Abstract
Body temperature increases when individuals experience salient, emotionally significant events. There is controversy concerning the contribution of nonshivering thermogenesis in brown adipose tissue (BAT) to emotional hyperthermia. In the present study we compared BAT, core body, and brain temperature, and tail blood flow, simultaneously measured, to determine whether BAT thermogenesis contributes to emotional hyperthermia in a resident Sprague-Dawley rat when an intruder rat, either freely-moving or confined to a small cage, is suddenly introduced into the cage of the resident rat for 30 min. Introduction of the intruder rat promptly increased BAT, body, and brain temperatures in the resident rat. For the caged intruder these temperature increases were 1.4 ± 0.2, 0.8 ± 0.1, 1.0 ± 0.1°C, respectively, with the increase in BAT temperature being significantly greater (P < 0.01) than the increases in body and brain. The initial 5-min slope of the BAT temperature record (0.18 ± 0.02°C/min) was significantly greater (P < 0.01) than the corresponding value for body (0.10 ± 0.01°C/min) and brain (0.09 ± 0.02°C/min). Tail artery pulse amplitude fell acutely when the intruder rat was introduced, possibly contributing to the increases in body and brain temperature. Prior blockade of β3 adrenoceptors (SR59230A 10 mg/kg ip) significantly reduced the amplitude of each temperature increase. Intruder-evoked increases in BAT temperature were similar in resident rats maintained at 11°C for 3 days. In the caged intruder situation there is no bodily contact between the rats, so the stimulus is psychological rather than physical. Our study thus demonstrates that BAT thermogenesis contributes to increases in body and brain temperature occurring during emotional hyperthermia.
Keywords: body temperature, brain temperature, cutaneous blood flow, stress-induced hyperthermia, fever
in mammals, birds, and reptiles, body temperature may increase when individuals are placed in salient, emotionally significant situations, those relevant to the life and survival of the individual, a response sometimes referred to as “stress-induced hyperthermia” or “psychological fever” (5, 8, 18, 31, 32, 35, 40, 44, 60). The amplitude of the stress-related body temperature increase is similar in animals maintained at thermoneutrality or in a cold environment, so the response is not simply secondary to increased metabolism in skeletal and cardiac muscle. Rather, the temperature increases are initiated from the brain via active central command, supplementing homeostatic thermoregulatory processes. This is readily apparent when a lizard behaviorally increases its temperature by selecting a hotter environment after being released from manual restraint (10). In mammals, salient, emotionally arousing events also trigger vasoconstriction in the thermoregulatory cutaneous vascular beds, potentially contributing to emotional hyperthermia by reducing heat loss from the body (for references see Ref. 36).
Most mammals have the capacity for facultative (nonshivering) thermogenesis such as that generated in brown adipose tissue (BAT), and heat produced by BAT is important for thermoregulation because of the amount of heat produced and because the heated blood flows to the rostral half of the body (12, 55, 56). In arousing hibernators, BAT metabolism accounts for as much as 50% of total energy expenditure (55). Thus BAT clearly has the potential to contribute to emotional hyperthermia, and its dense sympathetic innervation suggests that BAT thermogenesis is under direct central command.
However, very few studies have investigated whether heat produced in BAT actually contributes to emotional hyperthermia. Direct BAT and body temperature measurements with in situ thermistors, together with denervation and pharmacological blockade studies, suggest that hyperthermia due to restraint is partially due to BAT thermogenesis (45, 53, 54), as is the hyperthermia associated with social defeat (31). On the other hand, infrared thermographic measurements of interscapular BAT have been interpreted to suggest that BAT thermogenesis does not contribute to hyperthermia associated with conditioned contextual fear (34, 62).
Here we simultaneously measure BAT, core body, and brain temperatures to determine whether BAT thermogenesis contributes to the emotional hyperthermia that occurs in a resident Sprague-Dawley rat when an intruder Sprague-Dawley rat, either freely moving or confined to a small cage, is suddenly introduced into the home cage of the resident rat. To further assess the possible contribution of BAT thermogenesis to emotional hyperthermia, we determined whether SR59230A, a β3 adrenoceptor antagonist (33, 43, 47), reduces the increases in BAT, body, and brain temperatures in resident rats suddenly confronted with an intruder rat. In all resident rats we also monitored the thermoregulatory vascular bed by continuously measuring tail artery blood flow with a chronically implanted Doppler ultrasonic flow probe. To further confirm that BAT temperature changes occur independently of homeostatic thermoregulation, intruder experiments were conducted in resident rats maintained for 3 days at 11°C (32).
MATERIALS AND METHODS
Animals used and anesthesia for implantation of thermistors and tail artery Doppler probes.
Experiments were conducted in 37 male Sprague-Dawley rats (350–400 g), with procedures approved by the Animal Welfare Committee of Flinders University. The number of animals and their suffering were minimized, and individual animals were sometimes used in different experimental conditions with appropriate consideration of the problem of serial effects (see Measurement of physiological and environmental variables). Animals were instrumented (see Measurement of physiological and environmental variables) under general anesthesia (2% isoflurane in O2, Veterinary Companies of Australia, NSW, Australia). Analgesia (Caprofen, 5 mg/kg sc, Norbrook Laboratories, Melbourne, Australia) and antibiotics (Baytril, 0.1 ml sc, 15 mg/kg sc, Bayer Aust, Pymble, NSW, Australia) were administered. At the conclusion of the experiments animals were humanely euthanized by intraperitoneal injection of Lethabarb (pentobarbitone sodium, 180 mg/kg).
