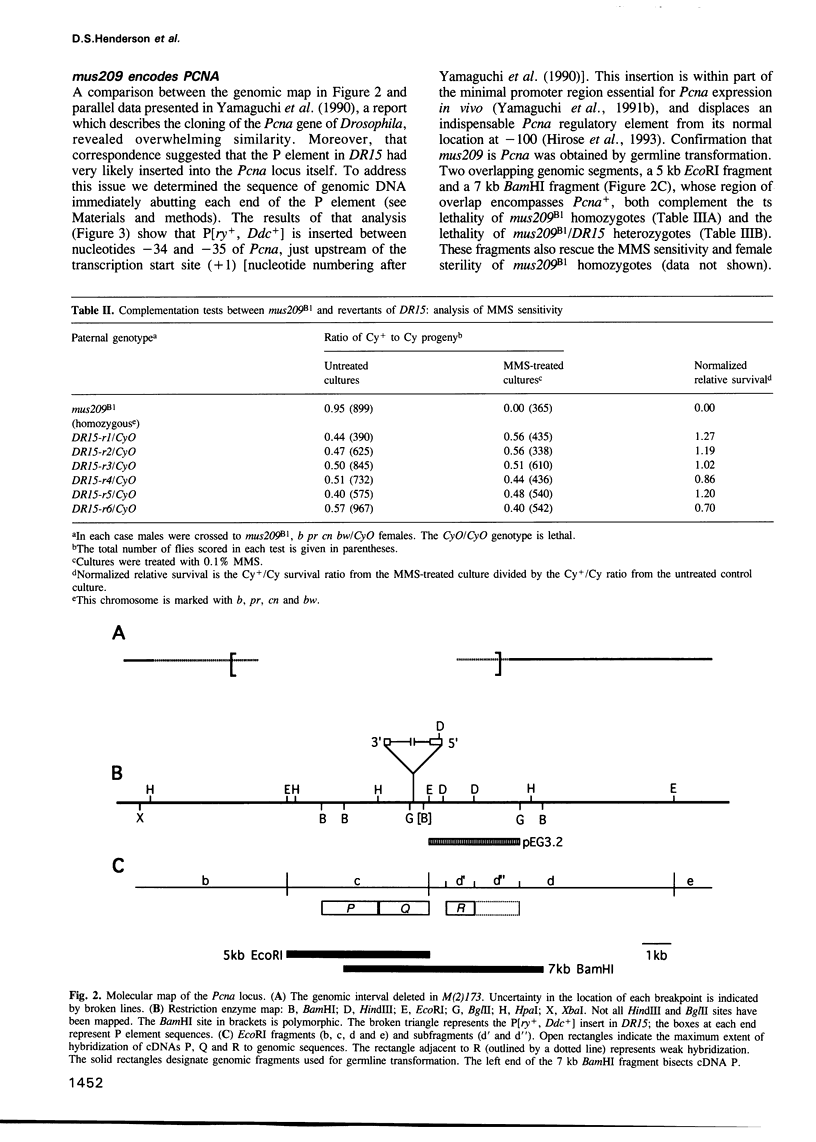
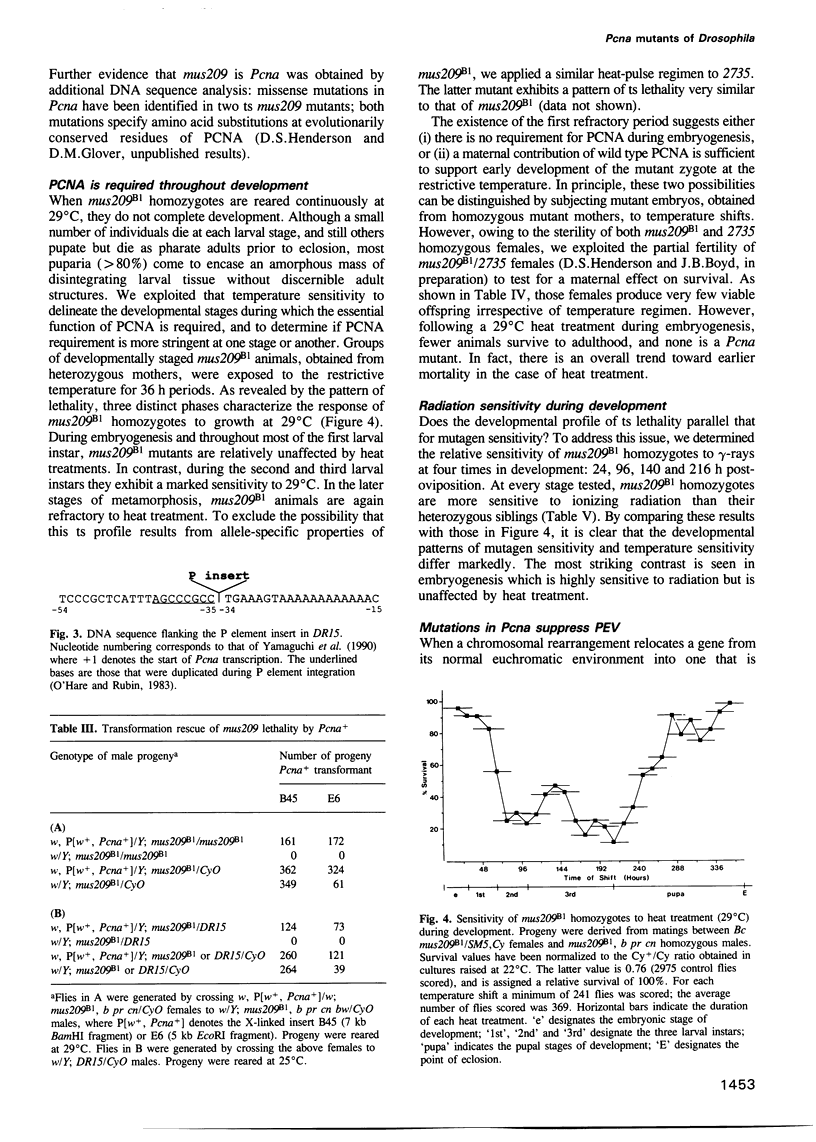
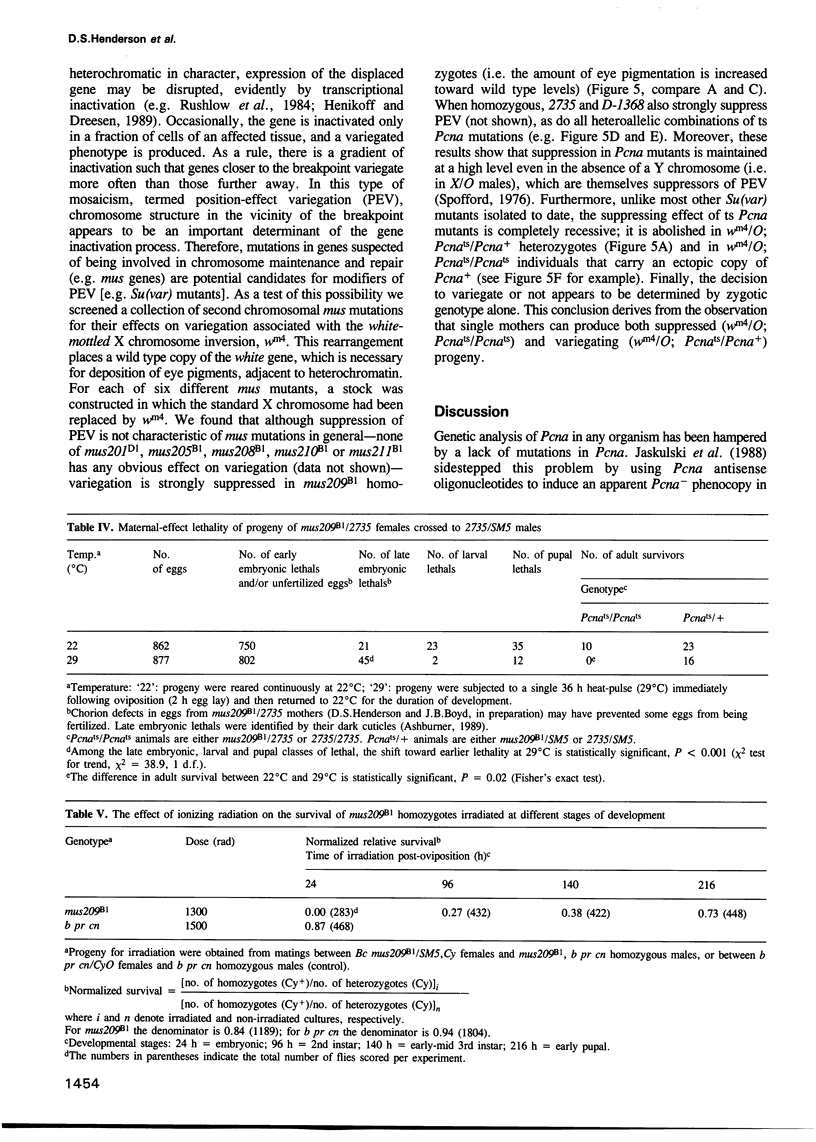
Abstract
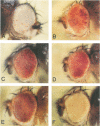
The mus209B1 mutant of Drosophila melanogaster exhibits a complex pleiotropy of temperature-sensitive (ts) lethality, hypersensitivity to DNA-damaging agents such as ionizing radiation and methyl methanesulfonate, suppression of position-effect variegation (PEV), and female sterility. Our discovery that mus209 encodes proliferating cell nuclear antigen (PCNA), which is an indispensable component of the DNA replication apparatus, suggests that alterations to chromosome replication may underlie that pleiotropy. Nine lethal mutations, three of them ts, genetically define the Pcna locus. Temperature shift studies reveal that the vital function of PCNA is required throughout virtually all stages of fly development, and that maternally encoded PCNA is essential for embryogenesis. All three ts mutants strongly suppress PEV, which suggests a role for PCNA in chromatin assembly or modification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R. Poly ADP-ribosylation: a histone shuttle mechanism in DNA excision repair. J Cell Sci. 1992 Aug;102(Pt 4):663–670. doi: 10.1242/jcs.102.4.663. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Smith D. A., Gatti M. Region-specific effects on chromosome integrity of mutations at essential loci in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1205–1209. doi: 10.1073/pnas.79.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Smith D. A. The effects of mutagen-sensitive mutants of Drosophila melanogaster in nonmutagenized cells. Genetics. 1979 Jul;92(3):833–847. doi: 10.1093/genetics/92.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker W. K. Position-effect variegation. Adv Genet. 1968;14:133–169. [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res. 1990 Jan 25;18(2):261–265. doi: 10.1093/nar/18.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase delta. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7506–7510. doi: 10.1073/pnas.85.20.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Mason J. M., Yamamoto A. H., Brodberg R. K., Banga S. S., Sakaguchi K. A genetic and molecular analysis of DNA repair in Drosophila. J Cell Sci Suppl. 