Summary
The past decades of biomedical research have yielded massive evidence for the contribution of microbiome in the development of a variety of chronic human diseases. There is emerging evidence that Porphyromonas gingivalis, a well-adapted opportunistic pathogen of the oral mucosa and prominent constituent of oral biofilms, best known for its involvement in periodontitis, may be an important mediator in the development of a number of multifactorial and seemingly unrelated chronic diseases, such as rheumatoid arthritis and orodigestive cancers. Orodigestive cancers represent a big portion of the total malignancies worldwide, and include cancers of the oral cavity, gastro-intestinal tract, and pancreas. For prevention and/or enhanced prognosis of these diseases, a good understanding of the pathophysiological mechanisms and the interaction between P. gingivalis and host is much needed. With this review, we introduce the currently accumulated knowledge on P. gingivalis’ plausible association with cancer as a risk modifier, and present the putative cancer promoting cellular and molecular mechanisms that this organism may influence in the oral mucosa.
“Knowledge is made by oblivion, and to purchase a clear and warrantable body of truth, we must forget and part with much we know”
Sir Thomas Browne (1605–1682)
Introduction
The last few decades of biomedical research have accumulated a large knowledge base on the etiopathogenic mechanisms of numerous chronic diseases and conditions of man. In many of these chronic pathologies, bacteria have been shown to play a major role either as singular etiologic factors, or as synergistic contributors in the multifaceted stimulation of the host immune system that can tip the scales towards chronic or autoimmune disease development. Recent advances in metagenomic tools have heightened the importance of resident microbiome in mammalian development, metabolism, immune response and behavior during all stages of life, which has led to the initiation of several world-wide projects to determine the species diversity and genetic make-up of the human microbiome in health and disease (Diaz Heijtz et al., 2011, Cox et al., 2013, Erturk-Hasdemir & Kasper, 2013, Guinane & Cotter, 2013, Mavrommatis et al., 2013, Ridaura et al., 2013). Multiple specific examples may be given for the growing role of the human microbiome in development of chronic pathological conditions. These include chronic inflammatory diseases of the lung, liver, cardiovascular and digestive systems, as well as of periodontium (Berry & Reinisch, 2013, Chassaing et al., 2013). The human microbiome contains a number of adaptable pathogenic species that are now identified to be closely involved in the etiology of various chronic diseases. One of the best documented examples is a Gram-negative bacterium, Helicobacter pylori, primarily known for its involvement in gastric and gastro-intestinal cancers, and more recently in other chronic diseases, including asthma, type2 diabetes, and obesity (Wang et al., 2013). Furthermore, increasing evidence suggests that the establishment of a variety of chronic diseases could result from a polymicrobial interactions within the underlying microbiome, in concert with host genetic and metabolic risk factors (Michaud, 2013, Wroblewski et al., 2010, Fox & Sheh, 2013).
The human body represents a multitude of mucosal sites, each comprising a specific milieu of highly adapted, commensal and opportunistic-pathogen organisms. One of the most complex and highly variable communities is the oral microbiome, which has long been implicated in the development of tooth decay and periodontal disease (Jenkinson, 2011). The oral microbiome represents a unique and vastly dynamic multidimensional biochemical network, formed by the interaction among the proteomes and metabolomes of the comprising organisms, the behavioral, hygienic and dietary habits of the host, and the genetic and immunologic host factors (Jenkinson, 2011, Khalili, 2008, Teles et al., 2013, Wright et al., 2013). Some of these interactions yield potentially pathogenic compounds that may have effect on the mucosal lining, the surrounding tissues and even on the distant organs and systems of the body (Hooper et al., 2009, Jenkinson, 2011, Ahn et al., 2012a). Those noxious metabolic products or molecules include lipids, lipopolysaccharides (LPS), free oxygen and nitrogen radicals, pro-inflammatory mediators and host and/or bacteria derived enzymes. Hence, the oral microbiome has been proposed to impact the etiology and progression of diabetes, cardiovascular disease, and lately a number of studies have indicated the association among distinct consortia of microbial species, periodontal disease and orodigestive cancers (Koren et al., 2011, Ahn et al., 2012a, Meyer et al., 2008). The evidence accumulated from those studies has specified strong correlation among a number of chronic periodontal bacteria containing Prevotella, Porphyromonas Fusobacterium, and Streptococcus spp., and squamous cell carcinoma of the orodigestive tract, which encompasses the oral cavity, gastro-intestinal tract and pancreas (Nagy et al. 1998, Mager et al., 2005,, Ahn et al., 2012b).
Among these specific species, an opportunistic pathogen, Porphyromonas gingivalis, has emerged as a potential mediator in the etiology of a variety of presumably unrelated chronic diseases, such as rheumatoid arthritis, cardiovascular diseases, diabetes, and more recently different types of orodigestive cancers (Ahn et al., 2012b, Hitchon et al., 2010, Totaro et al., 2013, Ishikawa et al., 2013, Aemaimanan et al., 2013, Katz et al., 2011,, Michaud, 2013, Hajishengallis et al., 2012). In the case of rheumatoid arthritis, P. gingivalis’ peptidylarginine deiminase (PAD) has been recently suggested to assume a key role in the citrullination of arginine residues in host proteins, thus altering their normal folding and creating conditions for the induction of auto-antibodies against host cartilage-specific antigens. P. gingivalis’ ability to activate host matrix metalloproteases (MMPs) and the organisms’ role in altering cytokine responses have also been implicated in the protein degradation, observed in arthritic joints (Mangat et al., 2010, Hitchon et al., 2010, Detert et al., 2010). Conjointly, prolonged systemic cytokine elevation and inflammation have been especially attributed to P. gingivalis in the context of the multifactorial periodontal disease as contributing and/or amplifying factor in the development and progression of diabetes mellitus. The poor glycemic control in diabetes, on the other hand, has been shown to exacerbate chronic periodontitis (Lalla & Papapanou, 2011). A significant statistical association has been also observed between P. gingivalis-carriage in saliva samples and non-alcoholic fatty liver disease, further strengthening P. gingivalis’ role as a risk factor in multiple chronic conditions (Yoneda et al., 2012).
