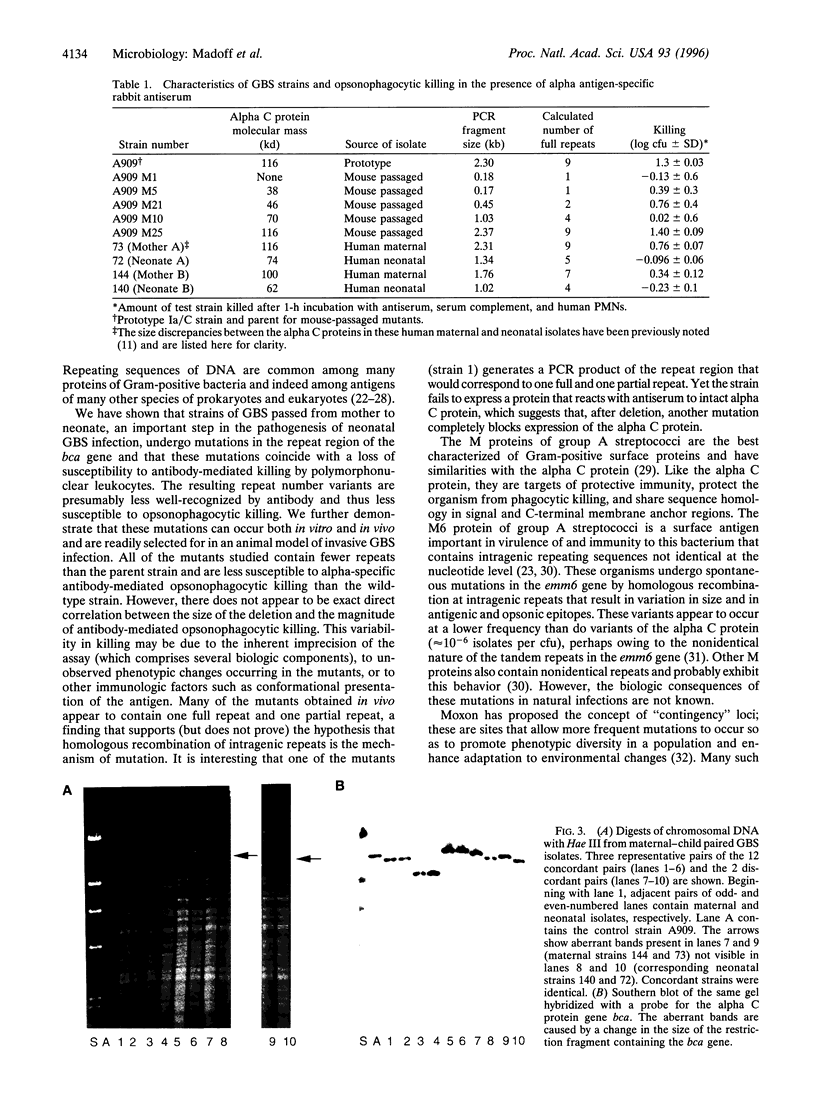
Abstract
Group B streptococci (GBS) are the most common cause of neonatal sepsis, pneumonia, and meningitis. The alpha C protein is a surface-associated antigen; the gene (bca) for this protein contains a series of tandem repeats (each encoding 82 aa) that are identical at the nucleotide level and express a protective epitope. We previously reported that GBS isolates from two of 14 human maternal and neonatal pairs differed in the number of repeats contained in their alpha C protein; in both pairs, the alpha C protein of the neonatal isolate was smaller in molecular size. We now demonstrate by PCR that the neonatal isolates contain fewer tandem repeats. Maternal isolates were susceptible to opsonophagocytic killing in the presence of alpha C protein-specific antiserum, whereas the discrepant neonatal isolates proliferated. An animal model was developed to further study this phenomenon. Adult mice passively immunized with antiserum to the alpha C protein were challenged with an alpha C protein-expressing strain of GBS. Splenic isolates of GBS from these mice showed a high frequency of mutation in bca--most commonly a decrease in repeat number. Isolates from non-immune mice were not altered. Spontaneous deletions in the repeat region were observed at a much lower frequency (6 x 10(-4)); thus, deletions in that region are selected for under specific antibody pressure and appear to lower the organism's susceptibility to killing by antibody specific to the alpha C protein. This mechanism of antigenic variation may provide a means whereby GBS evade host immunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Group B streptococcal vaccines. Rev Infect Dis. 1985 Jul-Aug;7(4):458–467. doi: 10.1093/clinids/7.4.458. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Barbour A. G., Carter C. J., Burman N., Freitag C. S., Garon C. F., Bergström S. Tandem insertion sequence-like elements define the expression site for variable antigen genes of Borrelia hermsii. Infect Immun. 1991 Jan;59(1):390–397. doi: 10.1128/iai.59.1.390-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevanger L., Naess A. I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):121–124. doi: 10.1111/j.1699-0463.1985.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Dramsi S., Dehoux P., Cossart P. Common features of gram-positive bacterial proteins involved in cell recognition. Mol Microbiol. 1993 Sep;9(5):1119–1121. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Farley M. M., Harvey R. C., Stull T., Smith J. D., Schuchat A., Wenger J. D., Stephens D. S. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med. 1993 Jun 24;328(25):1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- Fasola E., Livdahl C., Ferrieri P. Molecular analysis of multiple isolates of the major serotypes of group B streptococci. J Clin Microbiol. 1993 Oct;31(10):2616–2620. doi: 10.1128/jcm.31.10.2616-2620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein. Sci Am. 1991 Jun;264(6):58–65. doi: 10.1038/scientificamerican0691-58. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Hervás J. A., González L., Gil J., Paoletti L. C., Madoff L. C., Benedí V. J. Neonatal group B streptococcal infection in Mallorca, Spain. Clin Infect Dis. 1993 May;16(5):714–718. doi: 10.1093/clind/16.5.714. