Abstract
Brown-headed cowbirds (Molothrus ater) are obligate brood parasites. Only females search for host nests and they find host nests one or more days before placing eggs in them. Past work has shown that females have a larger hippocampus than males, but sex differences in spatial cognition have not been extensively investigated. We tested cowbirds for sex and seasonal differences in spatial memory on a foraging task with an ecologically relevant retention interval. Birds were trained to find one rewarded location among 25 after 24 h. Females made significantly fewer errors than males and took more direct paths to the rewarded location than males. Females and males showed similar search times, indicating there was no sex difference in motivation. This sex difference in spatial cognition is the reverse of that observed in some polygynous mammals and is consistent with the hypothesis that spatial cognition is adaptively specialized in this brood-parasitic species.
Keywords: brown-headed cowbird, Molothrus ater, cognition, sex differences, spatial memory
1. Introduction
Memory can have profound effects on fitness and survival, but it is unclear exactly how natural selection has affected the evolution of memory. The predominant hypothesis in neuroecology, the adaptive specialization hypothesis (ASH), proposes that the brain and cognition are adaptively specialized to solve specific ecological problems [1,2]. For example, mating systems may favour greater spatial abilities in one sex over the other. Polygynous male voles (Microtus spp.) have a larger home range, a larger hippocampus, and outperform females on a spatial memory task, whereas no sex differences exist in monogamous voles [3–5].
Brown-headed cowbirds (Molothrus ater) provide a strong test of the ASH, because they exhibit a sex difference in space use that is the reverse of most species, with greater spatial memory demands on females. Cowbirds are obligate generalist brood parasites. Only females locate, monitor and revisit the host nests that they parasitize; female reproductive success depends on spatial ability [6]. Females locate host nests by searching the canopy, watching host nest building activity and attempting to flush incubating hosts from their nests [7]. Female cowbirds, which spend their mornings in their egg-laying range either alone or followed by males, parasitize nests before sunrise when it is still dark and must, therefore, have an accurate memory of the locations of potential host nests [6,8]. Female brown-headed cowbirds have a larger hippocampus than males, whereas no sex difference exists in related species that are not brood parasites [9–11]. This difference in the hippocampus size between males and females may be present only in the breeding season [11]. Sex and seasonal differences in spatial cognition in cowbirds, however, are not well understood [12].
We tested male and female cowbirds’ spatial memory in breeding and non-breeding conditions using a delayed-matching-to-sample spatial memory task. Birds re-located a single covered cup containing food among 25 cups after a retention interval (RI) of 1 or 24 h. Although the rewarded cup was not a host nest and did not contain eggs, we take the ability to return to a baited cup location as a general test of memory for spatial location [3]. The 1 h RI mimicked the interval between laying an egg and returning to remove a host egg [13]. The 24 h RI mimicked the interval between discovering a potential host nest, monitoring it daily and parasitizing it [14,15]. We hypothesized that in response to the cognitive demands placed on female cowbirds by brood-parasitic breeding, females would make fewer errors than males and that the sex difference would be most pronounced in breeding condition, when females search for host nests in the wild.
2. Material and methods
We tested female (n = 7) and male (n = 7) brown-headed cowbirds in a 180 × 180 cm wire mesh enclosure (75 cm in height) with a door on each of the four sides. Twenty-five cups formed a 5 × 5 square array on the floor with 10 cm between cups (figure 1). In the sample phase of each trial, a bird entered the apparatus through one of the four doors determined at random and was then free to search the array of open cups to locate the one cup baited with millet and mealworms. The baited cup was randomly selected on each trial to be one of the eight interior cups, excluding the centre cup (figure 1). Once the food was located, the bird was permitted to eat for 2 min. Following a 1 or 24 h RI in its home cage, the bird re-entered the apparatus for the matching phase of the trial, again through one of the four randomly assigned doors. During this phase, the baited cup was in the same location, but all of the cups were individually covered with white paper lids. The bird’s task was to find the cup that matched the location of the cup that was baited in the sample phase. To reduce the possible use of olfactory cues to locate the baited cup, all cups were shaken with millet inside for 15 s. Once the food was located, the bird was given 5 min to eat the food as a reward. If a bird did not find the baited cup within 20 min, the trial was ended and scored as unsuccessful.
Figure 1.