On the cancer front, multiple clinical and experimental studies have displayed various degrees of associations between P. gingivalis and cancers of the oral cavity, orodigestive tract or pancreatic tissues (Katz et al., 2011, Groeger et al., 2011, Michaud, 2013, Michaud et al., 2012, Ahn et al., 2012b).
P. gingivalis, which has been shown to have the plenitude of virulence factors, can also be present in healthy individuals’ periodontal pockets, affluently survives within oral epithelial tissues, and is successfully able to evade the host immune response (Yilmaz, 2008, Choi et al., 2013, Yilmaz et al., 2008, Singh et al., 2011, Yao et al., 2010, Yilmaz et al., 2010). Accordingly, P. gingivalis has recently been attributed as a key-stone pathogen based on its ability to orchestrate inflammatory disease by remodeling the oral microbiome (Hajishengallis et al., 2012). P. gingivalis, can invade primary cultures of oral epithelial cells (OECs), render them resistant to cell death, and alter the chemokine and cytokine secretion (Spooner & Yilmaz, 2011, Choi et al., 2013, Yilmaz et al., 2010, Yao et al., 2010, Hung et al., 2013, Takeuchi et al., 2013). OECs that form the outer lining of the oral mucosa are the initial targets for colonizing bacteria, and stand an important arm of the host innate immune response by secreting antimicrobial, pro-inflammatory and chemotactic signals, and modulating apoptosis upon sensing invading organisms (Takeuchi et al., 2013, Yilmaz et al., 2010, Hung et al., 2013). Therefore, the ability of P. gingivalis to colonize in the oral cavity and modulate both the epithelial cell and systemic responses potentially places the organism not only in the category of central contributors in the multifactorial etiology of the periodontal disease, but also orodigestive cancers and other chronic diseases (Figure 1).
Figure 1.
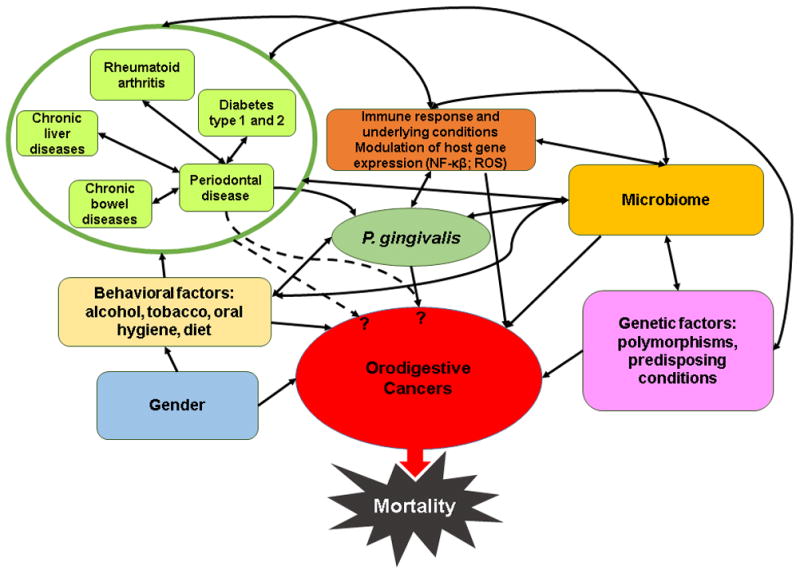
Schematic representation of the complex interrelationships between different human genetic, behavioral and immunologic factors, as well as microbiome-related factors, that are proposed to take part in the multifactorial etiology of orodigestive cancers and other possibly associated chronic diseases. P. gingivalis is implicated to play a specific role in these multi-directional links. Dashed arrows represent plausible associations, based on currently available epidemiologic, clinical, histological, and experimental studies.
The prospective multiple roles of P. gingivalis in the above mentioned conditions, however, remain incomplete and there is a clear need for mechanistic determination of these newly observed associations in order to better understand and possibly control those severe chronic conditions. Therefore, this review aims to offer a fresh perspective on the putative role of P. gingivalis in the orodigestive cancers, and its plausible use as a biomarker for the identification of at “high-risk” populations.
