Abstract
The evolution of conspicuous sexually selected traits, such as horns or antlers, has fascinated biologists for more than a century. Elaborate traits can only evolve if they substantially increase reproduction, because they probably incur survival costs to the bearer. Total selection on these traits, however, includes sexual selection on sires and viability selection on offspring and can be influenced by changes in each of these components. Non-random associations between paternal phenotype and offspring viability may thus affect total selection on sexually selected traits. Long-term data on wild bighorn sheep (Ovis canadensis) provide the first evidence in nature that association between paternal phenotype and lamb viability strengthens total selection on horn size of adult rams, a sexually selected trait. The association of paternal horn length and offspring viability was sexually antagonistic: long-horned males sired sons with high viability but daughters of low viability. These results shed new light on the evolutionary dynamics of an iconic sexually selected trait and have important implications for sustainable wildlife management.
Keywords: sexual antagonism, sexual selection, total selection, viability selection
1. Introduction
Fitness, the currency of evolution, can be empirically measured by the number of sexually mature offspring produced. Offspring survival to recruitment is therefore a critical component of parental fitness. Selection on offspring viability could strengthen sexual selection if offspring of males with more elaborated sexually selected traits had above-average survival. Conversely, decreased offspring viability as a function of paternal sexually selected traits could counterbalance sexual selection. Father–offspring phenotypic resemblance through genetic or non-genetic inheritance [1] may influence offspring fitness [2]. Identifying how paternal phenotype may be related to offspring viability is thus critical to understand the evolutionary dynamics of sexually selected traits. This is further underlined by a recent debate on how total selection on sexually selected traits in the wild may respond to ecological factors for example predation on offspring [3].
To investigate how the relationship between paternal phenotype and offspring viability influenced total selection on sexually selected traits, we used long-term data from wild bighorn sheep on Ram Mountain, Canada. In this polygynous mammal, rams with large horns achieve high rates of paternity. We used regression-based selection analyses to examine how successive selective events on mating success and offspring viability affected sexual and total selection [4] on paternal horn length and body mass. We had three objectives. First, we tested the hypothesis that offspring viability varied with paternal horn length and body mass. Second, we investigated whether the association between paternal phenotype and offspring viability varied according to offspring sex. Finally, to assess how viability selection affected total selection, we quantified selection on paternal traits over successive selective episodes by considering the number of lambs produced, weaned and recruited to 1 year.
2. Material and methods
(a). Animals and population
Bighorn sheep displays strong sexual size dimorphism and males are under sexual selection [5]. Sheep on Ram Mountain have been monitored since 1971 and caught in a corral trap baited with salt. Body mass (kg) and horn length (cm) were measured at each capture. Most sheep are first caught as lambs and their exact age is known [6]. Lamb tissue samples are genotyped to assess male reproductive success. Paternities of lambs that die before capture are unknown. Lamb survival is evaluated via repeated censuses from May to September. We considered that a lamb survived to weaning if it was alive in September, and to 1 year if it was seen the following May. As the resighting probability is nearly one [6], survival estimates are very accurate. The main survival selective event for lambs occurred during winter [7]. We used 21 years of data from 1988 to 1992 and 1996 to 2011, when DNA samples were collected and paternity assigned.
(b). Paternity assignment
Samples genotyped at 26 microsatellite loci [8] resolved 229 paternities among 61 candidate fathers aged 2 years and older, assigned with 95% confidence in CERVUS [9]. We excluded lambs conceived from 1993 to 1995 because samples were not collected in 1994–1996.