Measurement of physiological and environmental variables.
With the use of general anesthesia as described above, precalibrated thermistor probes (3, 46) were positioned in interscapular BAT near the vein of Sulzer (BAT temperature), in the anterior mediastinum ventral to the trachea (body temperature), and intracranially near the junction of sagittal and transverse sinuses, between the dura mater and the skull bone (brain temperature). We chose this extradural site to minimize traumatic damage associated with intracerebral probes. A Doppler ultrasonic probe (Iowa Doppler Products, Iowa City, IA) was chronically implanted around the base of the tail artery. Insulated wires from the temperature probes and the tail artery Doppler probes were passed subcutaneously and attached to a head socket screwed to the skull. After recovery from anesthesia, the animal was returned to the animal house and individually caged for at least 1 wk before experiments were carried out.
During the experimental period the home cage (35 × 40 × 45 cm) of the resident rat was situated within a ventilated commercial freezer unit, modified so that ambient temperature could be automatically controlled at a preset value (Biomedical Engineering, Flinders University). The freezer unit also isolated the resident rat from external visual and auditory stimuli. The ambient temperature of the unit was 24–26°C except for the cold ambient temperature study (see Intruder experimental design).The resident rat's head socket was connected to recording devices via a flexible cable and counter-balanced swivel (SL12C, PlasticsOne, Roanoke, VA) in the roof of the cage. Temperature signals were passed to a bridge amplifier (Biomedical Engineering, Flinders University) and then digitized (1 Hz) with PowerLab (ADInstruments, Castle Hill, NSW, Australia). The pulsatile tail artery Doppler flow signal was passed to an analyzer (model 200-202, Triton Technology, San Diego, CA) and then digitized (40 Hz) with PowerLab.
Rats were provided with food and water ad libitum. The food container was suspended from a high-frequency response strain gauge, so that it was possible to document the timing of eating and the amount of food eaten (3). A reverse light-dark cycle (lights off at 0700 h and lights on at 1900 h) was used, and the light intensity was recorded with PowerLab. Opening the lid of the freezer suddenly increased the light intensity signal within the cage of the resident rat, precisely documenting the commencement of the intruder period (see Intruder experimental design).In some experiments we used a CCD infrared video camera (DBK 21AF04, The Imaging Source, Taiwan) connected to the Powerlab MLS320/7 capture module to simultaneously record the behavior of the of the resident rat before and during the intruder period.
Intruder experimental design.
Instrumented rats were transferred to the residence cage and housed individually for at least 1 day before the intruder experiments were conducted. During this 24-h period the resident rat displayed episodic ultradian increases in BAT, body, and brain temperatures preceding food intake, with episodes occurring at ∼90-min intervals as we previously reported (3, 46). We introduced the intruder rat during the dark phase ∼20 min after the end of a meal when the rat was inactive and BAT, body, and brain temperatures were at low levels. The lid of the freezer was suddenly opened, the intruder rat was introduced into the cage of the resident rat, and the freezer lid was promptly closed. This process was usually completed within 30 s. After 30 min the freezer lid was again opened and the intruder was quickly removed.
Six categories of intruder-rat conditions were examined: 1) introduction of an unrestrained intruder Sprague-Dawley rat (ambient temperature 24–26°C); 2) introduction of an intruder Sprague-Dawley rat confined to a small (19 × 29 × 12 cm) cage (ambient temperature 24–26°C); 3) introduction of the small (washed) cage without any intruder rat (ambient temperature 24–26°C); 4) introduction of the caged intruder 1 h after administration of the β3 adrenoceptor antagonist SR59230A or vehicle; 5) introduction of the caged intruder Sprague-Dawley rat after ambient temperature had been maintained at 11°C for 3 days and nights; and 6) introduction of the caged intruder to the same resident rat on 3 separate occasions, with each introduction separated by an interval of 3 days.
Drugs.
The β3 adrenoceptor antagonist SR59230A (Tocris Bioscience, Bristol, UK) was sonicated and dissolved in warm water (35–37°C). The resident rat was gently held in the hands by one experimenter and the drug (10 mg/kg in 0.5 ml water) or vehicle was injected into the peritoneal cavity by a second experimenter.
Analysis, graphing, and statistical assessment of the measured signals.
Signals from PowerLab were imported into IgorPro (WaveMetrics, Lake Oswego, OR), and this software was used to analyze and graph the physiological signals. The maximum amplitude of the tail artery Doppler flow signal in sequential 0.25-s bins was calculated using IgorPro. Temperature and tail pulse amplitude traces were graphed as 0.1-Hz signals, with each trace commencing 10 min before introduction of the intruder, and extending for 30 min. Occasional transient artifacts were removed from the traces and replaced with the signal recorded just before or after the artifact using IgorPro software. Preintruder BAT, body, and brain temperature and tail flow pulse amplitudes for each resident rat in each experimental condition were calculated by averaging the signals from 3 to 1 min before introduction of the intruder rat. Postintruder values averaged the signals from 26 to 30 min after introduction of the intruder rat. We used IgorPro to calculate the average slope (°C/min) of the linear fit to the BAT, brain, and body temperature signals for individual resident rats for the period 0–5 min after introduction of the intruder rat.
Statistical analysis was performed using Statview 5 (SAS Institute Cary, NC). Group data were expressed as means ± SE. The statistical significance of mean differences was assessed with analysis of variance (ANOVA); factorial or repeated measures as appropriate (see figure and table legends for details). If the primary ANOVA treatment effects were significant at P ≤ 0.05, post-hoc analysis was performed using Fisher's protected t-test.