P. gingivalis and Cancer
The significance of P. gingivalis involvement in cancer has evolved within the last decade. Chronologically initial studies have comprised mostly epidemiological and clinical association between oral microbiome, periodontal disease or tooth loss, with orodigestive cancers including cancers of the oral cavity, gastrointestinal tract and pancreas (Hooper et al., 2009, Ahn et al., 2012a, Meyer et al., 2008, Katz et al., 2011, Groeger et al., 2011, Michaud, 2013, Ahn et al., 2012b). Among all observed cancer relations the most direct and strong association of P. gingivalis has been found with oral squamous cell carcinoma (OSCC) (Nagy et al., 1998, Mager et al., 2005, Katz et al., 2011). OSCC is one of the highly common cancers in the United States with estimated 35,000 newly diagnosed cases and over 7,500 deaths occurring yearly (Jemal et al., 2008). Currently there is a lack of reliable diagnostic tools for identification of high-risk persons, most OSCC cases have metastases already at presentation, and available therapies fail to prevent malignant progression (Mitka, 2013). All these factors support the necessity for early markers that can identify the risk populations and possibly be used for preventative care, before carcinogenesis develops.
1. Historical Perspective and Clinical Relevance
Among the first studies to examine association between periodontitis and cancer was a case-control study conducted between 1999 and 2005 in the USA that examined 473 patients (Tezal et al., 2009). The study measured periodontitis by alveolar bone loss and screened the patients for squamous cell carcinoma of the oral cavity, pharynx and larynx. Chronic periodontitis was associated with 4-fold increase in risk of any of the three examined types of carcinoma. The strength of the association was greatest with cancers of the oral cavity, followed by the oropharynx and larynx. The association persisted in subjects who never used tobacco and alcohol, which suggests chronic periodontitis to be an independent risk factor for the cancer development (Tezal et al., 2009).
One of the initial broad surveillance studies showing potential link between P. gingivalis and orodigestive cancers was the National Health and Nutrition Examination Survey III (Ahn et al., 2012b). This prospective study included a total of 12,605 men and women with clinically ascertained periodontitis of which 7,852 had serum IgG immune response to P. gingivalis. They were followed from 1988 through 2006 in relation to orodigestive cancer mortality, including cancers of the lip, oral cavity, gastrointestinal tract, pancreas and liver, and in that period 105 orodigestive cancer deaths occurred. Moderate or severe periodontitis was found to be associated with increased orodigestive cancer mortality relative risk. The mortality risk was also proportionally increased with increasing severity of periodontal disease. The highest association with periodontitis was found for colorectal and pancreatic cancer, whereas greater serum P. gingivalis IgG levels tended to be associated with increased orodigestive cancer mortality (Ahn et al., 2012b). Interestingly, P. gingivalis was also associated with 2.25-fold higher likelihood for orodigestive mortality in healthy subjects not exhibiting overt periodontal disease. Hence, this study was the first to illustrate direct correlation of orodigestive cancer mortality to P. gingivalis independently of periodontal disease, thus pinpointing that P. gingivalis could be a valuable biomarker for microbe-associated risk of orodigestive-cancer-death (Ahn et al., 2012b).
In 1998, a study compared biofilm samples from central ulcerating surfaces of OSCC lesions and healthy mucosa from 21 middle aged, prevalently male patients without antibiotic or tumor therapy (Nagy et al., 1998). The study found a significantly higher number of both specific anaerobic and aerobic bacterial species at the tumor sites compared to healthy mucosa. Interestingly the genus Porphyromonas was identified among the genera with highest rates of isolation at the tumor sites (Nagy et al., 1998). More than a decade later, in 2012, a similar study quantitated the 16S ribosomal DNA fragments from six periodontal bacteria including P. gingivalis in formalin-fixed, paraffin-embedded lymph nodes from 66 patients with histories of head and neck cancers, including OSCC (Amodini Rajakaruna et al., 2012). The lymph nodes selected for the study did not have metastatic invasion yet. The relationship between bacterial detection and cancer severity, gender, and the use of anti-cancer therapy was examined by Fisher’s exact test and was not found to be statistically significant. However, P. gingivalis was detected in the samples of submandibular and submental lymph nodes of ~ 20% of these patients, and the study suggested that translocation of periodontopathic bacteria may occur via lymphatic drainage, irrespective of the cancer disease status or therapy (Amodini Rajakaruna et al., 2012).
In a 2005 study an increased ratio of P. gingivalis in saliva samples of 45 OSCC patients compared to 229 healthy subjects was reported. While this increase was not statistically significant, this was perhaps due to the relatively small patient size (Mager et al., 2005). On the other hand, a recent analysis of oral tissue samples from 10 cases and 5 healthy individuals using anti-P. gingivalis (ATCC33277)-specific immunohistochemistry detected a statistically significant increase of P. gingivalis staining in OSCC tissues than normal tissues. The study used antibody staining against the commensal bacterium Streptococcus gordonii as a control, which did not show any significant distribution difference (Katz et al., 2011). This finding highlighted for the first time a noteworthy association of P. gingivalis with OSCC, despite the low sample size.
Another study from 2012 that measured plasma antibodies to 25 oral bacteria in pre-diagnostic blood samples from 405 pancreatic cancer patients and 416 matched controls also indirectly associated P. gingivalis with increased risk of pancreatic cancer. The study demonstrated high correlations for two strains of P. gingivalis (ATCC 53978 and 33277). Individuals with high levels of antibodies against P. gingivalis ATTC 53978 had a two-fold higher risk of pancreatic cancer than individuals with lower levels of anti-P. gingivalis antibodies (Michaud et al., 2012). A year later in a clinical study involving 37 cases of chronic atrophic gastritis, intestinal metaplasia, or dysplasia, DNA levels of periodontopathic bacteria, including P. gingivalis, were measured in plaque and saliva samples by real-time quantitative PCR (Salazar et al., 2013). An elevated but not statistically-significant odds ratio for gastric precancerous lesions was observed in relation to increasing colonization with P. gingivalis, Aggregatibacter actinomycetemcomitans and Treponema denticola measured in plaque, and thus the study associated high levels of periodontal-pathogen colonization with an increased risk of gastric precancerous lesions in individuals with periodontal disease (Salazar et al., 2013). A recent case-control study examined the fecal microbiome changes of 19 colorectal cancer patients in comparison to 20 healthy people using 16S rRNA pyro-sequencing (Wu et al., 2013). Interestingly, although the study was not designed to screen for specific bacterial species, it detected a highly statistically significant increase of Porphyromonadaceae family, along with Fusobacteriaceae, and 3 other operational taxonomic units in the colorectal cancer patients compared to the healthy subjects (Wu et al., 2013).
