Abstract
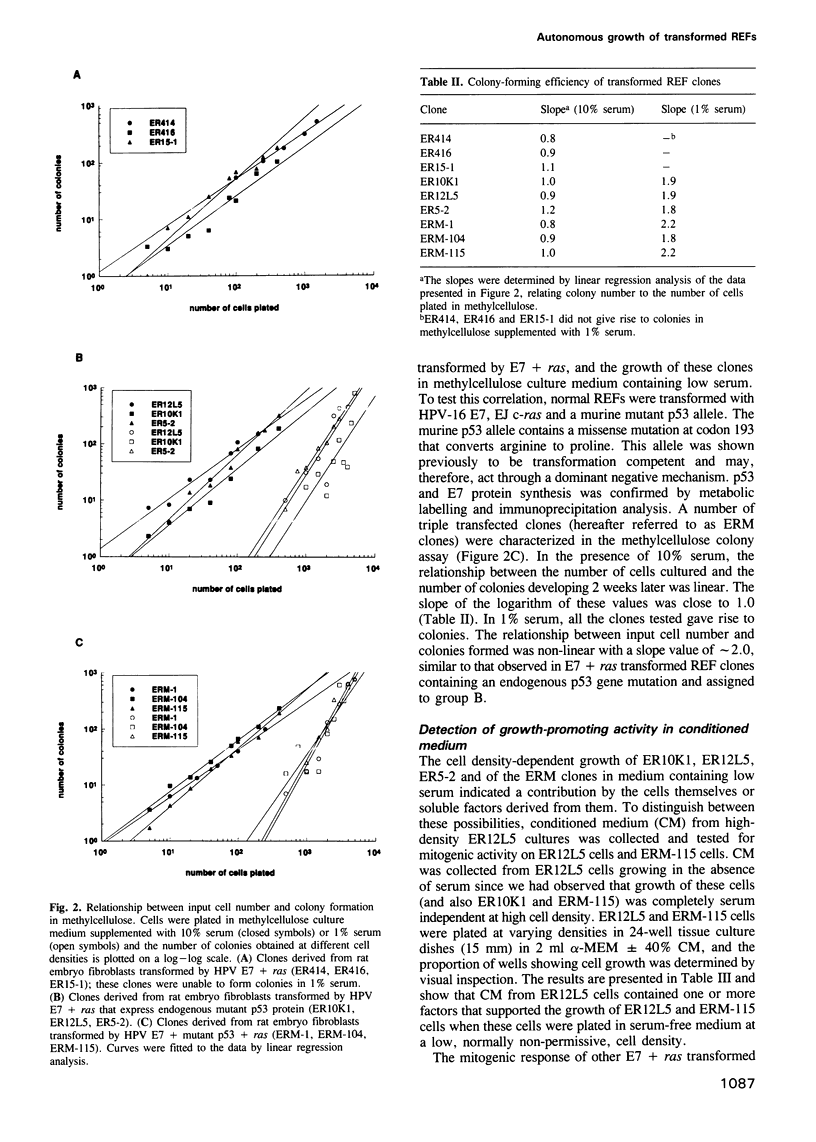
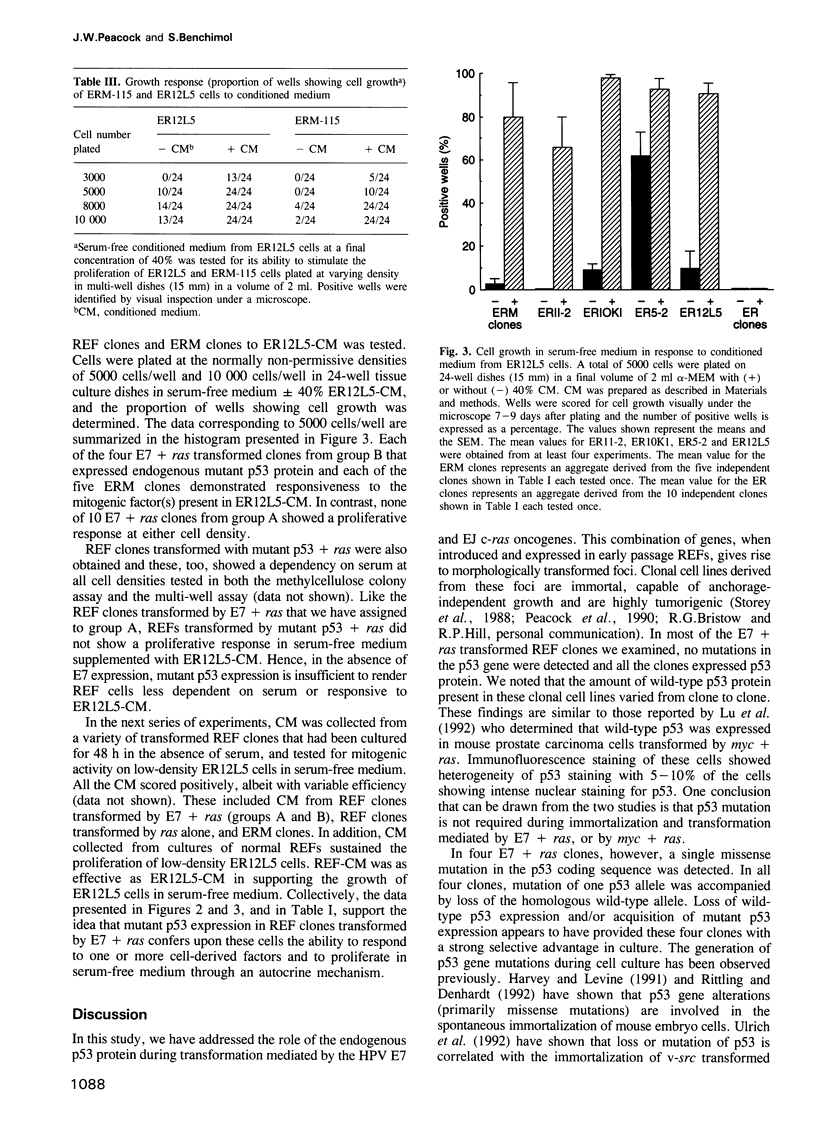
Rat embryo fibroblasts transformed with the HPV-16 E7 gene and the activated c-H-ras gene fall into two distinct phenotypic classes. At high cell density, clones of one class form colonies in methylcellulose supplemented with low serum; at low cell density, these cells display responsiveness to mitogenic factors present in serum-free conditioned medium from rat embryo fibroblasts. In contrast, clones of the second class exhibit an absolute dependency on growth factors present in serum at all cell densities in the methylcellulose colony assay and fail to respond to conditioned medium. We find that the status of the endogenous p53 gene is tightly correlated with these two classes of clones. Clones of the first class contain missense mutations in the p53 gene and have lost the wild-type allele. Clones of the second class express wild-type p53 protein. The importance of mutant p53 expression in reducing the growth factor dependency of transformed clones was confirmed in a separate series of experiments in which rat embryo fibroblasts were transformed with three genes, E7 + ras + mutant p53. The growth behaviour of these triply transfected clones was similar to that of the E7 + ras clones expressing endogenous mutant p53. We demonstrate that the enhanced proliferation of E7 + ras clones expressing mutant p53 protein involves an autocrine mechanism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja H., Bar-Eli M., Arlin Z., Advani S., Allen S. L., Goldman J., Snyder D., Foti A., Cline M. The spectrum of molecular alterations in the evolution of chronic myelocytic leukemia. J Clin Invest. 1991 Jun;87(6):2042–2047. doi: 10.1172/JCI115234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Berges R. R., Furuya Y., Remington L., English H. F., Jacks T., Isaacs J. T. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8910–8914. doi: 10.1073/pnas.90.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Crook T., Fisher C., Vousden K. H. Modulation of immortalizing properties of human papillomavirus type 16 E7 by p53 expression. J Virol. 1991 Jan;65(1):505–510. doi: 10.1128/jvi.65.1.505-510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Tidy J. A., Vousden K. H. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991 Nov 1;67(3):547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- Crook T., Vousden K. H. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J. 1992 Nov;11(11):3935–3940. doi: 10.1002/j.1460-2075.1992.tb05487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Wrede D., Tidy J. A., Mason W. P., Evans D. J., Vousden K. H. Clonal p53 mutation in primary cervical cancer: association with human-papillomavirus-negative tumours. Lancet. 1992 May 2;339(8801):1070–1073. doi: 10.1016/0140-6736(92)90662-m. [DOI] [PubMed] [Google Scholar]