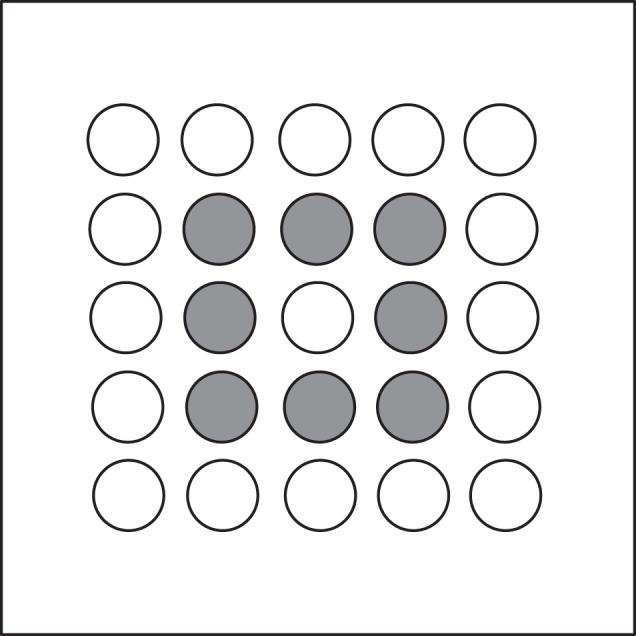
Diagram of the testing apparatus. Possible rewarded cups are shaded.
To assess memory in the matching phase for the location of the baited cup, we measured the number of errors before finding the baited cup and the time and path length between uncovering the first cup and uncovering the baited cup. Tortuosity of search was estimated as path length divided by the shortest possible path between entering the enclosure and the baited cup (the arc-chord ratio, equal to the inverse of the straightness index [16]). The expected number of errors to the first success is 25 if birds search at random and repeated visits to the same cup are scored as errors (i.e. sampling with replacement). If birds learn that only the eight interior cups are ever baited, the expected number of errors to the first success, scoring revisits as errors, would be 8. Because birds frequently revisited cups they had already opened, and these revisits were scored as errors, these values seem the best estimates of the number of errors expected by chance. If we assume instead that birds never revisit cups they have already opened (i.e. sample without replacement), then the number of errors expected by chance, based on the negative hypergeometric distribution, is 13 if birds searched all 25 cups and 4.5 if they searched only the interior eight cups.
Birds were tested every day; a full 24 h RI trial required 2 days to complete and a 1 h RI trial, a single day. Birds were tested first in non-breeding condition with the 1 h RI followed by the 24 h RI, and then retested in breeding condition with the 1 h RI followed by the 24 h RI. Birds learned the task during the 1 h RI in non-breeding condition and were given 10 practice trials before the 10 testing trials. During the 24 h RI in non-breeding condition and 1 and 24 h RIs in breeding condition, cowbirds were given 7, 5 and 5 training trials, respectively, before 10 testing trials. Acquisition was measured as the number of errors made across trials and the proportion of individuals who successfully completed the matching phase within the 20 min trial duration.
Non-breeding and breeding conditions were induced by manipulation of photoperiod, and breeding condition was verified by hormone assay and measurement of singing rates and gonads (see the electronic supplementary material, figure S1). Performance and acquisition data were analyzed using linear mixed models with PROC MIXED in SAS 9.3 (SAS Institute, Cary, NC, USA) with repeated measures for each subject across breeding conditions.
3. Results
(a). Task acquisition
Both sexes achieved better than chance performance (fewer than eight errors) on trial 2 of the 1 h RI in non-breeding condition with no effect of sex or sex by trial interaction (see the electronic supplementary material, figure S2). Over the 20 total trials (10 practice and 10 testing trials) on the 1 h RI in non-breeding, there was a significant increase in success across trials (F19,219 = 1.77, p = 0.028). There was no effect of sex (F1,12 = 0.23, p = 0.64) or sex by trial interaction (F19,219 = 0.69, p = 0.83). The proportion of successful individuals in the 10 testing trials did not differ among the four testing conditions (1 and 24 h RIs, non-breeding and breeding; F9,117 = 0.67, p = 0.73).