(c). Selection analyses
We estimated standardized selection differentials i on both horn length and body mass following [4] as : i = cov(trait,fitness), where trait was annually standardized to zero mean and unit variance, and fitness was the relative annual fitness obtained by dividing individual absolute fitness by annual mean fitness. The strength of linear selection was estimated by selection differentials obtained by regressing standardized phenotypic traits against relative fitness [4]. We used three proxies of fitness: (i) breeding success, the number of offspring sampled; (ii) weaning success, the number of offspring surviving to late September and (iii) recruitment, the number of offspring that survived to 1 year. We pooled all years to estimate the selection differentials on horn length and body mass for each fitness proxy. The standardization of individual horn length and body mass allowed a comparison of selection differentials. We performed a Z-test for each comparison of selection differentials. Z-scores were calculated as 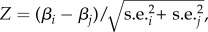 where β and s.e. were, respectively, the selection differential and their associated standard errors calculated for two different fitness proxies. A Z-score higher than 1.96 indicates a significant difference in a two-tailed test (p < 0.05). According to [10], the contribution of offspring viability to total selection was calculated as
where β and s.e. were, respectively, the selection differential and their associated standard errors calculated for two different fitness proxies. A Z-score higher than 1.96 indicates a significant difference in a two-tailed test (p < 0.05). According to [10], the contribution of offspring viability to total selection was calculated as 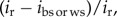 where the selection differential i was calculated via breeding success (ibs) or weaning success (iws) and recruitment (ir).
where the selection differential i was calculated via breeding success (ibs) or weaning success (iws) and recruitment (ir).
(d). Association between paternal phenotype and offspring viability
To evaluate whether paternal traits affect offspring viability, we modelled lamb survival to 1 year as a function of annually standardized paternal horn length or body mass, using separate generalized linear mixed effect models with a binomial error distribution and a logit link. These models also included the effects of lamb sex and an interaction between lamb sex and annually standardized paternal horn length or paternal body mass, lamb mass in September, population density, father's age, annually standardized maternal mass gain during summer and mother's age. Lamb mass in September affects survival over winter [11]. Standardized maternal mass gain during summer was interpreted as the ability of mothers to cope with environmental conditions and the energy costs of lactation. All continuous explanatory variables were scaled to zero mean and unit variance to allow comparison of their effect sizes. We also included year of birth, and mother and father identity as random variables to account for non-independence of offspring born from the same mother, father or in the same year. We used log-likelihood ratio test to assess the significance of random effects and to ensure that model fit was not reduced at each deletion step of the backward model simplification [10]. Inclusion of parental characteristics reduced the variance explained by random effects to statistical non-significance. We nevertheless retained these random effects to account for the hierarchical structure of the data. All statistical analyses were performed using R v. 2.14.1 [12], and generalized linear mixed models were fitted using the ‘lme4’ library [13].
3. Results
We found a sexually antagonistic non-random association between paternal phenotype and offspring viability (interaction paternal horn length—offspring sex: β = 1.189 ± 0.417 s.e., z = 2.851, p = 0.004, figure 1 and table 1). Survival from September to May of male and female lambs, respectively, increased by 15.4% and decreased by 11.4% per unit of standard deviation of paternal horn length. In absolute terms, survival of male and female lambs, respectively, increased by 1.12% and decreased by 0.83% per centimetre of paternal horn length. Selection on horn length was stronger via lamb recruitment (table 2) than via the number of lambs produced (Zrecruitment−lamb production = 12.111, p < 0.001) or weaned (Zrecruitment−weaning = 11.015, p < 0.001). Viability selection on offspring contributed 12.3% of the total selection on paternal horn length. Despite the strong correlation between horn length and body mass (r = 0.93, t = 50.744, d.f. = 430, p < 0.001), paternal mass did not affect lamb viability, although it had a non-significant effect similar to that of horn length (electronic supplementary material, table S1). The selection differential on paternal mass was stronger via lamb recruitment than via lambs produced or weaned (table 2). Population density or paternal age did not influence offspring viability (all ps > 0.23; electronic supplementary material, table S1). Horn length and body mass in males are genetically correlated and heritable [14]. As our models controlled for offspring body mass, which affects lamb winter survival [15], the relationship between paternal phenotype and offspring viability was not simply due to heavy and large-horned males producing large sons and small daughters.
Figure 1.