- Crook T., Wrede D., Tidy J., Scholefield J., Crawford L., Vousden K. H. Status of c-myc, p53 and retinoblastoma genes in human papillomavirus positive and negative squamous cell carcinomas of the anus. Oncogene. 1991 Jul;6(7):1251–1257. [PubMed] [Google Scholar]
- Dittmer D., Pati S., Zambetti G., Chu S., Teresky A. K., Moore M., Finlay C., Levine A. J. Gain of function mutations in p53. Nat Genet. 1993 May;4(1):42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinstein E., Cimino G., Gale R. P., Alimena G., Berthier R., Kishi K., Goldman J., Zaccaria A., Berrebi A., Canaani E. p53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6293–6297. doi: 10.1073/pnas.88.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman P. N., Chen X., Bargonetti J., Prives C. The p53 protein is an unusually shaped tetramer that binds directly to DNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3319–3323. doi: 10.1073/pnas.90.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M., Levine A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991 Dec;5(12B):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Yamashita J., Yamaguchi K. Timing and role of p53 gene mutation in the recurrence of glioma. Biochem Biophys Res Commun. 1991 Oct 31;180(2):1145–1150. doi: 10.1016/s0006-291x(05)81186-6. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Kaelbling M., Burk R. D., Atkin N. B., Johnson A. B., Klinger H. P. Loss of heterozygosity on chromosome 17p and mutant p53 in HPV-negative cervical carcinomas. Lancet. 1992 Jul 18;340(8812):140–142. doi: 10.1016/0140-6736(92)93214-8. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Kraiss S., Quaiser A., Oren M., Montenarh M. Oligomerization of oncoprotein p53. J Virol. 1988 Dec;62(12):4737–4744. doi: 10.1128/jvi.62.12.4737-4744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lu X., Park S. H., Thompson T. C., Lane D. P. Ras-induced hyperplasia occurs with mutation of p53, but activated ras and myc together can induce carcinoma without p53 mutation. Cell. 1992 Jul 10;70(1):153–161. doi: 10.1016/0092-8674(92)90541-j. [DOI] [PubMed] [Google Scholar]
- Mashal R., Shtalrid M., Talpaz M., Kantarjian H., Smith L., Beran M., Cork A., Trujillo J., Gutterman J., Deisseroth A. Rearrangement and expression of p53 in the chronic phase and blast crisis of chronic myelogenous leukemia. Blood. 1990 Jan 1;75(1):180–189. [PubMed] [Google Scholar]
- Mietz J. A., Unger T., Huibregtse J. M., Howley P. M. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992 Dec;11(13):5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A., Cook A. C. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991 Jan;11(1):12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll U. M., Riou G., Levine A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Peacock J. W., Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990 Jul;10(7):3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Hayashi K., Hirohashi S., Sekiya T. Aberrations of the tumor suppressor p53 and retinoblastoma genes in human hepatocellular carcinomas. Cancer Res. 1991 Oct 15;51(20):5520–5525. [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Peacock J. W., Matlashewski G. J., Benchimol S. Synergism between pairs of immortalizing genes in transformation assays of rat embryo fibroblasts. Oncogene. 1990 Dec;5(12):1769–1774. [PubMed] [Google Scholar]
- Rittling S. R., Denhardt D. T. p53 mutations in spontaneously immortalized 3T12 but not 3T3 mouse embryo cells. Oncogene. 1992 May;7(5):935–942. [PubMed] [Google Scholar]
- Rovinski B., Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988 May;2(5):445–452. [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Ben-Ze'ev A., Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol. 1990 Dec;10(12):6565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Mikkelsen T., Schwechheimer K., Rosenblum M. L., Cavanee W., Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Slingerland J. M., Jenkins J. R., Benchimol S. The transforming and suppressor functions of p53 alleles: effects of mutations that disrupt phosphorylation, oligomerization and nuclear translocation. EMBO J. 1993 Mar;12(3):1029–1037. doi: 10.1002/j.1460-2075.1993.tb05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., Breugnot C., May E. Nucleotide sequence of a cDNA encoding the rat p53 nuclear oncoprotein. Nucleic Acids Res. 1988 Dec 9;16(23):11384–11384. doi: 10.1093/nar/16.23.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Addison C., Rudge K., Remm M., Grimaldi M., Keenan E., Jenkins J. R. A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992 Aug;7(8):1513–1523. [PubMed] [Google Scholar]
- Taylor W. R., Egan S. E., Mowat M., Greenberg A. H., Wright J. A. Evidence for synergistic interactions between ras, myc and a mutant form of p53 in cellular transformation and tumor dissemination. Oncogene. 1992 Jul;7(7):1383–1390. [PubMed] [Google Scholar]
- Ulrich E., Boehmelt G., Bird A., Beug H. Immortalization of conditionally transformed chicken cells: loss of normal p53 expression is an early step that is independent of cell transformation. Genes Dev. 1992 May;6(5):876–887. doi: 10.1101/gad.6.5.876. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Harris N., Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984 Aug;38(1):119–126. doi: 10.1016/0092-8674(84)90532-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Yoshida T., Hayashi K., Sekiya T., Yokota J., Hirohashi S., Nakatani K., Nakano H., Sugimura T., Terada M. p53 gene mutations in gastric cancer metastases and in gastric cancer cell lines derived from metastases. Cancer Res. 1991 Nov 1;51(21):5800–5805. [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]



