico BR. Hermansky-Pudlak syndrome interstitial pneumonia: it's the epithelium, stupid! Am J Respir Crit Care Med 186: 939–940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahavadi P, Korfei M, Henneke I, Liebisch G, Schmitz G, Gochuico BR, Markart P, Bellusci S, Seeger W, Ruppert C, Guenther A. Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. Am J Respir Crit Care Med 182: 207–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi K, Yanagi S, Kawahara K, Nishio M, Tsubouchi H, Imazu Y, Koshida R, Matsumoto N, Taguchi A, Yamashita S, Suzuki A, Nakazato M. Epithelial Pten controls acute lung injury and fibrosis by regulating alveolar epithelial cell integrity. Am J Respir Crit Care Med 187: 262–275, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol 34: 1534–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Selman M, Pardo A. Alveolar epithelial cell disintegrity and subsequent activation: a key process in pulmonary fibrosis. Am J Respir Crit Care Med 186: 119–121, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5: e62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selman M, Pardo A, Richeldi L, Cerri S. Emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs 16: 341–362, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahlman MT, Besnard V, Wert SE, Weaver TE, Dingle S, Xu Y, von Zychlin K, Olson SJ, Whitsett JA. Expression of ABCA3 in developing lung and other tissues. J Histochem Cytochem 55: 71–83, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Stramer B, Martin P. Cell biology: master regulators of sealing and healing. Curr Biol 15: R425–R427, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Kanai F, Tada M, Tateishi R, Sanada M, Nannya Y, Ohta M, Asaoka Y, Seto M, Shiina S, Yoshida H, Kawabe T, Yokosuka O, Ogawa S, Omata M. Gain of GRHL2 is associated with early recurrence of hepatocellular carcinoma. J Hepatol 49: 746–757, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Tsang AR, Wyatt HD, Ting NS, Beattie TL. hTERT mutations associated with idiopathic pulmonary fibrosis affect telomerase activity, telomere length, and cell growth by distinct mechanisms. Aging Cell 11: 482–490, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Tsujino K, Takeda Y, Arai T, Shintani Y, Inagaki R, Saiga H, Iwasaki T, Tetsumoto S, Jin Y, Ihara S, Minami T, Suzuki M, Nagatomo I, Inoue K, Kida H, Kijima T, Ito M, Kitaichi M, Inoue Y, Tachibana I, Takeda K, Okumura M, Hemler ME, Kumanogoh A. Tetraspanin CD151 protects against pulmonary fibrosis by maintaining epithelial integrity. Am J Respir Crit Care Med 186: 170–180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varma S, Cao Y, Tagne JB, Lakshminarayanan M, Li J, Friedman TB, Morell RJ, Warburton D, Kotton DN, Ramirez MI. The transcription factors Grainyhead-like 2 and NK2-homeobox 1 form a regulatory loop that coordinates lung epithelial cell morphogenesis and differentiation. J Biol Chem 287: 37282–37295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varma S, Millen G, Wang J, Hinds A, Kotton D, Ramirez MI. Role of grainyhead-like transcription factors Grhl2 and Grhl3 in morphogenesis of alveolar epithelial cells (Abstract). Am J Respir Crit Care 179: A1871, 2009 [Google Scholar]
- 42.Xiang X, Deng Z, Zhuang X, Ju S, Mu J, Jiang H, Zhang L, Yan J, Miller D, Zhang HG. Grhl2 determines the epithelial phenotype of breast cancers and promotes tumor progression. PLoS One 7: e50781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan GR, Xu SH, Tan ZL, Liu L, He QY. Global identification of miR-373-regulated genes in breast cancer by quantitative proteomics. Proteomics 11: 912–920, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Vasudevan P, Parekh V, Penev A, Cunningham JM. Bridging cancer biology with the clinic: relative expression of a GRHL2-mediated gene-set pair predicts breast cancer metastasis. PLoS One 8: e56195, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Zhang Y, Tao B, Teng L, Li Y, Cao R, Gui Q, Ye M, Mou X, Cheng H, Hu H, Zhou R, Wu X, Xie Q, Ning W, Lai M, Shen H, Feng GS, Ke Y. Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB J 26: 2338–2350, 2012 [DOI] [PubMed] [Google Scholar]