All these studies underlined the plausible role of P. gingivalis in the variety of orodigestive cancers described, and prompt many questions on the possible mechanistic bases of the detected associations. Answers to such questions may be obtained through the use of physiologically relevant in vitro studies. The rest of the review is dedicated to describe the presently available work that exist in the literature and will provide further insights.
2. In Vitro Studies and Potential Mechanisms
Although there is mounting clinical evidence links P. gingivalis and/or periodontal disease with orodigestive cancer risk, there are currently only several in vitro reports that directly studied the carcinogenic potential of P. gingivalis. Based on the new information flowing and the available new technologies, there is a new charge for the scientific community to investigate the emerging role of P. gingivalis in the increased risk of cancer development.
2a. Findings in Vitro with Oral Cancer Cell Lines
In 2011, a study utilized two OSCC-derived cell lines, the metastogenic ‘SCC-25’ and non-metastatic ‘BHY’ cells, as well as human primary oral epithelial cells (OECs), to examine the expression of two human B7-homologue receptors, ‘B7-H1’ and ‘B7-DC’ (Groeger et al., 2011). These receptors have been found to be highly expressed in the majority of human cancers. Receptor B7-H1, mostly known as programmed death ligand 1, is an important regulator in cell-mediated immunity by promoting the development of regulatory T cells, and is thought to play a major role in suppressing the immune system during cancer (Greaves & Gribben, 2013). B7-DC, also known as programmed death ligand 2, is expressed mainly on dendritic cells and macrophages, although it can be induczed in a variety of cell types and is suggested to suppress T-cell survival, cytokine production and proliferation (Rozali et al., 2012). In the study, Groeger and colleagues demonstrated a significant upregulation of both B7-H1 and B7-DC receptors in the metastogenic SCC-25 cell line and in human primary OECs after infection with P. gingivalis strains ATCC 33277 or W83. In contrast, infection with Streptococcus salivarius K12, a commensal bacterium used as a control in the study, did not have any effect on the examined receptors that are important for cancer etiology (Groeger et al., 2011). These findings strongly suggest a potential role for P. gingivalis in contributing to the cellular / molecular changes promoting oral cancer, possibly through facilitating immune evasion.
An earlier in vitro study examined the effect of P. gingivalis’ LPS on cancer progression of an OSCC derived cell line, ‘YD-10B’, which has been shown to express functional Toll-like receptors (Park et al., 2010). The study showed no effect of P. gingivalis LPS on the cell proliferation, migration, invasion or angiogenesis, which are the mechanisms shown to be involved in various cancer development (Park et al., 2010). The study, however, used purified LPS, and was not designed to study the outcome of complete P. gingivalis infection on the cancer cell line, thus excluding the possible roles of other virulence factors of the bacterium in this model. This result may suggest that the reputed role of P. gingivalis is possibly not due to one singular component, but is likely a greatly concerted synergistic play, requiring other structural properties, and potentially dynamic bacterium-host interaction.
Very recently, another study examined the effect of P. gingivalis, using strain ATCC 33277, on the metastatic ability of two OSCC cell lines, the highly metastogenic ‘SAS’ cells and the low-metastogenic ‘Ca9-22’ cells (Inaba et al., 2013). Metastatic invasion was measured by the induction and activation of human matrix metalloproteinase 9 (MMP9) in the cells upon infection with P. gingivalis compared to another periodontal organism Fusobacterium nucleatum. In SAS cells wild-type P. gingivalis ATCC 33277 was able to increase basal expression of proMMP9 enzyme and subsequently to activate MMP9, whereas F. nucleatum did not alter expression or activation of proMMP9. In the less-metastatic Ca9-22 cell line, P. gingivalis infection did not have directly observed effect on pro-MMP9 expression or activation, although incubation of Ca9-22 cells with supernatant of P. gingivalis-infected SAS cells increased Ca9-22 cells’ metastogenic capacity significantly. Using gingipain mutant strains, the production and activation of proMMP9 was shown to be dependent on gingipains of P. gingivalis. The study also showed that proMMP9 production was controlled by the phosphorylation of the heat-shock-protein 27, HSP27, which was previously found to be over expressed in OSCC tissues (Turhani et al., 2006). P. gingivalis may hold the potential to contribute to the cancer progression and mediate enhanced host-cell metastatic potential by activation of MMP9 via its gingipain proteases (Inaba et al., 2013).