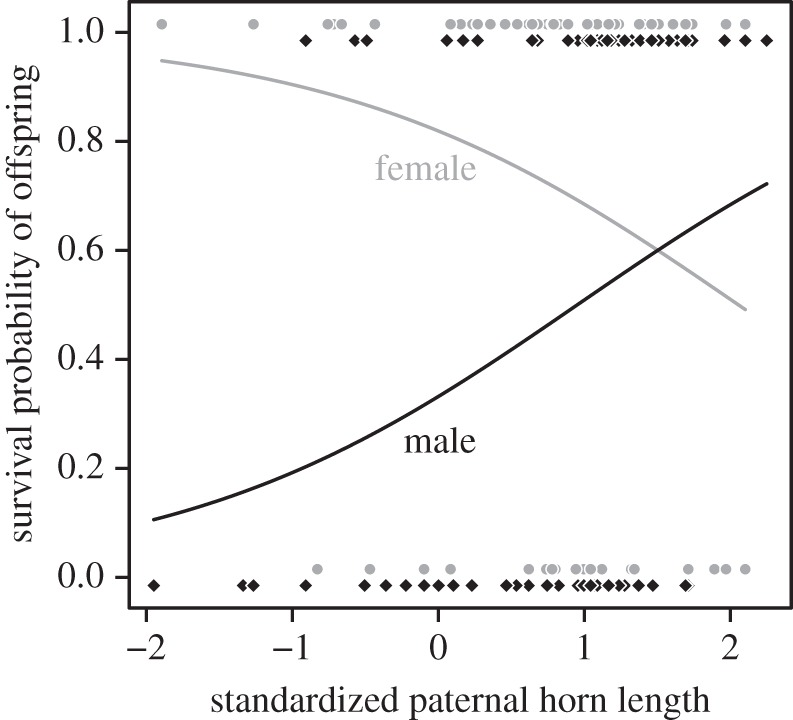
Bighorn lamb survival from September to May and paternal standardized horn length at Ram Mountain, Canada, 1988–1992 and 1996–2011. The grey and black solid lines are predictions of the model in table 1, respectively, for female and male lambs. Grey dots and black diamonds are data points for female and male offspring, respectively.
Table 1.
Effect of paternal horn length interacting with lamb sex on survival to 1 year of bighorn lambs at Ram Mountain, Canada, 1988–1992 and 1996–2011. Estimates are from a generalized linear mixed model with a binomial error distribution that included mother and father identity and year of birth as random effects. Female lambs were the sex of reference.
| variable | estimate | s.e. | z | p |
|---|---|---|---|---|
| intercept | 0.860 | 0.275 | 3.134 | 0.002 |
| father horn length | −0.594 | 0.308 | −1.928 | 0.054 |
| lamb sex (male) | −0.920 | 0.383 | −2.403 | 0.016 |
| mother mass gain | 0.657 | 0.202 | 3.245 | 0.001 |
| offspring mass | 0.419 | 0.205 | 2.047 | 0.041 |
| father horn length × lamb sex (male) | 1.189 | 0.417 | 2.851 | 0.004 |
Table 2.
Linear selection differentials calculated via the number of lambs produced, weaned and recruited, on horn length and body mass of bighorn rams at Ram Mountain, Canada, 1988–1992 and 1996–2011. Different superscripts indicate significant differences between selection differentials.
| traits | fitness proxy | linear selection differential ± s.e. | t | p |
|---|---|---|---|---|
| horn length | production | 0.934 ± 0.006a | 9.675 | <0.001 |
| horn length | weaning | 0.939 ± 0.007a | 9.403 | <0.001 |
| horn length | recruitment | 1.065 ± 0.009b | 7.004 | <0.001 |
| body mass | production | 0.853 ± 0.007c | 9.764 | <0.001 |
| body mass | weaning | 0.844 ± 0.008c | 9.352 | <0.001 |
| body mass | recruitment | 0.885 ± 0.010d | 7.188 | <0.001 |
4. Discussion
Only a handful of studies of vertebrates have examined the relationship between paternal phenotype and offspring viability in nature [2,16] and none investigated whether this relationship modifies selection on sexually selected traits. Because rams with the longest horns were sexually selected, sexual selection and offspring viability selection acted in concert. Our investigation of successive selection events thus revealed that a substantial part of total selection on paternal horn length was due to viability selection on offspring.