RESULTS
BAT, body, and brain temperatures in the resident rat after introduction of an intruder rat at ambient temperature.
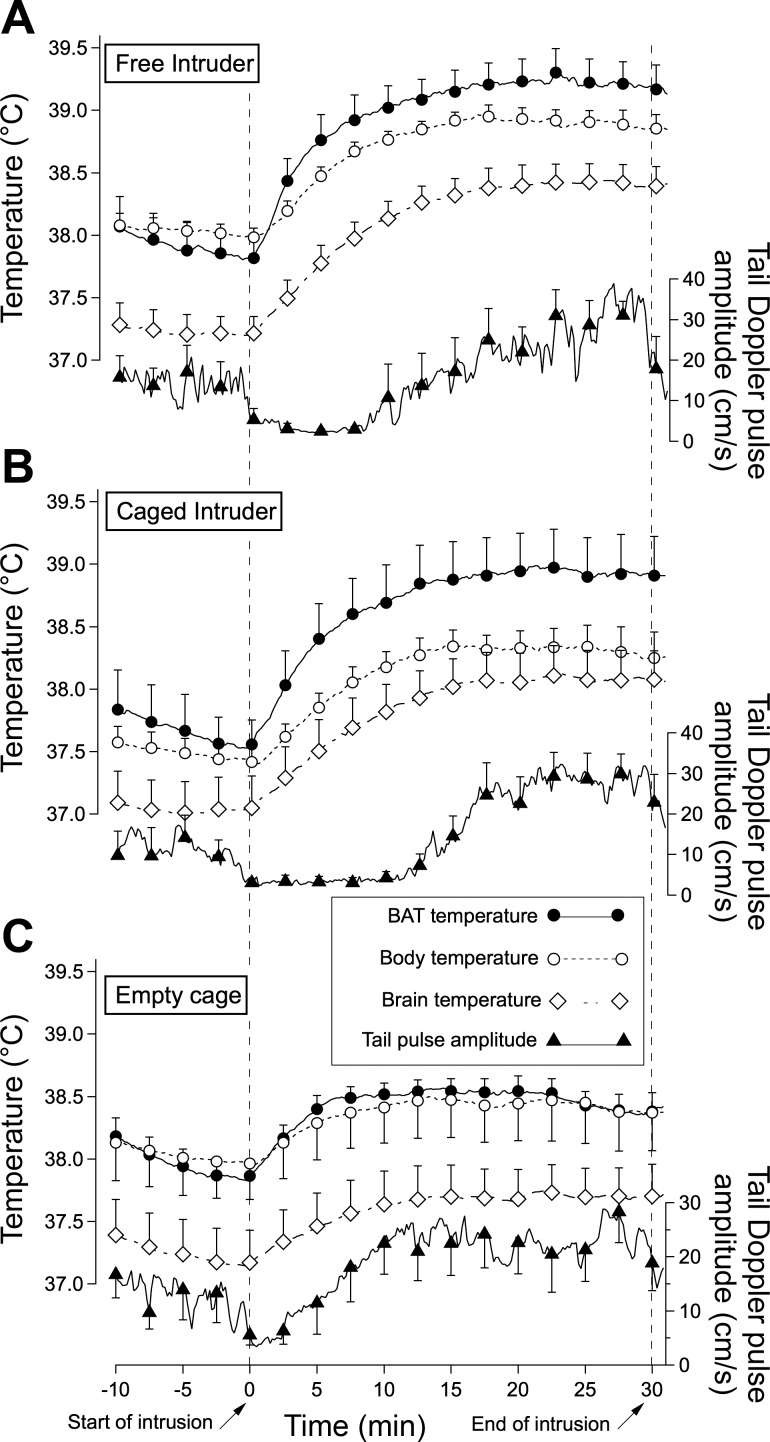
Promptly after introduction of the intruder rat, all three measured temperatures began to increase in the resident rat, both when the intruder was free to move around the cage of the resident rat and when the intruder was confined to a small cage, preventing physical contact with the resident rat (Fig. 1, A and B). Temperatures reached peak values after ∼15 min and remained elevated for the duration of the 30-min intruder period. The infrared video in the free intruder situation showed that both animals ran around the cage, often making physical contact with each other, without obvious fighting behavior. When the caged intruder was introduced, the resident rat observed the cage for a couple of minutes and then climbed over the cage for ∼10 min.
Fig. 1.
Grouped results showing averaged individual computer records of temperatures (original sampling frequency 1 Hz) and tail artery pulse amplitude (original sampling frequency 40 Hz) in the resident rat for preintruder and postintruder periods. Each trace is mean and SE of the original traces, with error bars shown at ∼2.5-min intervals, for clarity. A: intruder rat could move freely around the cage of the resident rat. B: intruder rat was confined to a small cage. C: empty small cage was introduced into the cage of the resident rat. Start and end of the intrusion period is indicated with slanted arrows on the time base. BAT, brown adipose tissue.
