Abstract
Epithelial-to-mesenchymal transition (EMT) of endocardial cells is a critical initial step in the formation of heart valves. The collagen gel in vitro model has provided significant information on the role of growth factors regulating EMT but has not permitted investigation of mechanical factors. Therefore we sought to develop a system to probe the effects of mechanical inputs on endocardial EMT by incorporating hyaluronic acid (HA), the primary component of endocardial cushions in developing heart valves, into the gel assay. This was achieved using a combination collagen and crosslinkable methacrylated HA hydrogel (Coll-MeHA). Avian atrioventricular canal explants on Coll-MeHA gels showed increased numbers of transformed cells. Analysis of the mechanical properties of Coll-MeHA gels show that stiffness does not directly affect EMT. Hydrogel deformation from the beating myocardium of explants directly led to higher levels of regional gel deformation and larger average strain magnitudes associated with invaded cells on Coll-MeHA gels. Inhibition of this contraction reduced EMT on all gel types, although to a lesser extent on Coll-MeHA gels. Using the system we have developed, which permits the manipulation of mechanical factors, we have demonstrated that active mechanical forces play a role in the regulation of endocardial EMT.
Keywords: collagen, epithelial-to-mesenchymal transition (EMT), heart valve, hyaluronic acid, mechanical properties
1. Introduction
Understanding how heart valves develop in utero, including the complex spatiotemporal regulation of signaling mechanisms and dynamic biomechanical environment, will aid in the creation of viable tissue engineered heart valves and novel treatment strategies for valve diseases. A crucial first step in the formation of heart valves is epithelial-to-mesenchymal transformation (EMT) of specialized endocardial cells, which gives rise to valvular interstitial cells (VICs) which remodel the immature cardiac cushions into mature valve leaflets and maintain adult valves throughout life [1–4]. Additionally, VICs are implicated in disease mechanisms including calcific valve disease and have recently been shown to be responsive to hyaluronic acid (HA) signaling [5]. Understanding the mechanical environment that generates VICs will elucidate cell behavior in both developmental and pathological states and potentially impact the creation of viable tissue engineered heart valve replacements [6].
In humans, mice, and chicks, the developing heart tube consists of a common atrium, ventricle, and outflow tract. An initial step of heart valve formation occurs when regions in the atrioventricular canal (AVC) and outflow tract swell, forming endocardial cushions extracelluar matrix (ECM) termed cardiac jelly. EMT occurs when endocardial cells lining the developing heart tube receive a signal to detach from the endocardial cell layer and elongate, before migrating into the cardiac jelly [1, 3, 6–7]. The cardiac jelly is primarily composed of HA but also contains other ECM and signaling molecules [1–2, 8–13]. Over time, the transformed cells that migrate into the endocardial cushions respond to these signals by remodeling the cardiac jelly into the highly structured ECM architecture of mature heart valve leaflets. The spatial and temporal regulation of this process is important for the proper formation of heart valves, and delays or alterations in this signaling can lead to significant impairment of the mature heart valve structure or function.
In vitro, endocardial EMT is studied via a collagen gel protocol developed nearly 30 years ago [14–15]. Briefly, stage HH16 avian embryos are harvested, and the AVC endocardial cushions are removed before explanting endocardium-side down onto a collagen hydrogel. Over 2 days, endocardial cells migrate out of the explant and onto the surface of the collagen gel, forming an endocardial cell sheet; some of these cells undergo EMT and migrate into the collagen gel. This assay has been performed in both mouse and chicken and demonstrates the high degree of conservation between signaling mechanisms governing endocardial EMT between species, despite the fact that mouse explants do not form endocardial sheets [1, 13, 16–18]. Also, as the ventricle of the developing heart tube does not undergo EMT, it is frequently used to test the ability for signaling molecules to induce EMT [19]. For a full review on endocardial EMT assays, see [18, 20]. After developing the original collagen gel assay, Bernanke et al. went on to show that soluble HA can affect the level of EMT that occurs in vitro, although this study was not pursued further [21]. Studies testing the effects of hyaluronate in embryonic rat hearts demonstrated that HA degradation prevented endocardial cushion formation [22]. Also, Camenish et al. showed that mice lacking HA-synthase 2 (HAS2−/−) fail to form heart valves, due to a lack of endocardial cushions, and die at approximately stage E11 with an absence of EMT [16]. However, in vitro, EMT can be rescued in HAS2−/− cells by the addition of soluble HA. Based on the role of HA as an important structural and signaling component in EMT, examination of its inclusion in the in vitro hydrogel assay to determine effects on EMT is warranted [23–25].