(b). Performance
(i). Errors across retention intervals and breeding conditions
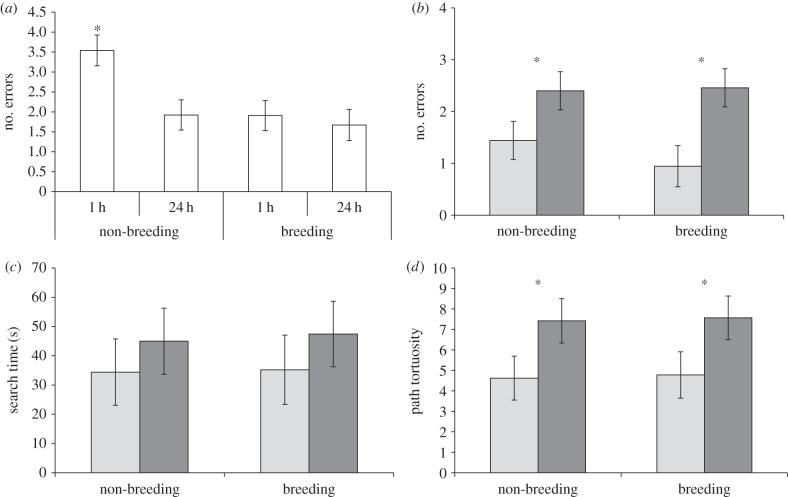
Birds improved as testing progressed (F3,38 = 6.98, p = 0.0007, figure 2a). Performance in the first condition (1 h non-breeding) was significantly different from the last three conditions probably because task acquisition continued during this initial condition (Tukey's tests: 24 h non-breeding, t38 = 3.56, p = 0.0053; 1 h breeding, t38 = −3.59, p = 0.0049; 24 h breeding, t38 = 4.03, p = 0.0014). Because performance was likely to be still improving during testing for the 1 h RI in non-breeding, the remainder of our analyses are based on the 24 h RI only.
Figure 2.
(a) Number of errors made by male and female brown-headed cowbirds across RIs and breeding conditions in testing order. Performance improved after the first condition (1 h non-breeding; asterisk indicates p < 0.05). (b) Number of errors before rewarded cup was opened during 24 h RI. Asterisks indicate a significant main effect of sex (p < 0.05). (c) Time required to find rewarded cup once searching commenced during the 24 h RI. (d) Path tortuosity: length of the path taken by the bird from the enclosure entrance to the correct cup divided by the shortest possible path from the entrance to the correct cup during the 24 h RI. Asterisks indicate a significant main effect of sex (p < 0.05). (b–d) Light grey bars indicate females and dark grey bars indicate males. (a-d) All values are means (±SE).
(ii). Number of errors
There was a significant effect of sex, with females making fewer errors than males (table 1 and figure 1b). There was no effect of breeding condition or interaction between breeding condition and sex (table 1 and figure 1b).
Table 1.
Summary of statistical effects of sex, breeding condition (BC) and their interaction at the 24 h RI for each measure of performance during the matching phase of the search task. Significant factors are in italic.
| factors | F | d.f. | p-value |
|---|---|---|---|
| number of errors | |||
| sex | 11.46 | 1,12 | 0.0054 |
| BC | 0.33 | 1,11 | 0.5775 |
| sex × BC | 0.52 | 1,11 | 0.4850 |
| search time | |||
| sex | 1.48 | 1,12 | 0.2475 |
| BC | 0.01 | 1,11 | 0.9048 |
| sex × BC | 0.00 | 1,11 | 0.9514 |
| path tortuosity | |||
| sex | 6.26 | 1,12 | 0.0278 |
| BC | 0.02 | 1,11 | 0.8869 |
| sex × BC | 0.00 | 1,11 | 0.9987 |
(iii). Search time
There was no effect of sex, breeding condition or interaction between sex and breeding condition (table 1 and figure 2c).
(iv). Path tortuosity
There was a significant effect of sex, with females having a less tortuous path than males (table 1 and figure 2d). There was no effect of breeding condition or interaction between breeding condition and sex (table 1 and figure 2d).
4. Discussion
Females made fewer spatial memory errors than males and took more direct paths to the rewarded cup (table 1 and figure 2b,d). There was no effect of sex for search time (figure 2c) indicating that females did not differ from males in motivation to search for the baited cup. This sex-specific effect may reflect an adaptation for brood parasitism because only females monitor host nests daily in the breeding season to appropriately time the parasitism event and maximize fitness [6,8,13,15]. We did not find a significant effect of breeding condition for any of the factors measured (table 1 and figure 2). However, because captivity may differentially affect the hippocampus, we cannot preclude the existence of seasonal differences in free-living birds [17]. Regardless, where sex differences in spatial cognition are found in animals, it is usually males who have better spatial ability [3]. In contrast, we show superior female spatial ability in a system with sex-role-reversed use of space.
Although our spatial memory task did not specifically test memory for host nests, our task likely tested for common underlying cognitive mechanisms which tap into the abilities that females use to re-visit host nests. Using food as a reward was necessary to ensure that both sexes would perform the task. Laboratory tests of spatial memory allow us to perform controlled tests of cognitive abilities that animals probably use in their natural environment.
Female brown-headed cowbirds have a larger hippocampus than males, unlike related species that are not brood parasites [9,10]. Here, we show that this difference in brain morphology is associated with superior spatial memory in females as predicted from behavioural sex differences observed in the wild [6,8]. Female superiority in memory for spatial locations in brown-headed cowbirds suggests that spatial ability in this species has been adaptively modified for a brood-parasitic mode of reproduction.