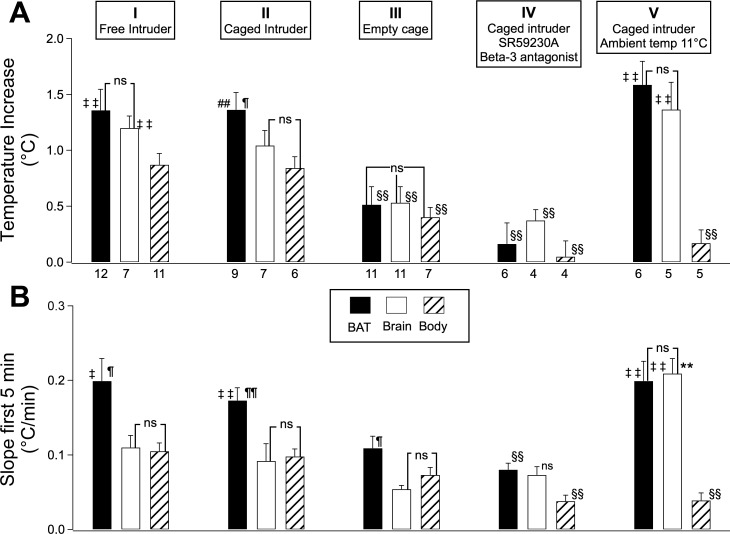
For both the free and caged intruder situations, the amplitude of the increase in BAT temperature was greater than the corresponding value in body temperature, and for the caged intruder the increase in BAT temperature was also greater than the increase in brain temperature (Fig. 2A). The slope of the initial 5-min increase in BAT temperature was greater than the corresponding slope for body and brain temperature in both the free and caged intruder situations (Fig. 2B). In the free intruder situation, the increase in brain temperature was also greater than the increase in body temperature, but these two temperature increases were similar in the caged intruder situation (Fig. 2B). There was no significant difference between body and brain in the initial 5-min slope values for either the free or caged intruder situation (Fig. 2B). Introduction of the empty cage also increased BAT, body, and brain temperatures, but the increases were minor compared with those observed when there was a rat in the cage (Figs. 1C and 2).
Fig. 2.
Group data (means ± SE) of BAT (solid bars), brain (open bars), and body (slanting striped patterns) temperatures (A) and initial 5-min slope values (B) for the five experimental conditions depicted at the top of the figure. Numbers below the columns in A indicate the number of rats in each experimental condition, for both A and B. ‡Significantly greater than corresponding value for body, P < 0.05, repeated measures ANOVA. ‡‡Significantly greater than corresponding value for body, P < 0.01, repeated measures ANOVA. ¶Significantly greater than corresponding value for brain, P < 0.05, repeated measures ANOVA. ¶¶Significantly greater than corresponding value for brain, P < 0.01, repeated measures ANOVA. §§Significantly less than corresponding value for caged intruder at 24–26°C, P < 0.01, factorial ANOVA. **Significantly greater than corresponding value for caged intruder at 24–26°C, P < 0.01, factorial ANOVA. ns, Not significantly different from indicated comparison, P > 0.05, repeated measures ANOVA. In B, column IV, ns defined as not significantly different from indicated comparison, P > 0.05, factorial ANOVA.
BAT, body, and brain temperatures in the resident rat after introduction of a caged intruder rat after pretreatment of the resident rat with β3 adrenergic receptor antagonist SR59230A.
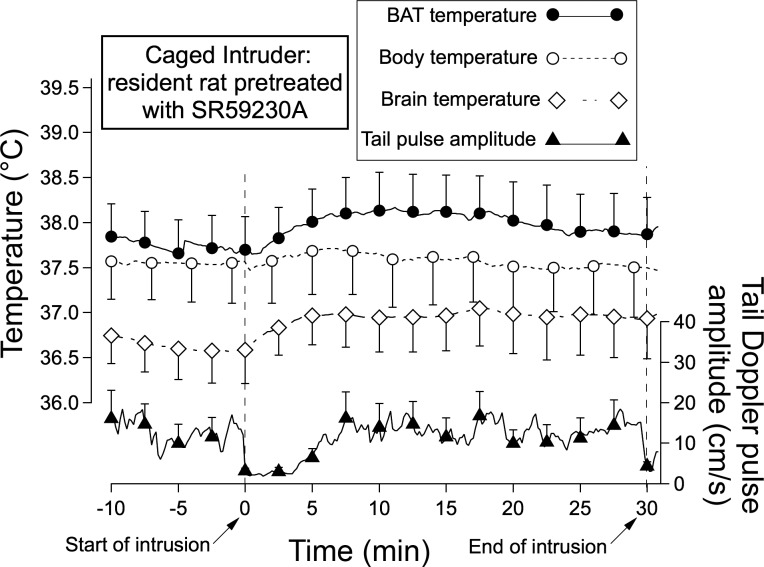
Pretreatment with SR59230A (10 mg/kg ip) 1 h before introduction of the caged intruder substantially reduced the amplitude of the caged intruder-evoked increases in BAT, body, and brain temperature compared with no treatment (Figs. 2, A and B, and 3). SR59230A also substantially reduced the initial 5-min slope of the BAT and body temperature increases, but the slope of the brain temperature increase was not significantly reduced (Fig. 2B). When vehicle was substituted for SR59230A the values for the amplitude and slopes of the temperature increases for BAT, body, and brain were +1.0 ± 0.1°C and 0.15 ± 0.02°C (n = 5), +0.7 ± 0.1°C and 0.07 ± 0.01°C (n = 5), and +0.8 ± 0.1°C and 0.08 ± 0.02°C (n = 4), respectively. There was no significant change in these values (P > 0.05 for all changes, factorial ANOVA) compared with the corresponding no treatment condition.
Fig. 3.