1987;6:39–60. doi: 10.1242/jcs.1984.supplement_6.3. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P., Celis A., Nielsen H. V., Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987 Aug 10;220(1):1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Madsen P. Increased nuclear cyclin/PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986 Dec 15;209(2):277–283. doi: 10.1016/0014-5793(86)81127-9. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C. Position effect variegation in Drosophila: towards a genetics of chromatin assembly. Bioessays. 1989 Jul;11(1):14–17. doi: 10.1002/bies.950110105. [DOI] [PubMed] [Google Scholar]
- Fairman M. P. DNA polymerase delta/PCNA: actions and interactions. J Cell Sci. 1990 Jan;95(Pt 1):1–4. doi: 10.1242/jcs.95.3.1. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Gatti M. Genetic control of chromosome breakage and rejoining in Drosophila melanogaster: spontaneous chromosome aberrations in X-linked mutants defective in DNA metabolism. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1377–1381. doi: 10.1073/pnas.76.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M., Smith D. A., Baker B. S. A gene controlling condensation of heterochromatin in Drosophila melanogaster. Science. 1983 Jul 1;221(4605):83–85. doi: 10.1126/science.6407113. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Karpen G. H., Spradling A. C. Replication forks are not found in a Drosophila minichromosome demonstrating a gradient of polytenization. Chromosoma. 1992 Dec;102(1):15–19. doi: 10.1007/BF00352285. [DOI] [PubMed] [Google Scholar]
- Henderson D. S., Bailey D. A., Sinclair D. A., Grigliatti T. A. Isolation and characterization of second chromosome mutagen-sensitive mutations in Drosophila melanogaster. Mutat Res. 1987 Mar;177(1):83–93. doi: 10.1016/0027-5107(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Dreesen T. D. Trans-inactivation of the Drosophila brown gene: evidence for transcriptional repression and somatic pairing dependence. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6704–6708. doi: 10.1073/pnas.86.17.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Position-effect variegation after 60 years. Trends Genet. 