Collectively, these studies substantiate the growing plausible role of P. gingivalis in cancer etiology, rather than being a bystander, although there has been a view that the opportunistic bacteria may come after the disease-induced suppression in immunity and compromised mucosal integrity (Cummins & Tangney, 2013). Therefore, it is important to recognize that studying pre-cancerous lesions of the oral mucosa, before malignancy, is perhaps the most relevant stage to examine the direct involvement of pathogens in cancer formation. The following section of this review will illustrate at present knowledge derived from human primary OECs and oncogenesis-related pathways, and highlight plausible mechanisms for P. gingivalis’ connection in the cellular / molecular mechanisms that are cancer promoting.
2b. Relevant Findings with Human Primary Oral Epithelial Cells
P. gingivalis-host interaction studied in the primary cultures of the OECs has proven to be an invaluable model to appreciate the highly orchestrated, complex nature of the interface between the chronic organism and the oral epithelium, as the OECs can emulate the in vivo characteristics of human mucosal epithelium most closely. During those studies, the human primary OEC model provided substantial mechanistic insights on the P. gingivalis’ persistence strategies in the oral epithelium that may well be pertinent in the orodigestive cancer etiology and/or advancement.
P. gingivalis was shown to rapidly invade the primary OECs in culture by using the β1-integrin and downstream signaling pathways, and can spread intercellularly later in the infection using actin cytoskeleton, thus evading recognition by the immune system (Yilmaz, 2008). Using P. gingivalis strain ATCC 33277 the organism was demonstrated to inhibit OEC programmed cell death induced by potent pro-apoptotic agents. The characterized epithelial-host-survival molecular actions of P. gingivalis include blocking of mitochondrion-dependent apoptosis (by inhibition of cytochrome-c release, mitochondrial membrane depolarization, balancing of pro-apoptotic Bax and anti-apoptotic Bcl-2 expression), activation of the phosphatidylinositol 3 kinase (PI3K) / Akt and survivin / Stat-3 survival pathways, inhibition of caspase-3 and 9 activation, and inactivation of pro-apoptotic Bad-signaling (Nakhjiri et al., 2001, Mao et al., 2007, Yilmaz, 2008, Yao et al., 2010). PI3K / Akt and Survivin / Stat-3 are well described as key regulators of cellular processes that can lead to initiation and/or maintenance of carcinogenesis. Both of the pathways are currently evaluated as major therapeutic targets in cancer (Altieri, 2003). The studies with the human primary OECs also revealed that P. gingivalis infection promotes pro-survival phenotype in the host cells by accelerating the progression through the S-phase of the cell cycle via the modulation of pathways involving cyclins and p53 (Yilmaz, 2008, Kuboniwa, M et al., 2008). The infection by the organism is able to alter the expression of ‘p53 tumor suppressor’, which is known to be involved in DNA-damage-response and in tumor suppression (Ozaki et al., 2013). The aforementioned actions of P. gingivalis appeared to be dependent on presence of the major fimbriae (FimA) of the organism (Kuboniwa et al., 2008). Furthermore, P. gingivalis can inhibit OEC cell death induced by an important physiological pro-apoptotic molecule, extracellular ATP. ATP elicits its activity through the purinergic cell surface receptor, P2X7,. The ATP coupled P2X7 signaling has direct implications for regulating of a variety of specific host immune response elements that are lately shown to be critically involved in pathways towards carcinogenesis (Adinolfi et al., 2012, Chadet et al., 2014, Di Virgilio, 2012, Jelassi et al., 2013, Roger & Pelegrin, 2011). Some of these actions include the modulation of intracellular reactive oxygen species (ROS), controlling of apoptosis, activation of inflammasome, and secretion of pro-inflammatory cytokines. The recent efforts in the treatment and invasiveness of several types of cancers with high mortalities are directly targeted against P2X7 / ATP-signaling, and P2X7 expression has been found in diverse tumors and has newly been proposed as a potential cancer cell biomarker (Huang et al., 2013, Roger & Pelegrin, 2011). P. gingivalis’ inhibition of ATP / P2X7-induced cell-death in OECs is mediated by secretion of an ATP-utilizing enzyme, nucleoside diphosphate kinase (NDK) (Spooner & Yilmaz, 2012, Choi et al., 2013). NDKs have recently emerged as a novel secreted effector for successful persistence by chronic opportunistic pathogens, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa (Yilmaz, 2008). A new study further demonstrated that P. gingivalis can modulate ATP-induced cytosolic and mitochondrial ROS as well as antioxidant glutathione response generated through P2X7 / NADPH-oxidase interactome via temporal secretion of the NDK (Choi et al., 2013). ROS can serve as a key mediator in activation of a selection of cancer / inflammation associated transcription factors (Spooner & Yilmaz, 2011).
Intriguingly, NDK species, including the human homologues known as ‘non-metastatic cells expressed proteins’ (NME), encoded by nm23 genes, are recently widely studied for their involvement in various forms of human cancers, such as breast, lung, thyroid and neuroblastoma cancers, as well as for metastasis and regulation of p53-mediated transcription (Prabhu et al., 2012, Marino et al., 2012, Boissan & Lacombe, 2011). Most interestingly, the human NDK was shown to be over expressed in surgically excised tumor-tissues of OSCC patients, compared to healthy mucosa from the same patients (Turhani et al., 2006). The study identified only 20 proteins with altered expression in OSCC tissue and intriguingly human NME1 and HSP27 were among the 14 proteins with significantly increased levels.