Acknowledgements
We thank Mark Conboy from Queen's University Biological Station, Stuart Mackenzie from Bird Studies Canada, Sarah Brodie and Robert Fearman.
Animal testing was performed under Canadian Council on Animal Care guidelines and University of Western Ontario Animal User Protocol 2007-001-03.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.b45bm [18].
Funding statement
Financial support was provided by NSERC Discovery grants to S.A.M-S. and D.F.S. and an NSERC postgraduate scholarship to M.F.G.
References
- 1.Sherry DF. 2006. Neuroecology. Annu. Rev. Psychol. 57, 167–197 (doi:10.1146/annurev.psych.56.091103.070324) [DOI] [PubMed] [Google Scholar]
- 2.Roth TC, Pravosudov VV. 2009. Hippocampal volume and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405 (doi:10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaulin SJC, FitzGerald RW. 1986. Sex differences in spatial ability: an evolutionary hypothesis and test. Am. Nat. 127, 74–88 (doi:10.1086/284468) [Google Scholar]
- 4.Gaulin SJC, FitzGerald RW. 1989. Sexual selection for spatial-learning ability. Anim. Behav. 37, 322–331 (doi:10.1016/0003-3472(89)90121-8) [Google Scholar]
- 5.Jacobs LF, Gaulin SJC, Sherry DF, Hoffman GE. 1990. Evolution of spatial cognition: sex-specific patterns of spatial behaviour predict hippocampal size. Proc. Natl Acad. Sci. USA 87, 6349–6352 (doi:10.1073/pnas.87.16.6349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothstein SI, Yokel DA, Fleischer RC. 1987. Social dominance, mating and spacing systems, female fecundity, and vocal dialects in captive and free-ranging brown-headed cowbirds. In Current ornithology, vol. 3 (ed. Johnston RF.), pp. 127–185 New York, NY: Plenum [Google Scholar]
- 7.Norman RF, Robertson RJ. 1975. Nest-searching behavior in the brown-headed cowbird. Auk 92, 610–611 (doi:10.2307/4084631) [Google Scholar]
- 8.Gates JE, Evans DR. 1998. Cowbirds breeding in the central Appalachians: spatial and temporal patterns and habitat selection. Ecol. Appl. 8, 27–40 (doi:10.2307/2641309) [Google Scholar]
- 9.Sherry DF, Forbes MR, Khurgel M, Ivy GO. 1993. Females have a larger hippocampus than males in the brood-parasitic brown-headed cowbird. Proc. Natl Acad. Sci. USA 90, 7839–7843 (doi:10.1073/pnas.90.16.7839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reboreda JC, Clayton NS, Kacelnik A. 1996. Species and sex differences in hippocampus size between parasitic and non-parasitic cowbirds. Neuroreport 7, 505–508 (doi:10.1097/00001756-199601310-00031) [DOI] [PubMed] [Google Scholar]
- 11.Clayton NS, Reboreda JC, Kacelnik A. 1997. Seasonal changes in hippocampus volume in parasitic cowbirds. Behav. Proc. 41, 237–243 (doi:10.1016/S0376-6357(97)00050-8) [DOI] [PubMed] [Google Scholar]
- 12.Astié AA, Kacelnik A, Reboreda JC. 1998. Sexual differences in memory in shiny cowbirds. Anim. Cogn. 1, 77–82 (doi:10.1007/s100710050011) [DOI] [PubMed] [Google Scholar]
- 13.Sealy SG. 1992. Removal of yellow warbler eggs in association with cowbird parasitism. Condor 94, 40–54 (doi:10.2307/1368794) [Google Scholar]
- 14.Sealy SG. 1995. Burial of cowbird eggs by parasitized yellow warblers: an empirical and experimental study. Anim. Behav. 49, 877–889 (doi:10.1006/anbe.1995.0120) [Google Scholar]
- 15.White DF, Ho L, Freed-Brown G. 2009. Counting chicks before they hatch: female cowbirds can time readiness of a host nest for parasitism. Psychol. Sci. 20, 1140–1145 (doi:10.1111/j.1467-9280.2009.02418.x) [DOI] [PubMed] [Google Scholar]
- 16.Benhamou S. 2004. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J. Theor. Biol. 229, 209–220 (doi:10.1016/j.jtbi.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 17.Day LB, Guerra M, Schlinger BA, Rothstein SI. 2008. Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater). Behav. Neurosci. 122, 527–534 (doi:10.1037/0735-7044.122.3.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guigueno MF, Snow DA, MacDougall-Shackleton SA, Sherry DF. 2014. Data from: Female cowbirds have more accurate spatial memory than males. Dryad Digital Repository. (doi:10.5061/dryad.b45bm) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.b45bm [18].