1990 Dec;6(12):422–426. doi: 10.1016/0168-9525(90)90304-o. [DOI] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Barry J., Alberts B. M., Geiduschek E. P. Enhancement of bacteriophage T4 late transcription by components of the T4 DNA replication apparatus. Science. 1989 Sep 1;245(4921):952–958. doi: 10.1126/science.2672335. [DOI] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Geiduschek E. P. A transcriptional enhancer whose function imposes a requirement that proteins track along DNA. Science. 1992 May 29;256(5061):1298–1303. doi: 10.1126/science.1598572. [DOI] [PubMed] [Google Scholar]
- Hirose F., Yamaguchi M., Handa H., Inomata Y., Matsukage A. Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase alpha and proliferating cell nuclear antigen. J Biol Chem. 1993 Jan 25;268(3):2092–2099. [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Spradling A. C. Reduced DNA polytenization of a minichromosome region undergoing position-effect variegation in Drosophila. Cell. 1990 Oct 5;63(1):97–107. doi: 10.1016/0092-8674(90)90291-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khesin R. B., Leibovitch B. A. Influence of deficiency of the histone gene-containing 38B-40 region on X-chromosome template activity and the white gene position effect variegation in Drosophila melanogaster. Mol Gen Genet. 1978 Jul 4;162(3):323–328. doi: 10.1007/BF00268858. [DOI] [PubMed] [Google Scholar]
- Kill I. R., Bridger J. M., Campbell K. H., Maldonado-Codina G., Hutchison C. J. The timing of the formation and usage of replicase clusters in S-phase nuclei of human diploid fibroblasts. J Cell Sci. 1991 Dec;100(Pt 4):869–876. doi: 10.1242/jcs.100.4.869. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Chromatin structure and transcription. Annu Rev Cell Biol. 1992;8:563–587. doi: 10.1146/annurev.cb.08.110192.003023. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Pan Z. Q., Kwong A. D., Burgers P. M., Hurwitz J. Synthesis of DNA by DNA polymerase epsilon in vitro. J Biol Chem. 1991 Nov 25;266(33):22707–22717. [PubMed] [Google Scholar]
- Miura M., Domon M., Sasaki T., Takasaki Y. Induction of proliferating cell nuclear antigen (PCNA) complex formation in quiescent fibroblasts from a xeroderma pigmentosum patient. J Cell Physiol. 1992 Feb;150(2):370–376. doi: 10.1002/jcp.1041500221. [DOI] [PubMed] [Google Scholar]
