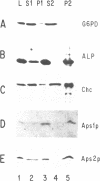
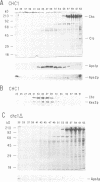
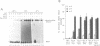Abstract
Clathrin-associated protein (AP) complexes have been implicated in the assembly of clathrin coats and the selectivity of clathrin-mediated protein transport processes. We have identified a yeast gene, APS1, encoding a homolog of the small (referred to herein as sigma) subunits of the mammalian AP-1 complex. Sequence comparisons have shown that Aps1p is more similar to the sigma subunit of the Golgi-localized mammalian AP-1 complex than Aps2p, which is more related to the plasma membrane AP-2 sigma subunit. Like their mammalian counterparts, Aps1p and Aps2p are components of distinct, large (> 200 kDa) complexes and a significant portion of the Aps proteins co-fractionate with clathrin-coated vesicles during gel filtration chromatography. Unexpectedly, even though the evolutionary conservation of AP small subunits is substantial (50% identity between mammalian and yeast proteins), disruptions of APS1 (aps1 delta) and APS2 (aps2 delta), individually or in combination, elicit no detectable mutant phenotypes. These data indicate that the Aps proteins are not absolutely required for clathrin-mediated selective protein transport in cells expressing wild type clathrin. However, aps1 delta accentuated the slow growth and alpha-factor pheromone maturation defect of cells carrying a temperature-sensitive allele of clathrin heavy chain (Chc) (chc1-ts). In contrast, aps1 delta did not influence the effects of chc1-ts on vacuolar protein sorting or receptor-mediated endocytosis. The aps2 delta mutation resulted in a slight effect on chc1-ts cell growth but had no additional effects. The growth defect of cells completely lacking Chc was compounded by aps1 delta but not aps2 delta. These results comprise evidence that Aps1p is involved in a subset of clathrin functions at the Golgi apparatus. The effect of aps1 delta on cells devoid of clathrin function suggests that Aps1p also participates in clathrin-independent processes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988 Apr;7(4):919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K. A., Chang M., Brodsky F. M., Keen J. H. Clathrin assembly protein AP-2 induces aggregation of membrane vesicles: a possible role for AP-2 in endosome formation. J Cell Biol. 1992 Nov;119(4):787–796. doi: 10.1083/jcb.119.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bush G. L., Tassin A. M., Fridén H., Meyer D. I. Secretion in yeast. Purification and in vitro translocation of chemical amounts of prepro-alpha-factor. J Biol Chem. 1991 Jul 25;266(21):13811–13814. [PubMed] [Google Scholar]
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Straubinger R. M., Acton S., Näthke I., Brodsky F. M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V., Egerton M., Elguindi I., Raths S., Singer B., Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A. J., Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989 Oct 27;246(4929):482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993 Apr;121(1):61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Keen J. Deep-etch visualization of proteins involved in clathrin assembly. J Cell Biol. 1988 Sep;107(3):877–886. doi: 10.1083/jcb.107.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H. Clathrin and associated assembly and disassembly proteins. Annu Rev Biochem. 1990;59:415–438. doi: 10.1146/annurev.bi.59.070190.002215. [DOI] [PubMed] [Google Scholar]
- Keen J. H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987 Nov;105(5):1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Davis A. C., Frucht S., Greco B. O., Payne G. S., Tubb B. AP17 and AP19, the mammalian small chains of the clathrin-associated protein complexes show homology to Yap17p, their putative homolog in yeast. J Biol Chem. 1991 Jun 15;266(17):11153–11157. [PubMed] [Google Scholar]
- Kirchhausen T. Identification of a putative yeast homolog of the mammalian beta chains of the clathrin-associated protein complexes. Mol Cell Biol. 1990 Nov;10(11):6089–6090. doi: 10.1128/mcb.10.11.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Nathanson K. L., Matsui W., Vaisberg A., Chow E. P., Burne C., Keen J. H., Davis A. E. Structural and functional division into two domains of the large (100- to 115-kDa) chains of the clathrin-associated protein complex AP-2. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2612–2616. doi: 10.1073/pnas.86.8.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Jones E. W. Clathrin requirement for normal growth of yeast. Science. 1987 Oct 23;238(4826):504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Pellicena-Palle A., Conley K., Freund C. L. Sequence of the clathrin heavy chain from Saccharomyces cerevisiae and requirement of the COOH terminus for clathrin function. J Cell Biol. 1991 Jan;112(1):65–80. doi: 10.1083/jcb.112.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Moore M. S., Sanan D. A., Anderson R. G. Reconstitution of clathrin-coated pit budding from plasma membranes. J Cell Biol. 1991 Sep;114(5):881–891. doi: 10.1083/jcb.114.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey D. T., Peeler J. S., Brodsky F. M., Anderson R. G. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990 Sep 25;265(27):16514–16520. [PubMed] [Google Scholar]
