Abstract
Cell recognition and mating in the smut fungus Ustilago maydis have been proposed to involve specific pheromones and pheromone receptors. The respective structural genes are located in the a mating type locus that exists in the alleles a1 and a2. We demonstrate that binding of pheromone to the receptor can induce a morphological switch from yeast-like to filamentous growth in certain strains. Using this as biological assay we were able to purify both the a1 and a2 pheromone. The structure of the secreted pheromones was determined to be 13 amino acids for a1 and nine amino acids for a2. Both pheromones are post-translationally modified by farnesylation and carboxyl methyl esterification of the C-terminal cysteine. An unmodified a1 peptide exhibits dramatically reduced activity. The pheromone alone is able to induce characteristic conjugation tubes in cells of opposite mating type and confers mating competence; even cells of the same mating type undergo fusion. We discuss the role of pheromones in initiating filamentous growth and pathogenic development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Kusaka I., Fukui S. Morphological change in the early stages of the mating process of Rhodosporidium toruloides. J Bacteriol. 1975 May;122(2):710–718. doi: 10.1128/jb.122.2.710-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akada R., Minomi K., Kai J., Yamashita I., Miyakawa T., Fukui S. Multiple genes coding for precursors of rhodotorucine A, a farnesyl peptide mating pheromone of the basidiomycetous yeast Rhodosporidium toruloides. Mol Cell Biol. 1989 Aug;9(8):3491–3498. doi: 10.1128/mcb.9.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
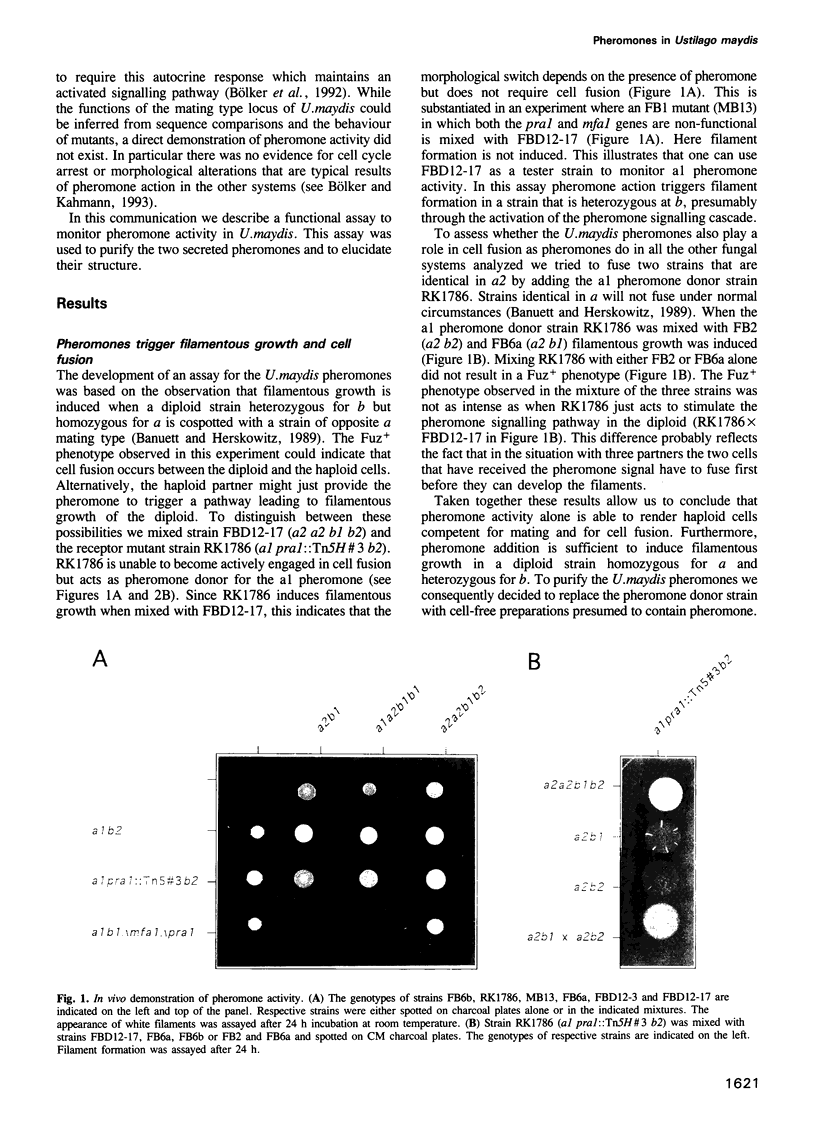
- Banuett F., Herskowitz I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5878–5882. doi: 10.1073/pnas.86.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Ustilago maydis, the delightful blight. Trends Genet. 1992 May;8(5):174–180. doi: 10.1016/0168-9525(92)90220-x. [DOI] [PubMed] [Google Scholar]