- Moore G. D., Procunier J. D., Cross D. P., Grigliatti T. A. Histone gene deficiencies and position--effect variegation in Drosophila. Nature. 1979 Nov 15;282(5736):312–314. doi: 10.1038/282312a0. [DOI] [PubMed] [Google Scholar]
- Moore G. D., Sinclair D. A., Grigliatti T. A. Histone Gene Multiplicity and Position Effect Variegation in DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):327–344. doi: 10.1093/genetics/105.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Ng L., Prelich G., Anderson C. W., Stillman B., Fisher P. A. Drosophila proliferating cell nuclear antigen. Structural and functional homology with its mammalian counterpart. J Biol Chem. 1990 Jul 15;265(20):11948–11954. [PubMed] [Google Scholar]
- Nichols A. F., Sancar A. Purification of PCNA as a nucleotide excision repair protein. Nucleic Acids Res. 1992 Jul 11;20(13):2441–2446. doi: 10.1093/nar/20.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Reuter G., Spierer P. Position effect variegation and chromatin proteins. Bioessays. 1992 Sep;14(9):605–612. doi: 10.1002/bies.950140907. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow C. A., Bender W., Chovnick A. Studies on the mechanism of heterochromatic position effect at the rosy locus of Drosophila melanogaster. Genetics. 1984 Nov;108(3):603–615. doi: 10.1093/genetics/108.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Selig S., Okumura K., Ward D. C., Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992 Mar;11(3):1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Sinclair D. A., Suzuki D. T., Grigliatti T. A. Genetic and developmental analysis of a temperature-sensitive minute mutation of Drosophila melanogaster. Genetics. 1981 Mar-Apr;97(3-4):581–606. doi: 10.1093/genetics/97.3-4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989 Jul 14;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 1991 Apr;10(4):971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Karpen G. H. Sixty years of mystery. Genetics. 1990 Dec;126(4):779–784. doi: 10.1093/genetics/126.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D., Bishop C., Jones M., Hobbs C. A., Locke J. Towards an understanding of position effect variegation. Dev Genet. 1989;10(3):162–176. doi: 10.1002/dvg.1020100306. [DOI] [PubMed] [Google Scholar]