The primary limitation of the collagen gel assay is that it fails to mimic the in vivo environment of the developing heart valves in composition (chiefly, lacking HA) which has been recently identified as a key mechanotransduction protein [26]. Further, significant progress has been made in elucidating the signaling mechanisms important for driving EMT, but little work has been done in the biomechanical aspects of this process. Since the biomechanical properties of early and mature heart valve tissues directly relate to valve function, the current lack of knowledge about the relationship between EMT and mechanics needs to be addressed [27–28]. The goal of our study was to create a hydrogel platform incorporating collagen and HA which can be used to study the mechanical context of EMT, in order to test our hypothesis that mechanical factors may play a previously unrecognized role in the regulation of endocardial EMT.
2. Materials and Methods
2.1 Gel synthesis
Coll-MeHA gels were synthesized following previously established protocols [25, 29–30]. Briefly, HA was modified to contain methacrylate crosslinking groups by reacting HA (#53747, Sigma Aldrich, St. Louis MO, ~1.6MDa) with methacrylic anhydride. Coll-MeHA gels were formed by mixing type I collagen (#354249, BD Biosciences, San Jose CA) with MeHA stock solution and neutralizing with 0.1M NaOH. For crosslinking, 0.1wt% (to total gel weight) of Irgacure 2529 (#410896, Sigma Aldrich, St. Louis MO) was mixed into the solution. Gels were crosslinked for 5min under a 365nm UV wand before incubation at 37°C and 5% CO2 to complete collagen gelation. Prior to explant experiments, Coll-MeHA gels were equilibrated overnight in complete media.
2.2 Mechanical analysis of gels
AFM was utilized to measure the moduli of Coll-MeHA and collagen only gels. Samples were analyzed using a BioScope Catalyst AFM (Bruker AXS, Santa Barbara CA) operated in Peak Force – Quantitative Nanomechanical Mapping mode in fluid using a calibration protocol developed in our lab. Gels were fully hydrated in PBS prior to AFM measurements. Multiple scans on multiple gels were used to generate average moduli values (n ≥ 8 per gel composition).
2.3 Gel topography analysis
The fiber structure and overall gel topography were visualized using a Hitachi S-4200 Scanning Electron Microscope (SEM) (Pleasanton, CA). Samples were mounted on SEM posts using conductive tape. Before imaging, scaffolds were coated with gold for 20s using a sputter coater Model-108 (Cressington Scientific, Watford, UK).
2.4 Chick AVC explant harvesting and culture
Stage HH16–17 were harvested as described elsewhere [15, 31]. Briefly, AVCs were excised from embryos, bisected, and seeded endocardium-side down onto fully hydrated gel surfaces. As controls, 0.12wt% collagen only gels were utilized; this composition corresponds to the standard collagen gel assay [14–15, 21]. Explants were given M199 media with 1% FBS, 1% antibiotic/antimycotic, and 1% insulin-selenium-transferrin solution, on the morning following seeding and fed every 2d after that until 7d total culture time was reached. Explants were imaged using a Nikon-Ti300 inverted microscope (Nikon Inc., Melville NY) equipped with Hoffman Modulated Optics. A minimum of 8 explants were seeded per condition and experiments were repeated with at least 3 different batches of eggs to ensure reproducibility. For mechanical analysis, stage HH16–17 embryos were selected and fresh frozen in OCT according to established protocols [31].
2.5 EMT quantification
The surface of the gels was imaged to measure endocardial sheet size, which directly relates to the number of endocardial cells that have migrated out of the explant. To quantify cell transformation, images were taken every 50μm throughout the depth of the gel where cell bodies were present. Both endocardial sheet size and number of transformed cells were quantified using ImageJ.
2.6 Proliferation assay
Proliferation of cells from explants on gels was measured using BrdU. Briefly, gels were incubated with 1:1000 BrdU (RPN201V1, Amersham Biosciences, Pittsburgh PA) for 1hr at 37°C before fixation with 4% paraformaldehyde and stained via a previously established protocol [13]. Images were taken on a Nikon E800 (Nikon Inc., Melville NY) and processed using ImageJ.