- Bölker M., Kahmann R. Sexual pheromones and mating responses in fungi. Plant Cell. 1993 Oct;5(10):1461–1469. doi: 10.1105/tpc.5.10.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölker M., Urban M., Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992 Feb 7;68(3):441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Davey J. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 1992 Mar;11(3):951–960. doi: 10.1002/j.1460-2075.1992.tb05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger E. H., Leong S. A. The a mating-type alleles of Ustilago maydis are idiomorphs. Gene. 1991 Apr;100:113–122. doi: 10.1016/0378-1119(91)90356-g. [DOI] [PubMed] [Google Scholar]
- Gillissen B., Bergemann J., Sandmann C., Schroeer B., Bölker M., Kahmann R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell. 1992 Feb 21;68(4):647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., McCaffrey G., Sprague G. F., Jr Evidence the yeast STE3 gene encodes a receptor for the peptide pheromone a factor: gene sequence and implications for the structure of the presumed receptor. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1418–1422. doi: 10.1073/pnas.83.5.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
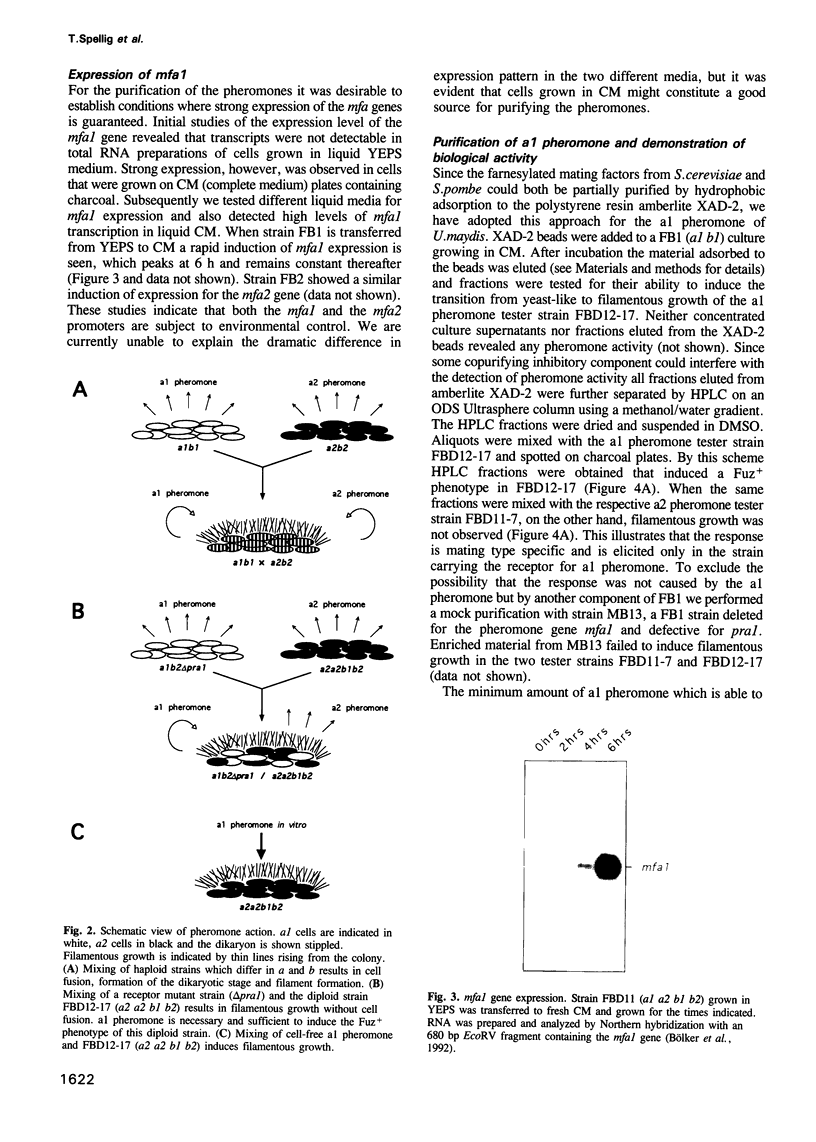
- Kamiya Y., Sakurai A., Tamura S., Takahashi N. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1077–1083. doi: 10.1016/0006-291x(78)91505-x. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Leong S. A. Isolation of two alleles of the b locus of Ustilago maydis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):978–982. doi: 10.1073/pnas.86.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Leong S. A. The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev. 1990 Aug;4(8):1384–1395. doi: 10.1101/gad.4.8.1384. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Caldwell G. A., Miller D., Xue C. B., Naider F., Becker J. M. Significance of C-terminal cysteine modifications to the biological activity of the Saccharomyces cerevisiae a-factor mating pheromone. Mol Cell Biol. 1991 Jul;11(7):3603–3612. doi: 10.1128/mcb.11.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. D., Edman J. C. The alpha-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol Cell Biol. 1993 Mar;13(3):1962–1970. doi: 10.1128/mcb.13.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