Means and SE of original computer records of temperatures and tail artery pulse amplitude for preintruder and postintruder (caged intruder) periods in resident rats pretreated with the β3 adrenoceptor antagonist SR59230A (10 mg/kg ip) Details of the traces are the same as in Fig. 1.
Pretreatment with SR59230A (10 mg/kg ip) did not prevent the acute fall in tail artery blood flow elicited by the introduction of the intruder, as can be seen in Fig. 3. Tail artery blood flow Doppler signal measured 2.5 min after introduction of the intruder was 3 ± 1 cm/s (n = 5), not significantly different (P > 0.05, factorial ANOVA) from 3 ± 2 cm/s (n = 5), the corresponding values for the no treatment condition.
Introduction of a caged intruder rat after maintaining the resident for 3 days at 11°C.
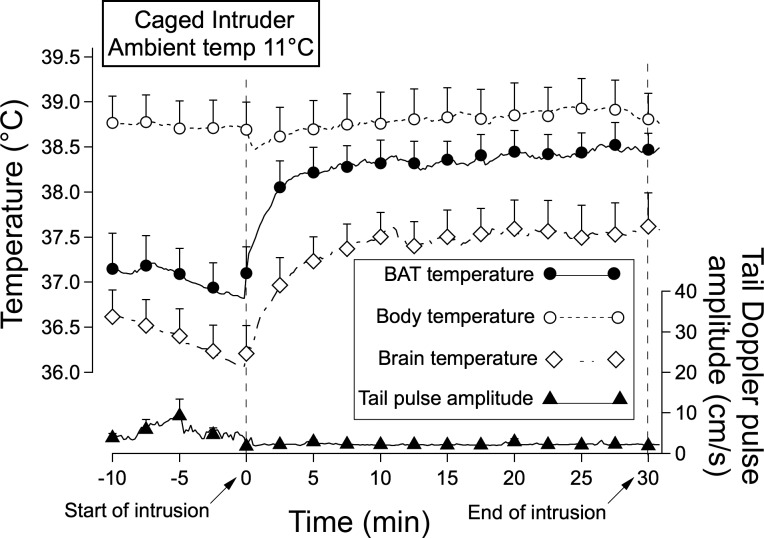
After 3 days at ambient temperature 11°C, rats exhibited ultradian variability in BAT, brain, and body temperature, with episodes commencing ∼15 min before food intake (Blessing W, unpublished data),as previously reported for rats maintained at 24–26°C (2, 3). Intramediastinal body temperature during inactive times of the basic rest-activity cycle was 38.7 ± 0.3°C compared with 37.9 ± 0.1°C in rats maintained at 24–26°C (P < 0.05). In contrast, basal temperature readings for BAT and brain were ∼0.5–1°C lower than corresponding values recorded at ambient temperatures 24–26°C (Figs. 1 and 4).
Fig. 4.
Means and SE of original computer records of temperatures and tail artery pulse amplitude for preintruder and postintruder (caged intruder) periods in resident rats maintained at 11°C ambient temperature for 3 days. Details of the traces are the same as in Fig. 1.
Introduction of a caged intruder rat after 3 days at 11°C substantially increased BAT and brain temperatures, with little or no change in body temperature (Figs. 2A and 4). The amplitude of the BAT and brain temperature increases was similar to the corresponding amplitude recorded at 24–26°C. The time course of the increase in BAT temperature was also similar, so that the initial 5-min slope of the increase was similar to that the corresponding values at 24–26°C ambient temperature (Fig. 2B). The initial slope of the brain temperature increase at 11°C was actually greater than the corresponding slope at 24–25°C (Fig. 2B).
Tail artery blood flow pulse amplitude.
Introduction of the free or caged intruder rat at ambient temperature caused a rapid fall in tail artery pulse amplitude to zero or near-zero levels (Fig. 1, A and B). After 10–15 min the flow increased, reaching or exceeding preintruder levels during the 20- to 30-min period after introduction of the intruder. There was also a prompt fall in tail artery blood flow pulse amplitude when the empty cage was introduced, but in this situation flow commenced returning toward preintroduction levels within 5 min (Fig. 1C). In rats maintained at ambient temperature 11°C for 3 days tail artery pulse amplitude was at near-zero levels before the caged intruder was introduced (Fig. 4). Pulse amplitude remained at zero or near-zero levels during the intrusion period (Fig. 3).
Repeated introduction of the caged intruder.
In a separate series of animals, a caged intruder rat was introduced to the same resident rat on 3 occasions, with 3 days between each introduction. The intruder-provoked increases in BAT, brain, and body temperature, and the initial slopes of the temperature traces, were similar on all 3 occasions (see Table 1), with no evidence of habituation with repeated introductions of the intruder rat.
Table 1.
Temperature changes observed when the caged intruder was introduced 3 times to the same resident rat, with 3 days between each intruder introduction
| First Introduction of Caged Intruder | Second Introduction of Caged Intruder | Third Introduction of Caged Intruder | NS | |
|---|---|---|---|---|
| BAT temp increase, °C | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | n = 5 |
| BAT slope, °C/min | 0.13 ± 0.02 | 0.11 ± 0.01 | 0.13 ± 0.01 | |
| Brain temp increase, °C | 0.5 ± 0.1 | 0.9 ± 0.3 | 0.9 ± 0.2 | n = 4 |
| Brain slope, °C/min | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.01 | |
| Body temp increase, °C | 0.6 ± 0.08 | 0.8 ± 0.15 | 0.7 ± 0.09 | n = 5 |
| Body slope, °C/min | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.02 |
Data are means±SE. NS; no significant difference between corresponding values observed on each of the 3 occasions, P > 0.05, repeated measures ANOVA. Brown adipose tissue (BAT) temperature increase and initial 5-min slope are significantly greater than corresponding values for brain and body, P < 0.01 repeated measures ANOVA, results for each occasion pooled.