Notably, ATP / P2X7-signaling is lately associated with the development of multiple pathologic conditions in addition to cancer such as diabetes, obesity, multiple sclerosis, joint disease, pancreatic disease, and kidney diseases. The significance of P2X7 receptor’s splice and polymorphism variants in chronic human diseases is also becoming an active area of research (Hillman et al., 2005, Adinolfi et al., 2012, Di Virgilio, 2012, Chen et al., 2011, Garcia-Hernandez et al., 2011, Sun et al., 2012, Oyanguren-Desez et al., 2011, Lopez-Castejon et al., 2010). P2X7 is known to play a critical role in promoting cell growth, neovascularization, tumor-host interactions, and metastasis, and can induce release of cathepsins by macrophages (involved in joint diseases) markedly increased in chronic and inflammatory conditions (kidney disease, diabetes). It is worth emphasizing that this assembly of diseases is similar to the array of chronic conditions recently being associated with presence of P. gingivalis (Aemaimanan et al., 2013, Ahn et al., 2012b, Hitchon et al., 2010, Katz et al., 2011, Michaud, 2013). Moreover, molecular actions of P. gingivalis have been shown to be highly cell-type dependent and different outcomes may be stimulated in different cell types. These interactions are further complicated by the fact that P. gingivalis’ colonization in the oral mucosa is closely accompanied by multiple bacterial species. Intriguingly, among those oral organisms, F. nucleatum is newly associated with progression of malignant tumors of intestinal system particularly colorectal cancer (Kostic et al., 2013, Rubinstein et al., 2013), thus making the entire host-pathogen interaction much more complex.
Taken together, P. gingivalis’ shown ability to modulate ATP / P2X7-signaling, to secrete an anti-apoptotic enzyme, NDK during the infection of primary OECs, and to express other virulence factors (e.g. fimbriae, gingipains, PAD) may serve as potential etiologic links with orodigestive cancers and possibly other chronic diseases. The postulated stepwise transformation of the normal epithelium to the precancerous and cancerous lesions in the presence of P. gingivalis infection and the plausible associated pathways are sketched in Figure 2.
Figure 2.
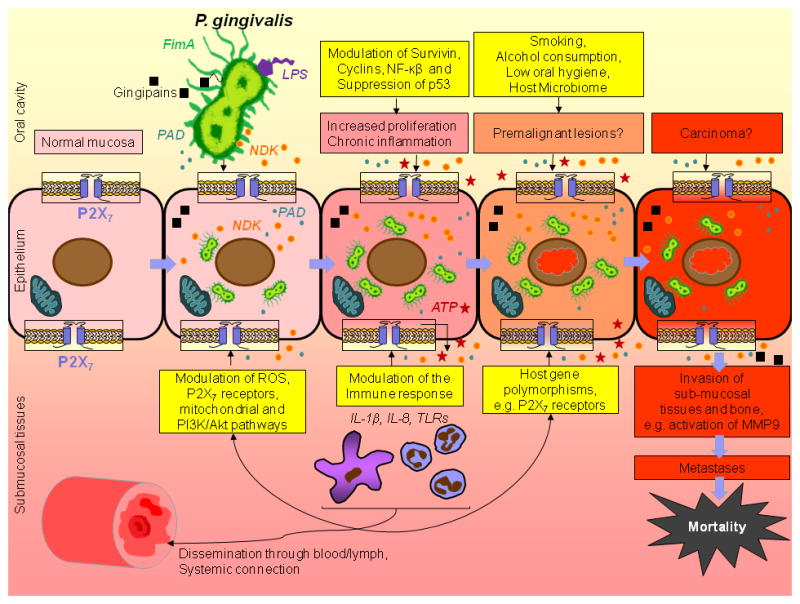
Postulated pro-cancer molecular circuitry of P. gingivalis and OECs interface that may affect the increased risk of orodigestive cancers and their poor prognosis. Over the period of its co-evolution with human oral mucosa, P. gingivalis has established multiple virulence factors such as distinctive fimbriae (FimA), cysteine proteases (gingipains), lipopolysaccharide (LPS), peptidyl-arginine deiminase (PAD), and nucleoside diphosphate kinase (NDK). These molecules, in concert with genetic predisposition, behavioral factors and possibly in synergy with other microbiome components, can elicit multiple pro-survival effects on the epithelial cells that may lead to predisposition for the cancerous transformation. Additionally, gingipains are suggested to play a role in the activation of MMPs, particularly MMP9, which are shown be associated in the metastatic dissemination of carcinoma cells.
Conclusion and Future Studies
The potential significance of P. gingivalis is rapidly rising in the OSCC etiology and the other cancers of the orodigestive tract. P. gingivalis is thought to have co-evolved with humans since the divergence of the Old World monkeys of Africa around 6–7 million years ago (Mikkelsen et al., 2008). One can speculate this long co-existence may have brought the excellent adaptation of this successful persistent bacterium to the human oral mucosa. Cancer is considered to be a disease of civilization. It is possible that P. gingivalis has recently evolved to become a risk factor in the cancer etiology. Conceivably, the advancements in society, the global ecological changes, and increase in human longevity have allowed the increase of many of the current chronic diseases, and stimulated the rise of a range of currently unknown virulence factors of P. gingivalis. Nevertheless, the aforementioned possibilities remain to be further researched.