2.7 Deformation measurements
Sequence images of beating explants were taken and a pair of images for each explant were extracted, representing fully relaxed and fully contracted myocardium. These images were digitally subtracted using MATLAB and color coded to show deformation of cells and gels surrounding the explants. The area of this deformation was quantified and normalized to total endocardial sheet area of the relaxed myocardium at the time point of interest, resulting in regional gel deformation for each of the explants. Digital image correlation software was used to generate images of strain fields induced by myocardial beating; these images were overlaid with projections of total invaded cells counts to determine correlations between local deformation magnitude and EMT.
2.8 Inhibition of contractile forces
Explant contraction was inhibited using 1.5mM ethyl 3-aminobenzoate methanesulfonate salt (A5040, Sigma-Aldrich), otherwise known as the sodium channel inhibitor tricaine [32]. Explants were allowed to adhere overnight to gels before administration of the drug. Physical removal of the myocardium was tested by carefully using forceps to detach the explant from the surface of the gel after the explant had adhered overnight. The explant remained in the well, unattached, for the duration of the experiment. A control group had explants that were removed from the gel surface and then discarded.
2.9 Statistical analysis
All results from chick explant experiments were reported as average value plus or minus standard error of the mean. For mechanical analyses, the median value of each AFM scan was collected and aggregated into an average median value to represent the sample [31]. Weighted average deformations associated with invaded cells were calculated by weighting the local deformation magnitude in a 25×25 pixel volume by the total number of invaded cells present in that volume and dividing that value by total invaded cells. The average values of all groups were compared with ANOVA, while pairwise multiple comparisons were made using the Holm-Sidak post-hoc testing method.
3. Results
3.1 EMT on cross-linked Coll-MeHA hydrogels
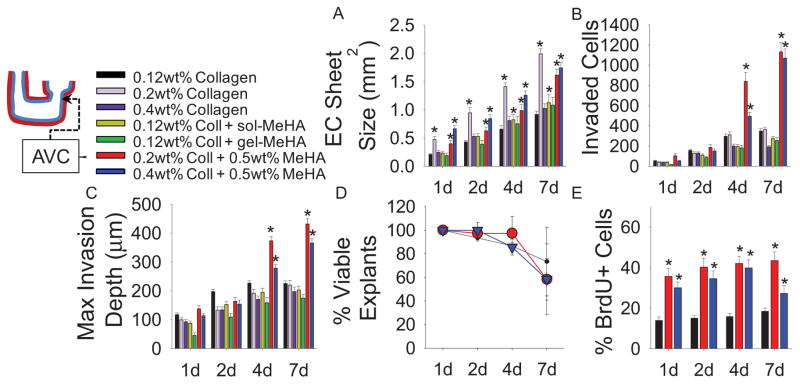
Combinations of collagen and HA were tested for AVC explant viability and attachment prior to detailed analyses. For these studies, a modified HA containing methacrylate groups (MeHA) was synthesized [25, 29–30]. Most notably, combination crosslinked Coll-MeHA gels that contained only 0.12wt% collagen (the standard collagen concentration) with any amount of MeHA demonstrated poor explant attachment. This may be due to a minimum requirement for collagen presentation in the context of HA gels, as a collagen concentration of at least 0.2wt% was required for explant attachment; likewise, gels with greater than 0.5wt% MeHA demonstrated poor explant attachment. The Coll-MeHA compositions reported in this study gave optimum explant attachment. After 4d in culture, AVCs explanted onto composite Coll-MeHA gels exhibited larger endocardial cell sheets (Fig. 1A) and an increased number of transformed cells compared to explants on collagen only gels (Fig. 1B). Endocardial sheets on 0.2wt% collagen gels were significantly larger than those on controls and comparable to sheets seen on Coll-MeHA gels; however there was no increase in EMT (Fig. 1B). In addition to increased EMT, maximum invasion depth is significantly increased on Coll-MeHA gels after 4d in culture (Fig. 1C). MeHA incorporated into 0.12wt% collagen gels uncrosslinked at 0.5mg/mL (+gel-MeHA) or as a supplement to the media at 0.5mg/mL (+sol-MeHA) showed no increased cell invasion compared to control groups (Fig. 1B,C).
Fig. 1.
EMT behaviors as a function of collagen and MeHA presentation. (A) Endocardial (EC) sheet sizes. (B) Quantification of EMT via counts of invaded cells. (C) Maximum invasion depth of invaded cells. (D) Viability of explants seeded on different gel compositions. (E) Proliferation of cells on collagen and Coll-MeHA gels as measured by BrdU. All data presented as average ± SEM and represents 3 technical replicates with n ≥ 10 biological replicates. *p < 0.05 vs. 0.12wt% collagen at same time point.