DISCUSSION
BAT thermogenesis contributes to emotional hyperthermia.
Body and brain temperatures promptly increased when the intruder rat was suddenly introduced into the normally quiet environment of the resident rat. Our demonstration of emotional hyperthermia confirms the observations of many previous colleagues, documenting that salient, stressful, or emotive environmental events increase body temperature. In the caged intruder model, the amplitude and the slope of the increases in BAT temperature were greater than corresponding values for body and brain temperature, and pharmacological blockade of β3 adrenoceptors with SR59230A substantially reduced the intruder-induced increases in BAT, body temperature, and brain temperatures. Our study therefore provides strong evidence that BAT thermogenesis contributes to the increases in body and brain temperatures in the intruder rat model of emotional hyperthermia.
Previous studies also support our conclusion. BAT temperature increases more than body temperature in a restraint-stress model, and interruption of the sympathetic innervation of BAT abolishes the increase in BAT temperature and reduces the corresponding increase in body temperature (53, 54). Our laboratory also reported that BAT temperature increases more than body temperature in a restraint stress model (45). In a social defeat model of emotional hyperthermia, pharmacological blockade of β3 adrenoceptors with SR59230A also reduces the body temperature increase, so that BAT thermogenesis contributes to the hyperthermia in this model (31). Other investigators concluded that BAT thermogenesis does not contribute to the emotional hyperthermia that occurs in a conditioned contextual fear model (34, 62). Both of these studies used infrared thermography to measure the temperature of interscapular BAT. We consider that this conclusion should be verified with a thermistor or thermocouple probe chronically implanted in the BAT tissue.
Brain temperature and emotional hyperthermia.
The brain does no mechanical work, so that increases in brain metabolism are especially likely to appear as heat. Summarizing evidence from his own work (23–26) and from previous studies (4, 15), Kiyatkin (25) concludes that increases in brain temperature during emotional hyperthermia result from increases in the brain's own metabolism, not from heat transferred to the brain via the arterial supply. There are contrasting views. Moser and colleagues (38) observed that brain temperature increases when rats explore the environment. Complementary studies using focal brain stimulation in anesthetized animals led Moser and Matheisen (39) to conclude that the most of the exploration-related increase in brain temperature results from heat produced in peripheral organs. Other investigators have also stressed the importance of the temperature of the incoming cerebral arterial blood in the regulation of brain temperature, noting rapid increases in both parameters in monkeys when, for example, the door of their chamber is suddenly opened and the animal is confronted by the human observer (17). This is consistent with the idea that BAT thermogenesis contributes to increases in brain temperature during emotional hyperthermia.
Kiyatkin (25) notes that, in awake animals, “the brain is always warmer than the arterial blood supply and thus cannot be warmed by arterial blood.” However, if the brain is perfused with arterial blood that has itself been warmed, the final temperature of the brain will be higher than it would have been if perfused with arterial blood that has not been warmed. Our own results strongly suggest that BAT thermogenesis, triggered from the brain via the sympathetic outflow, contributes to the increases in brain temperature occurring during emotional hyperthermia. This is important because it suggests that the temperature increases have a functional role.
Possible functional role of the increases in body and brain temperature.
In the present study we observed the resident rat for at least 24 h, recording the coordinated behavioral and physiological events that occur when the rat engages with the external environment as part of the basic rest-activity cycle (2, 3, 46, 47). This enabled us to introduce the intruder rat when temperatures were at a basal level. The coordinated behavioral and physiological events evoked by the intruder rat are remarkably similar to those occurring, apparently spontaneously, during active phases of the basic rest-activity cycle. The contribution of BAT thermogenesis to the temperature increases is also similar in the two situations.
Increases in body temperature facilitate cardiac and skeletal muscle functioning associated with activity (1). Neural processing is both metabolically expensive and temperature sensitive (20, 21, 29, 38), so that increases in the temperature of the brain may be important for its own intrinsic functions. We have suggested that increases in brain temperature could facilitate the complex synaptic processing that underlies cognitive processes associated with exploration of the environment (2). Mechanisms whereby this occurs are still under investigation. The hippocampus expresses theta rhythm when an animal actively explores the environment (7, 49, 61). Higher physiological temperatures facilitate this rhythm because hippocampal neurons express transient receptor potential vanilloid 4 (TRPV4) receptors, and activation of these receptors by physiological increases in brain temperature increases neuronal excitability (51, 52). Temperature-sensitive TRPV4 and TRPV1 receptors are also physiologically important in magnocellular neurosecretory neurons (58).
The intruder model of emotional hyperthermia.
Our intruder rat procedure has a number of advantages as a model of emotional hyperthermia in the resident rat. The procedure is technically simple and economical. The intruder-induced increases in temperature are substantial and reliable, with a stable temporal pattern. Habituation does not occur when the intruder procedure is repeated. Measuring the physiological variables in the resident rat means that it is possible to obtain continuous records without the problem of reconnecting the recording system, as is necessary when measurements are made in a “socially defeated” intruder rat, or when the cage-switch paradigm is used.
The free intruder rat sometimes disturbs the swivel connection and headpiece attached to the resident rat, and with this model it is harder to differentiate physical and psychological aspects of the intruder stimulation. The caged intruder model has the advantage of being a purer psychological stimulus.