Our results have important implications for evolutionary biology, wildlife management and conservation. In long-lived species, attention should be paid to how successive selective episodes affect total selection, because selection on quantitative traits can vary over the lifespan in changing environments [17]. Evaluating how different fitness components may influence the overall strength of selection is critical for understanding the temporal dynamics of selection on phenotypic traits. Given the potentially important role of viability selection in total selection on sexually selected traits, sexual selection analyses alone may not fully reveal the evolutionary dynamics of these traits. Predictions of evolutionary changes in sexually selected traits should consider possible correlations of these traits with other fitness components. Finally, trophy hunting exerts strong artificial selection with potentially undesirable evolutionary effects by selectively removing large-horned males [14]. Our result suggests that trophy hunting may also have sex-specific effects on juvenile survival. Selective hunting may thus have indirect effects on population sex ratio, an important factor for population dynamics, recruitment and wildlife management [18].
Acknowledgements
We thank A. Hubbs, C. Feder and J. Jorgenson for their support of the Ram Mountain research programme, all assistants and students who worked on Ram Mountain over decades, J. Hogg for initiating tissue sample collections and analyses, and P. Bergeron and D. Garant for discussions.
This research project was approved by the Animal Care Committee of the Université de Sherbrooke (MFB2009-01 and FP2012-01), an affiliate of the Canadian Council on Animal Care.
Data accessibility
After a one year embargo, data will be available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.j4n82.
Funding statement
M.F.B., D. C. and F.P. are financially supported by NSERC. F.P. holds the Canada Research Chair in Evolutionary Demography and Conservation. Our research is also supported by the Government of Alberta and the Alberta Conservation Association.
References
- 1.Danchin É, Charmantier A, Champagne F, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486 (doi:10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 2.Qvarnström A, Price TD. 2001. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100 (doi:10.1016/S0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 3.Bergeron P, Martin AM, Garant D, Pelletier F. 2013. Comment on ‘Bateman in nature: predation on offspring reduces the potential for sexual selection’. Science 340, 549 (doi:10.1126/science.1233246) [DOI] [PubMed] [Google Scholar]
- 4.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 5.Coltman DW, Festa-Bianchet M, Jorgenson JT, Strobeck C. 2002. Age-dependent sexual selection in bighorn rams. Proc. R. Soc. Lond. B 269, 165–172 (doi:10.1098/rspb.2001.1851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgenson JT, Festa-Bianchet M, Gaillard J-M, Wishart WD. 1997. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78, 1019–1032 (doi:10.1890/0012-9658(1997)078[1019:EOASDA]2.0.CO;2) [Google Scholar]
- 7.Portier C, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Yoccoz NG. 1998. Effects of density and weather on survival of bighorn sheep lambs (Ovis canadensis). J. Zool. 245, 271–278 (doi:10.1017/s0952836998007043) [Google Scholar]
- 8.Coltman DW, O'Donoghue P, Hogg JT, Festa-Bianchet M. 2005. Selection and genetic (co)variance in bighorn sheep. Evolution 59, 1372–1382 [PubMed] [Google Scholar]
- 9.Marshall TC, Slate J, Kruuk LEB, Pemberton JM. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655 (doi:10.1046/j.1365-294x.1998.00374.x) [DOI] [PubMed] [Google Scholar]
- 10.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, New York, NY: Springer [Google Scholar]
- 11.Rioux-Paquette E, Festa-Bianchet M, Coltman DW. 2011. Sex-differential effects of inbreeding on overwinter survival, birth date and mass of bighorn lambs. J. Evol. Biol. 24, 121–131 (doi:10.1111/j.1420-9101.2010.02154.x) [DOI] [PubMed] [Google Scholar]
- 12.R Development Core Team 2011. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/). [Google Scholar]
- 13.Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes R package version 0999375–42 See http://CRANR-projectorg/package=lme4
- 14.Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 (doi:10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 15.Feder C, Martin JGa, Festa-Bianchet M, Bérubé C, Jorgenson J. 2008. Never too late? Consequences of late birthdate for mass and survival of bighorn lambs. Oecologia 156, 773–781 (doi:10.1007/s00442-008-1035-9) [DOI] [PubMed] [Google Scholar]
- 16.Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB. 2007. Sexually antagonistic genetic variation for fitness in red deer. Nature 447, 1107–1110 (doi:10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 17.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 18.Milner JM, Nilsen EB, Andreassen HP. 2007. Demographic side effects of selective hunting in ungulates and carnivores. Conserv. Biol. 21, 36–47 (doi:10.1111/j.1523-1739.2006.00591.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After a one year embargo, data will be available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.j4n82.