- Tartof K. D., Hobbs C., Jones M. A structural basis for variegating position effects. Cell. 1984 Jul;37(3):869–878. doi: 10.1016/0092-8674(84)90422-7. [DOI] [PubMed] [Google Scholar]
- Toschi L., Bravo R. Changes in cyclin/proliferating cell nuclear antigen distribution during DNA repair synthesis. J Cell Biol. 1988 Nov;107(5):1623–1628. doi: 10.1083/jcb.107.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Functions of replication factor C and proliferating-cell nuclear antigen: functional similarity of DNA polymerase accessory proteins from human cells and bacteriophage T4. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1023–1027. doi: 10.1073/pnas.87.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Labib K., Nurse P., Lane D. P. Isolation and analysis of the fission yeast gene encoding polymerase delta accessory protein PCNA. EMBO J. 1992 Dec;11(13):5111–5120. doi: 10.1002/j.1460-2075.1992.tb05618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. New insights into chromatin function in transcriptional control. FASEB J. 1992 Dec;6(15):3354–3361. doi: 10.1096/fasebj.6.15.1464369. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Date T., Matsukage A. Distribution of PCNA in Drosophila embryo during nuclear division cycles. J Cell Sci. 1991 Dec;100(Pt 4):729–733. doi: 10.1242/jcs.100.4.729. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hirose F., Nishida Y., Matsukage A. Repression of the Drosophila proliferating-cell nuclear antigen gene promoter by zerknüllt protein. Mol Cell Biol. 1991 Oct;11(10):4909–4917. doi: 10.1128/mcb.11.10.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Nishida Y., Moriuchi T., Hirose F., Hui C. C., Suzuki Y., Matsukage A. Drosophila proliferating cell nuclear antigen (cyclin) gene: structure, expression during development, and specific binding of homeodomain proteins to its 5'-flanking region. Mol Cell Biol. 1990 Mar;10(3):872–879. doi: 10.1128/mcb.10.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xiong Y., Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993 Sep;4(9):897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]