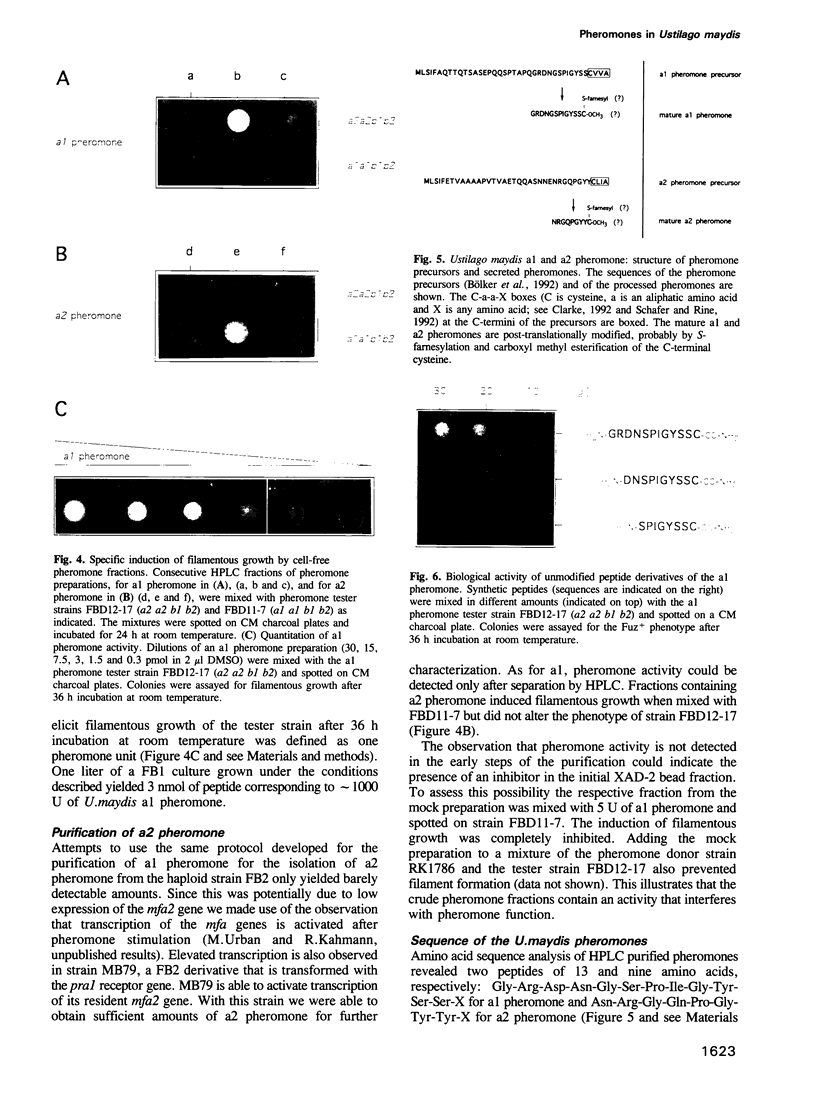
- Moores S. L., Schaber M. D., Mosser S. D., Rands E., O'Hara M. B., Garsky V. M., Marshall M. S., Pompliano D. L., Gibbs J. B. Sequence dependence of protein isoprenylation. J Biol Chem. 1991 Aug 5;266(22):14603–14610. [PubMed] [Google Scholar]
- Nakayama N., Miyajima A., Arai K. Nucleotide sequences of STE2 and STE3, cell type-specific sterile genes from Saccharomyces cerevisiae. EMBO J. 1985 Oct;4(10):2643–2648. doi: 10.1002/j.1460-2075.1985.tb03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhalla J. E. Compatibility reactions on solid medium and interstrain inhibition in Ustilago maydis. Genetics. 1968 Nov;60(3):461–474. doi: 10.1093/genetics/60.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami Y., Yoshida M., Isogai A., Suzuki A. Peptidal Sex Hormones Inducing Conjugation Tube Formation in Compatible Mating-Type Cells of Tremella mesenterica. Science. 1981 Jun 26;212(4502):1525–1527. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Rine J. Protein prenylation: genes, enzymes, targets, and functions. Annu Rev Genet. 1992;26:209–237. doi: 10.1146/annurev.ge.26.120192.001233. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B., Banuett F., Dahl M., Schlesinger R., Schäfer W., Martin T., Herskowitz I., Kahmann R. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell. 1990 Jan 26;60(2):295–306. doi: 10.1016/0092-8674(90)90744-y. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr Assay of yeast mating reaction. Methods Enzymol. 1991;194:77–93. doi: 10.1016/0076-6879(91)94008-z. [DOI] [PubMed] [Google Scholar]
- Strazdis J. R., MacKay V. L. Reproducible and rapid methods for the isolation and assay of a-factor, a yeast mating hormone. J Bacteriol. 1982 Sep;151(3):1153–1161. doi: 10.1128/jb.151.3.1153-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya E., Fukui S., Kamiya Y., Sakagami Y., Fujino M. Requirements of chemical structure of hormonal activity of lipopeptidyl factors inducing sexual differentiation in vegetative cells of heterobasidiomycetous yeasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):459–463. doi: 10.1016/s0006-291x(78)80064-3. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]