To determine if Coll-MeHA gels may promote EMT in endocardial cells that do not typically undergo EMT, ventricle explants from stage HH16–17 chick hearts were explanted on Coll-MeHA gels, using collagen only gels as a control. Ventricular explants exhibited no difference in endocardial sheet size or number of invaded cells compared to ventricle explants on collagen only gels (Supplemental Fig. 1 A,B). As expected, both endocardial sheet size and number of transformed cells were significantly smaller in ventricle samples compared to AVC explants.
3.2 Cell proliferation on Coll-MeHA gels
Differences in EMT did not result from differences in explant viability, as defined by the presence of myocardial contractions (Fig. 1D). Proliferation was increased on both compositions of Coll-MeHA gels across all time points when compared to samples on collagen only gels (Fig. 1E).
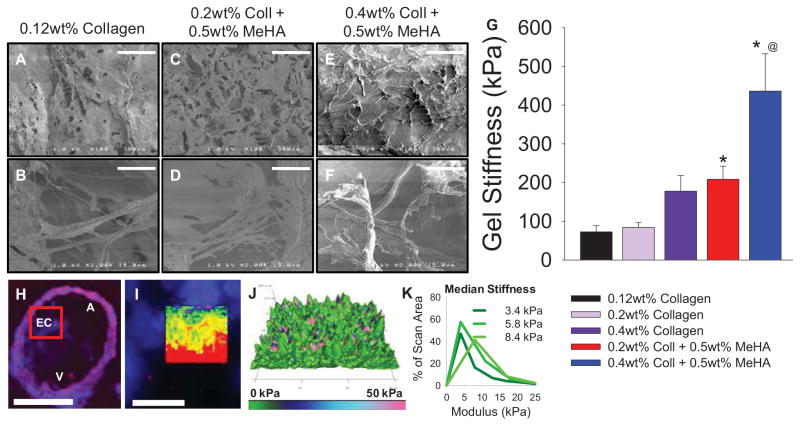
3.3 Scaffold characterization
Collagen only gels exhibited a highly fibrous structure, with little organization or alignment of fibers (Fig. 2A,B). Combination Coll-MeHA gels also exhibited similar collagen fibrous structures, interspersed with sheets of MeHA (Fig. 2C–D, E–F). Mechanical analysis of gels by atomic force microscopy (AFM) showed that the addition of crosslinked MeHA and increasing collagen content increase modulus, as expected (Fig. 2G). However, results from AFM of chick AVC explants indicated an average modulus of 7.2 ± 1.9 kPa for endocardial cushions (Fig. 2H–K), indicating that all gels used in this study are much stiffer than the AVC stiffness.
Fig. 2.
Gel characterization. (A–F) SEM images of 0.12wt% collagen (A,B), 0.2wt% Coll + 0.5wt% MeHA (C,D), and 0.4wt% Coll + 0.5wt% MeHA (E,F). Scale bar = 300μm (A,C,E); Scale Bar = 15μm (B,D,F). (G) Modulus data of gels obtained via AFM. All data presented as average ± SEM with n ≥ 8 scans per composition on N ≥ 2 different gels. (H–I) Fluorescent images of avian AVC scanned by AFM. Scale bar = 100μm (H) and 25μm (I). Inset in (I) highlights scanned area. (J) 3d topographical map with modulus value overlaid. (K) Representative distributions of modulus vs. scan area. Inset numbers represent median values. N = 3 embryos. * p<0.05 vs. 0.12wt% collagen; @ p<0.01 vs. 0.2wt% Coll + 0.5wt% MeHA.