Emotional hyperthermia and the concept of fever.
It has been claimed that a rise in temperature “must be defined as a fever” if it involves activation of thermoeffector mechanisms that would normally be inhibited during an increase in temperature resulting from increased heat load (5). In our study the sympathetic outflow to the tail artery was activated in association with the increases in temperature induced by the intruder rat. BAT thermogenesis was also activated, with the similar BAT temperature increases at 24–26°C and at 11°C. Thus the temperature increase observed in our study could/should be classified as a fever according to strictly applied set-point criteria (5, 9, 11, 27, 32, 57). However, there is a strong medical association between “fever” and underlying life-threatening processes, including infection and occult neoplasm, that increase temperature by cytokine-related mechanisms. Thus, when Renbourn (48) demonstrated that the body temperature of young boxers is higher before an actual boxing match than before a period of equivalent exercise, the boxing-related temperature increase was described as “emotional hyperthermia” rather than “psychogenic fever.”
Nevertheless the idea that emotional hyperthermia is a true fever has motivated many studies investigating whether it is prevented or reversed by agents that inhibit synthesis of prostaglandins. Kluger and colleagues (28) demonstrated a partial reversal of cage-switching hyperthermia after aspirin and indomethacin. However, the modern consensus is that emotional hyperthermia is not sensitive to inhibitors of cyclooxygenases and is therefore distinct from cytokine-initiated fevers mediated by prostaglandin synthesis (31). Clearly the set point concept is not useful for differentiating emotionally induced and cytokine-induced increases in temperature. There are other theoretical problems with the set-point concept (37) and we consider that emotional hyperthermia is a better term than psychogenic fever.
Perspectives and Significance
We show in rats that BAT thermogenesis, initiated reasonably independently of thermoregulatory homeostasis via brain central command, contributes to increases in temperature that presumably facilitate planning and action. Our present study and our earlier investigations in rabbits and rats (6, 14, 36, 63, 64) demonstrate that salient or emotional events also vigorously reduce thermoregulatory cutaneous blood flow, and this is also the case in humans (6, 13, 16, 22). Emotional hyperthermia also occurs in humans, as first convincingly demonstrated by Renbourn (48). BAT thermogenesis occurs in adult humans and is under sympathetic control (30, 41, 42, 50, 59). Human basal metabolic rate is particularly sensitive to emotionally significant or salient events (19). It will be important to determine whether BAT thermogenesis also contributes to emotional hyperthermia in humans.
GRANTS
This study was supported by the National Health and Medical Research Council Project Grant 1051826 and by the Flinders Medical Centre Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M. performed experiments; M.M., Y.O., and W.W.B. analyzed data; M.M., Y.O., and W.W.B. interpreted results of experiments; M.M., Y.O., and W.W.B. edited and revised manuscript; M.M., Y.O., and W.W.B. approved final version of manuscript; Y.O. and W.W.B. conception and design of research; W.W.B. prepared figures; W.W.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Jessie Moore and Sarah Tamang for technical assistance and Esther Blessing for critically reviewing our manuscript.
REFERENCES
- 1.Bennett AF. Temperature and muscle. J Exp Biol 115: 333–344, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Blessing W, Mohammed M, Ootsuka Y. Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol Behav 121: 61–69, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Blessing W, Mohammed M, Ootsuka Y. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol Behav 105: 966–974, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Blumberg MS, Mennella JA, Moltz H. Hypothalamic temperature and deep body temperature during copulation in the male rat. Physiol Behav 39: 367–370, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Briese E, Cabanac M. Stress hyperthermia: physiological arguments that it is a fever. Physiol Behav 49: 1153–1157, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Brown R, James C, Henderson LA, Macefield VG. Autonomic markers of emotional processing: skin sympathetic nerve activity in humans during exposure to emotionally charged images. Front Physiol 3: 394, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16: 130–138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabanac A, Cabanac M. Heart rate response to gentle handling of frog and lizard. Behav Proc 52: 89–95, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Cabanac M. Adjustable set point: to honor Harold T. Hammel. J Appl Physiol 100: 1338–1346, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cabanac M, Gosselin F. Emotional fever in the lizard Callopistes maculatus (Teiidae). Animal Behav 46: 200–202, 1993 [Google Scholar]
- 11.Cabanac M, Massonnet B. Temperature regulation during fever: change of set point or change of gain? A tentative answer from a behavioural study in man. J Physiol 238: 561–568, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Darrow CW. The galvanic skin-reflex and finger volume changes. Am J Physiol 88: 219–229, 1929 [Google Scholar]
- 14.de Menezes RC, Ootsuka Y, Blessing WW. Sympathetic cutaneous vasomotor alerting responses (SCVARs) are associated with hippocampal theta rhythm in non-moving conscious rats. Brain Res 1298: 123–130, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Delgado J, Hanai T. Intracerebral temperatures in free-moving cats. Am J Physiol 211: 755–769, 1966 [DOI] [PubMed] [Google Scholar]
- 16.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972 [DOI] [PubMed] [Google Scholar]
- 17.Hayward JN, Baker MA. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol 215: 389–403, 1968 [DOI] [PubMed] [Google Scholar]
- 18.Hetem RS, Mitchell D, de Witt BA, Fick LG, Meyer LC, Maloney SK, Fuller A. Cheetah do not abandon hunts because they overheat. Biol Lett 9: 20130472, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingbar SH, Woeber KA. The thyroid gland. In: Textbook of Endocrinology, edited by Williams RH. Philadelphia, PA: Saunders, 1968, p. 168 [Google Scholar]