In summary, oral mucosa harbors vast numbers of naturally occurring microbes on the epithelial surfaces, which might have direct synergistic or antagonistic effects on the occurrence and or advancement of precancerous and cancerous formations in the oral cavity. The centrality of P. gingivalis to orodigestive cancer is still debated, however, in light of the recent findings, we propose the organism is perhaps an important predisposing factor in the microenvironment that directs the development or poor prognosis of orodigestive cancers and possibly other chronic diseases. Future large-scale integrated clinical and in vitro mechanistic studies accompanied by in vivo validation assays may determine the specific microbial components and the associated microbiome populations that may well participate in cancer and the complex bacteria-host interaction contributing to these devastating chronic diseases.
Acknowledgments
The authors would like to acknowledge the support of the NIDCR grant R01DE016593.
References
- Adinolfi E, Amoroso F, Giuliani AL. P2X7 Receptor Function in Bone-Related Cancer. J Osteoporos. 2012:637863. doi: 10.1155/2012/637863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aemaimanan P, Amimanan P, Taweechaisupapong S. Quantification of key periodontal pathogens in insulin-dependent type 2 diabetic and non-diabetic patients with generalized chronic periodontitis. Anaerobe. 2013;22:64–68. doi: 10.1016/j.anaerobe.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012a;23:399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012b;33:1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Amodini Rajakaruna G, Umeda M, Uchida K, Furukawa A, Yuan B, Suzuki Y, Noriko E, Izumi Y, Eishi Y. Possible translocation of periodontal pathogens into the lymph nodes draining the oral cavity. J Microbiol. 2012;50:827–836. doi: 10.1007/s12275-012-2030-8. [DOI] [PubMed] [Google Scholar]
- Berry D, Reinisch W. Intestinal microbiota: a source of novel biomarkers in inflammatory bowel diseases? Best practice & research. Clinical gastroenterology. 2013;27:47–58. doi: 10.1016/j.bpg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Boissan M, Lacombe ML. Learning about the functions of NME/NM23: lessons from knockout mice to silencing strategies. N-S Arch Pharmacol. 2011;384:421–431. doi: 10.1007/s00210-011-0649-3. [DOI] [PubMed] [Google Scholar]
- Chadet S, Jelassi B, Wannous R, Angoulvant D, Chevalier S, Besson P, Roger S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis. 2014 doi: 10.1093/carcin/bgt493. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2013 doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Scheuplein F, Driver JP, Hewes AA, Reifsnyder PC, Leiter EH, Serreze DV. Testing the role of P2X7 receptors in the development of type 1 diabetes in nonobese diabetic mice. J Immunol. 2011;186:4278–4284. doi: 10.4049/jimmunol.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, Yilmaz O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol. 2013;15:961–976. doi: 10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Cookson WO, Moffatt MF. Sequencing the human microbiome in health and disease. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt398. [DOI] [PubMed] [Google Scholar]
- Cummins J, Tangney M. Bacteria and tumours: causative agents or opportunistic inhabitants? Infect Agent Cancer. 2013;8:11. doi: 10.1186/1750-9378-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detert J, Pischon N, Burmester GR, Buttgereit F. The association between rheumatoid arthritis and periodontal disease. Arthritis Research & Therapy. 2010:12. doi: 10.1186/ar3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, Kasper DL. Resident commensals shaping immunity. Current opinion in immunology. 2013 doi: 10.1016/j.coi.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Sheh A. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4 doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez MH, Portales-Cervantes L, Cortez-Espinosa N, Vargas-Morales JM, Fritche Salazar JF, Rivera-Lopez E, Rodriguez-Rivera JG, Quezada-Calvillo R, Portales-Perez DP. Expression and function of P2X(7) receptor and CD39/Entpd1 in patients with type 2 diabetes and their association with biochemical parameters. Cell Immunol. 2011;269:135–143. doi: 10.1016/j.cellimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology. 2011;216:1302–1310. doi: 10.1016/j.imbio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therapeutic advances in gastroenterology. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol. 2005;101:e24–30. doi: 10.1159/000086036. [DOI] [PubMed] [Google Scholar]
- Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, Markland J, Robinson D, Elias B, Newkirk M, Toes RM, Huizinga TW, El-Gabalawy HS. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37:1105–1112. doi: 10.3899/jrheum.091323. [DOI] [PubMed] [Google Scholar]
- Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009;31:1228–1239. doi: 10.1002/hed.21140. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen Y, Wu W, Ouyang N, Chen J, Li H, Liu X, Su F, Lin L, Yao Y. miR-150 Promotes Human Breast Cancer Growth and Malignant Behavior by Targeting the Pro-Apoptotic Purinergic P2X7 Receptor. PloS one. 2013;8:e80707. doi: 10.1371/journal.pone.0080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PloS one. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, Morisaki I, Lamont RJ, Amano A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2013 doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, Ozaki K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3beta signaling pathway. Biochim Biophys Acta. 2013;1832:2035–2043. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li JY, Gore J, Jiang LH, Roger S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34:1487–1496. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jenkinson HF. Beyond the oral microbiome. Environ Microbiol. 2011;13:3077–3087. doi: 10.1111/j.1462-2920.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–215. doi: 10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili J. Oral cancer: risk factors, prevention and diagnostic. Exp Oncol. 2008;30:259–264. [PubMed] [Google Scholar]
- Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Hasegawa Y, Mao S, Shizukuishi S, Amano A, Lamont RJ, Yilmaz O. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185:2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3 doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangat P, Wegner N, Venables PJ, Potempa J. Bacterial and human peptidylarginine deiminases: targets for inhibiting the autoimmune response in rheumatoid arthritis? Arthritis Res Ther. 2010;12:209. doi: 10.1186/ar3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, Stavropoulos MF, Yilmaz O, Lamont RJ. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino N, Nakayama J, Collins JW, Steeg PS. Insights into the biology and prevention of tumor metastasis provided by the Nm23 metastasis suppressor gene. Cancer Metast Rev. 2012;31:593–603. doi: 10.1007/s10555-012-9374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrommatis B, Young GR, Kassiotis G. Counterpoise between the microbiome, host immune activation and pathology. Current opinion in immunology. 2013 doi: 10.1016/j.coi.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013 doi: 10.1093/carcin/bgt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Kaaks R, Boeing H, Foerster J, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Sieri S, Palli D, Tumino R, Panico S, Siersema PD, Peeters PH, Lund E, Barricarte A, Huerta JM, Molina-Montes E, Dorronsoro M, Quiros JR, Duell EJ, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Travis RC, Vineis P, Bueno-de-Mesquita HB, Riboli E. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2012 doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen D, Milinovich GJ, Burrell PC, Huynh SC, Pettett LM, Blackall LL, Trott DJ, Bird PS. Phylogenetic analysis of Porphyromonas species isolated from the oral cavity of Australian marsupials. Environ Microbiol. 2008;10:2425–2432. doi: 10.1111/j.1462-2920.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- Mitka M. Evidence lacking for benefit from oral cancer screening. JAMA. 2013;309:1884. doi: 10.1001/jama.2013.4913. [DOI] [PubMed] [Google Scholar]
- Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–308. [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, Park K, Lamont RJ. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS microbiology letters. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Oyanguren-Desez O, Rodriguez-Antiguedad A, Villoslada P, Domercq M, Alberdi E, Matute C. Gain-of-function of P2X7 receptor gene variants in multiple sclerosis. Cell Calcium. 2011;50:468–472. doi: 10.1016/j.ceca.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nakagawara A, Nagase H. RUNX Family Participates in the Regulation of p53-Dependent DNA Damage Response. Int J Genomics. 2013;2013:271347. doi: 10.1155/2013/271347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Yoon HE, Jeon DI, Ahn SG, Yoon JH. Activation of TLR2 and TLR5 did not affect tumor progression of an oral squamous cell carcinoma, YD-10B cells. J Oral Pathol Med. 2010;39:781–785. doi: 10.1111/j.1600-0714.2010.00900.x. [DOI] [PubMed] [Google Scholar]
- Prabhu Siddikuzzaman VV, Grace VM, Guruvayoorappan C. Targeting tumor metastasis by regulating Nm23 gene expression. Asian Pac J Cancer Prev. 2012;13:3539–3548. doi: 10.7314/apjcp.2012.13.8.3539. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Inv Drug. 2011;20:875–880. doi: 10.1517/13543784.2011.583918. [DOI] [PubMed] [Google Scholar]
- Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar CR, Sun JH, Li YH, Francois F, Corby P, Perez-Perez G, Dasanayake A, Pei ZH, Chen Y. Association between Selected Oral Pathogens and Gastric Precancerous Lesions. PloS one. 2013;8 doi: 10.1371/journal.pone.0051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Wyant T, Anaya-Bergman C, Aduse-Opoku J, Brunner J, Laine ML, Curtis MA, Lewis JP. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun. 2011;79:4533–4542. doi: 10.1128/IAI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. International journal of molecular sciences. 2011;12:334–352. doi: 10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner R, Yilmaz O. Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes Infect. 2012;14:228–237. doi: 10.1016/j.micinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Xia S, Ji Y, Kersten S, Qi L. The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes. 2012;61:1471–1478. doi: 10.2337/db11-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. Plos Pathog. 2013;9:e1003326. doi: 10.1371/journal.ppat.1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000. 2013;62:95–162. doi: 10.1111/prd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, Lillis C, Wactawski-Wende J, Scannapieco FA. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- Totaro MC, Cattani P, Ria F, Tolusso B, Gremese E, Fedele AL, D’Onghia S, Marchetti S, Sante GD, Canestri S, Ferraccioli G. Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: analysis of various compartments including the synovial tissue. Arthritis Res Ther. 2013;15:R66. doi: 10.1186/ar4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhani D, Krapfenbauer K, Thurnher D, Langen H, Fountoulakis M. Identification of differentially expressed, tumor-associated proteins in oral squamous cell carcinoma by proteomic analysis. Electrophoresis. 2006;27:1417–1423. doi: 10.1002/elps.200500510. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu CR, Sun Y. The Association Between Asthma and Helicobacter pylori: A Meta-Analysis. Helicobacter. 2013;18:41–53. doi: 10.1111/hel.12012. [DOI] [PubMed] [Google Scholar]
- Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, Lamont RJ, Jenkinson HF. Microbial interactions in building of communities. Molecular oral microbiology. 2013;28:83–101. doi: 10.1111/omi.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Yang X, Zhang RF, Li J, Xiao X, Hu YF, Chen YF, Yang FL, Lu N, Wang ZY, Luan CG, Liu YL, Wang BH, Xiang C, Wang YZ, Zhao FQ, Gao GF, Wang SY, Li LJ, Zhang HZ, Zhu BL. Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients. Microb Ecol. 2013;66:462–470. doi: 10.1007/s00248-013-0245-9. [DOI] [PubMed] [Google Scholar]
- Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, Yilmaz O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Molecular oral microbiology. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