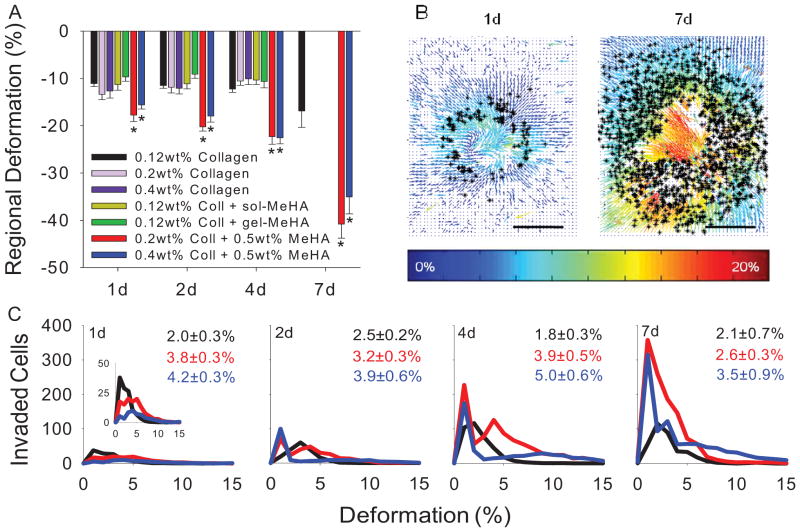
3.4 EMT regulation via active contractile forces
To investigate the role of contractile forces due to the beating myocardium in regulating EMT, we measured the strain transduced into collagen and Coll-MeHA gels. At all time points, explants on Coll-MeHA gels underwent larger deformations compared to explants on control collagen gels (Fig. 3A). This was unexpected because gels with HA have comparable or greater stiffness than collagen gels, indicating they would resist deformation. Gel deformations generated during myocardial contractions were not significantly different from control samples in the case of either soluble or uncrosslinked MeHA or increased collagen content, indicating it is biomechanical properties of MeHA and not simply HA signaling increasing cell transformation. By overlaying images of invaded cells with strain field maps generated with MATLAB, we were able to quantify the number of invaded cells at a specific deformation magnitude (Fig. 3B). These values were aggregated and used to generate histograms depicting the relationship between gel deformation magnitude and EMT (Fig. 3C). We additionally calculated weighted average deformation magnitudes associated with invaded cells to compare between sample groups (Fig. 3C, inset numbers). Higher average magnitudes on Coll-MeHA gels shows more invaded cells present in higher deformation regions on these gels. Furthermore, for all gels permutations and time points, our results indicated over 98% of all transformed cells were present in areas where deformation magnitudes are greater than zero.
Fig. 3.
Mechanical regulation of EMT. (A) Regional gel deformation for different presentations of collagen and MeHA. 7d data unavailable for some samples due to slow myocardium beating. All data presented as average ± SEM and represents 3 technical replicates with ≥ 4 biological replicates. (B) Strain field maps induced by beating myocardial overlaid with position of invaded cells (*) Scale bar = 200μm. (C) Histograms showing number of invaded cells as a function of local strain magnitudes. Inset in 1d is re-scaled to show detail. Numbers correspond to weighted average strain ± SEM with n ≥ 3 replicates (see A for color legend). *p < 0.05 vs. 0.12wt% collagen at same time point.
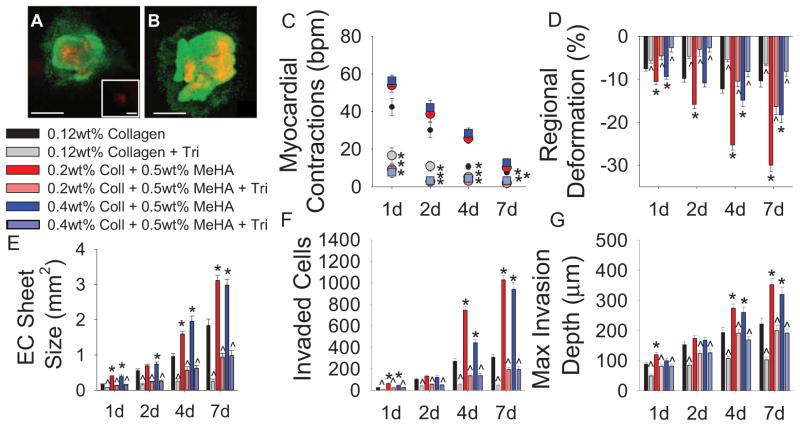
3.5 Decreased EMT with pharmacological inhibition of myocardial contraction
Preventing explant beating by administration of the sodium channel inhibitor, tricaine, demonstrated that myocardial contraction significantly contributes to endocardial EMT. Explants incubated with 1.5mM tricaine demonstrated similar viability staining compared to control groups (Fig. 4A,B) but significantly reduced myocardial contraction frequency and force (Fig. 4C,D). Endocardial sheet size, number of invaded cells, and maximum invasion depth are all significantly decreased when explants in the presence of tricaine for all gel permutations (Fig. 4E–G). Incubation with tricaine did not significantly inhibit cell migration, as verified by a wound assay with mouse embryonic fibroblasts incubated with 1.5mM tricaine overnight (Supplemental Fig. 2). This is the first study showing inhibiting myocardial contractions inhibits endocardial EMT.