- 20.Janssen R. Thermal influences on nervous system function. Neurosci Biobehav Rev 16: 399–413, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Kim JA, Connors BW. High temperatures alter physiological properties of pyramidal cells and inhibitory interneurons in hippocampus. Front Cell Neurosci 6: 27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kistler A, Mariauzouls C, von Berlepsch K. Fingertip temperature as an indicator for sympathetic responses. Int J Psychophysiol 29: 35–41, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kiyatkin E, Brown P, Wise R. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 16: 164–168, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kiyatkin E, Mitchum R. Fluctuations in brain temperature during sexual interaction in male rats: an approach for evaluating neural activity underlying motivated behavior. Neuroscience 119: 1169–1183, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci 15: 73–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav 84: 563–570, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kluger MJ. Fever vs. hyperthermia. N Engl J Med 299: 555, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Kluger MJ, O'Reilly B, Shope TR, Vander AJ. Further evidence that stress hyperthermia is a fever. Physiol Behav 39: 763–766, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci 1: 36–41, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lebron L, Chou AJ, Carrasquillo JA. Interesting image. Unilateral F-18 FDG uptake in the neck, in patients with sympathetic denervation. Clin Nucl Med 35: 899–901, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 34: 1442–1452, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Long N, Vander A, Kluger M. Stress-induced rise of body temperature in rats is the same in warm and cool environments. Physiol Behav 47: 773–775, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, Giudice A, Guzzi U, Landi M, Le Fur G. Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol 117: 435–442, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marks A, Vianna DM, Carrive P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am J Physiol Regul Integr Comp Physiol 296: R1239–R1247, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Meyer LC, Fick L, Matthee A, Mitchell D, Fuller A. Hyperthermia in captured impala (Aepyceros melampus): a fright not flight response. J Wildl Dis 44: 404–416, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Mohammed M, Kulasekara K, De Menezes RC, Ootsuka Y, Blessing WW. Inactivation of neuronal function in the amygdaloid region reduces tail artery blood flow alerting responses in conscious rats. Neuroscience 228: 13–22, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Morrison SF, Blessing WW. Central nervous system regulation of body temperature. In: Central Regulation of Autonomic Functions, edited by Llewellyn-Smith IJ, Verberne AJM. New York: Oxford University Press, 2011, p. 324–344 [Google Scholar]
- 38.Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science 259: 1324–1326, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Moser EI, Mathiesen LI. Relationship between neuronal activity and brain temperature in rats. Neuroreport 7: 1876–1880, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann NY Acad Sci 1212: E20–E36, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective beta 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol 49: 7–14, 1996 [PubMed] [Google Scholar]
- 44.Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med 63: 476–486, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 11: 125–133, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience 164: 849–861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ootsuka Y, Kulasekara K, de Menezes RC, Blessing WW. SR59230A, a β3 adrenoceptor antagonist, inhibits ultradian brown adipose tissue thermogenesis and interrupts associated episodic brain and body heating. Am J Physiol Regul Integr Comp Physiol 301: R987–R994, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Renbourn ET. Body temperature and pulse rate in boys and young men prior to sporting contests. A study of emotional hyperthermia: with a review of the literature. J Psychosomat Res 4: 149–175, 1960 [DOI] [PubMed] [Google Scholar]
- 49.Sainsbury RS, Heynen A, Montoya CP. Behavioral correlates of hippocampal type 2 theta in the rat. Physiol Behav 39: 513–519, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 27: 1566–1575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibasaki K, Yamada K, Miwa H, Tominaga M, Ishisaki Y. Brain temperature is a critical determinant of neuronal excitablility through TRPV4 activation. In: 37th Congress of IUPS. Birmingham, AL: 2013, p. PCD123 [Google Scholar]
- 53.Shibata H, Nagasaka T. Contribution of nonshivering thermogenesis to stress-induced hyperthermia in rats. Jpn J Physiol 32: 991–995, 1982 [DOI] [PubMed] [Google Scholar]
- 54.Shibata H, Nagasaka T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn J Physiol 34: 103–111, 1984 [DOI] [PubMed] [Google Scholar]
- 55.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 49: 330–425, 1969 [DOI] [PubMed] [Google Scholar]
- 56.Smith RE, Roberts JC. Thermogenesis of brown adipose tissue in cold-acclimated rats. Am J Physiol 206: 143–148, 1964 [DOI] [PubMed] [Google Scholar]
- 57.Stitt JT. Fever versus hyperthermia. Fed Proc 38: 39–43, 1979 [PubMed] [Google Scholar]
- 58.Sudbury JR, Bourque CW. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J Neurosci 33: 17160–17165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, Iwanaga T, Kameya T, Kawai Y, Saito M. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br J Nutr 110: 733–738, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Thompson CI, Brannon AJ, Heck AL. Emotional fever after habituation to the temperature-recording procedure. Physiol Behav 80: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev 3: 173–200, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 21: 2505–2512, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Yu YH, Blessing WW. Amygdala co-ordinates sudden falls in ear pinna blood flow in response to unconditioned salient stimuli in conscious rabbits. Neuroscience 93: 135–141, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Yu YH, Blessing WW. Neurons in amygdala mediate ear pinna vasoconstriction elicited by unconditioned salient stimuli in conscious rabbits. Autono Neurosci 87: 236–242, 2001 [DOI] [PubMed] [Google Scholar]