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Ibañez C. F., Affranchino J. L., Macina R. A., Reyes M. B., Leguizamon S., Camargo M. E., Aslund L., Pettersson U., Frasch A. C. Multiple Trypanosoma cruzi antigens containing tandemly repeated amino acid sequence motifs. Mol Biochem Parasitol. 1988 Jul;30(1):27–33. doi: 10.1016/0166-6851(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Jones K. F., Hollingshead S. K., Scott J. R., Fischetti V. A. Spontaneous M6 protein size mutants of group A streptococci display variation in antigenic and opsonogenic epitopes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8271–8275. doi: 10.1073/pnas.85.21.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Hori S., Michel J. L., Baker C. J., Kasper D. L. Phenotypic diversity in the alpha C protein of group B streptococci. Infect Immun. 1991 Aug;59(8):2638–2644. doi: 10.1128/iai.59.8.2638-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Michel J. L., Kasper D. L. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991 Jan;59(1):204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Michel J. L., Kasper D. L. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991 Jan;59(1):204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. L., Madoff L. C., Kling D. E., Kasper D. L., Ausubel F. M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991 Jun;59(6):2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. L., Madoff L. C., Olson K., Kling D. E., Kasper D. L., Ausubel F. M. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10060–10064. doi: 10.1073/pnas.89.21.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R. Molecular basis of invasive Haemophilus influenzae type b disease. J Infect Dis. 1992 Jun;165 (Suppl 1):S77–S81. doi: 10.1093/infdis/165-supplement_1-s77. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Rainey P. B., Nowak M. A., Lenski R. E. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994 Jan 1;4(1):24–33. doi: 10.1016/s0960-9822(00)00005-1. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Wright C. J., Jerse A. E., Cohen M. S., Cannon J. G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest. 1994 Jun;93(6):2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålhammar-Carlemalm M., Stenberg L., Lindahl G. Protein rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993 Jun 1;177(6):1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis A. E., McMaster W. R. Identification of Leishmania genes encoding proteins containing tandemly repeating peptides. J Exp Med. 1987 Dec 1;166(6):1814–1824. doi: 10.1084/jem.166.6.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Dunny G. M. Identification of a Streptococcus agalactiae protein antigen associated with bovine mastitis isolates. Infect Immun. 1987 May;55(5):1170–1175. doi: 10.1128/iai.55.5.1170-1175.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Briles D. E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992 Jan;174(2):601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Teng L. J., Watson H. L., Glass J. I., Blanchard A., Cassell G. H. Small repeating units within the Ureaplasma urealyticum MB antigen gene encode serovar specificity and are associated with antigen size variation. Infect Immun. 1995 Mar;63(3):891–898. doi: 10.1128/iai.63.3.891-898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]