- Manfredi J. J., Bazari W. L. Purification and characterization of two distinct complexes of assembly polypeptides from calf brain coated vesicles that differ in their polypeptide composition and kinase activities. J Biol Chem. 1987 Sep 5;262(25):12182–12188. [PubMed] [Google Scholar]
- Matsui W., Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990 Dec 4;29(48):10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Ahle S., Ungewickell E. Clathrin-coated vesicles. Curr Opin Cell Biol. 1989 Aug;1(4):684–690. doi: 10.1016/0955-0674(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Branton D. Identification of coated vesicles in Saccharomyces cerevisiae. J Cell Biol. 1984 Jan;98(1):341–346. doi: 10.1083/jcb.98.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A. L., Silveira L., Elgort M., Payne G. S. Viability of clathrin heavy-chain-deficient Saccharomyces cerevisiae is compromised by mutations at numerous loci: implications for the suppression hypothesis. Mol Cell Biol. 1991 Aug;11(8):3868–3878. doi: 10.1128/mcb.11.8.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y., Goebl M., O'Brine Greco B., Lemmon S., Pingchang Chow E., Kirchhausen T. The medium chains of the mammalian clathrin-associated proteins have a homolog in yeast. Eur J Biochem. 1991 Dec 5;202(2):569–574. doi: 10.1111/j.1432-1033.1991.tb16409.x. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Baker D., van Tuinen E., Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988 May;106(5):1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S. Genetic analysis of clathrin function in yeast. J Membr Biol. 1990 Jun;116(2):93–105. doi: 10.1007/BF01868668. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Hasson T. B., Hasson M. S., Schekman R. Genetic and biochemical characterization of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):3888–3898. doi: 10.1128/mcb.7.11.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985 Nov 29;230(4729):1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989 Sep 22;245(4924):1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984 Sep;3(9):1951–1957. doi: 10.1002/j.1460-2075.1984.tb02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler J. S., Donzell W. C., Anderson R. G. The appendage domain of the AP-2 subunit is not required for assembly or invagination of clathrin-coated pits. J Cell Biol. 1993 Jan;120(1):47–54. doi: 10.1083/jcb.120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S., Robinson M. S., Jackson A. P., Peiperl L., Parham P. Conservation and diversity in families of coated vesicle adaptins. J Biol Chem. 1990 Mar 25;265(9):4814–4820. [PubMed] [Google Scholar]
- Prasad K., Keen J. H. Interaction of assembly protein AP-2 and its isolated subunits with clathrin. Biochemistry. 1991 Jun 4;30(22):5590–5597. doi: 10.1021/bi00236a036. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptins. Trends Cell Biol. 1992 Oct;2(10):293–297. doi: 10.1016/0962-8924(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Cloning and expression of gamma-adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol. 1990 Dec;111(6 Pt 1):2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (alpha-adaptins). J Cell Biol. 1989 Mar;108(3):833–842. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991 Sep;114(5):869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Kao C. C., Pei R., Berk A. J. Yeast TATA-box transcription factor gene. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7785–7789. doi: 10.1073/pnas.86.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Payne G. S. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992 Aug;11(8):2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Payne G. S. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J Cell Biol. 1992 Aug;118(3):531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira L. A., Wong D. H., Masiarz F. R., Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990 Oct;111(4):1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tan P. K., Davis N. G., Sprague G. F., Payne G. S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993 Dec;123(6 Pt 2):1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurieau C., Brosius J., Burne C., Jolles P., Keen J. H., Mattaliano R. J., Chow E. P., Ramachandran K. L., Kirchhausen T. Molecular cloning and complete amino acid sequence of AP50, an assembly protein associated with clathrin-coated vesicles. DNA. 1988 Dec;7(10):663–669. doi: 10.1089/dna.1988.7.663. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Virshup D. M., Bennett V. Clathrin-coated vesicle assembly polypeptides: physical properties and reconstitution studies with brain membranes. J Cell Biol. 1988 Jan;106(1):39–50. doi: 10.1083/jcb.106.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]
- Zaremba S., Keen J. H. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983 Nov;97(5 Pt 1):1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]