Fig. 4.
Pharmacological inhibition of myocardial contraction. (A–B) Live(green)-Dead(red) staining on explants and cells seeded on 0.12wt% collagen with either no treatment (A) or 1.5mM tricaine (B). Inset in (A) shows negative control, 4% PFA treatment for 5min. (C) Quantification of myocardial contractions with tricaine treatment. (D) Regional gel deformation induced by explants treated with tricaine. (E) EC sheet areas for tricaine treated explants. (F) EMT quantification via number of invaded cells in the presence of tricaine. (G) Maximum invasion depth of transformed cells. All data presented as average ± SEM and represents 3 technical replicates with n ≥ 6 biological replicates. *p < 0.05 vs. 0.12wt% collagen at same timepoint. ^p < 0.05 vs. same gel type at same time point.
3.6 Partial EMT rescue with myocardial signaling without contraction
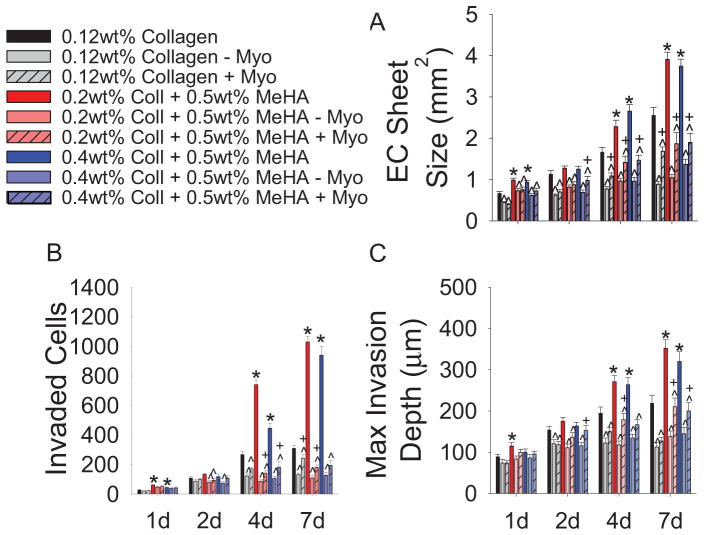
To disassociate the roles of soluble signaling and mechanical factors from the myocardium, explants were removed from the surface of the gel after 1d in culture and either discarded (− Myo) or allowed to remain unattached in the media (+ Myo). Significantly smaller endocardial sheets sizes, fewer invaded cells, and decreased invasion depths are observed when the myocardium is physically removed (Fig. 5A–C). However, if the explant was allowed to remain free floating in the media for the duration of the experiment, some recovery of EMT occurred. Since the presence of soluble signaling factors from the myocardium is not sufficient to fully rescue EMT, it indicates that myocardial contraction is the source of increased EMT in this study.
Fig. 5.
Physical inhibition of myocardial contractions. (A) EC sheet sizes as a function of myocardium presence. Note: These EC sheet values include area covered by explant. (B) EMT quantification via invaded cell counts with or without myocardium. (C) Maximum invasion depth of transformed cells with or without myocardium. All data presented as average ± SEM and represents 3 technical replicates with n ≥ 10 biological replicates. *p < 0.05 vs. 0.12wt% collagen at same timepoint. ^p < 0.05 vs. same gel type at same time point. +p < 0.05 vs. “−Myo” on same gel type at same time point.
4. Discussion
The objective of this study was to examine the contribution of HA in endocardial EMT and determine if mechanical factors play a role in EMT regulation. Specifically, the Coll-MeHA system allows for more control of mechanical factors compared to the traditional collagen only system, which provides another tunable variable in the scheme of biomaterials for directing cell fate. We found that combination crosslinked Coll-MeHA gels promote more endocardial cell outgrowth and proliferation, as well as enhance levels of EMT from chick AVC explants compared to the standard collagen gels. Further, we found enhanced mechanical transduction between the AVC cushion and Coll-MeHA gels such that there was greater contractile strain transfer to the gels, which resulted in mechano-influenced EMT.
4.1 Biochemical signaling and endocardial EMT
Soluble MeHA in media or uncrosslinked MeHA in 0.12wt% collagen gels did not increase EMT-related behaviors. This seems to contradict studies showing that soluble HA can induce nearly twice the number of invaded cells in a collagen gel assay [21]. However, the molecular weight of the HA used in the previous study was not discussed and has since been shown to be an important factor in HA signaling [23, 33]. Ventricular explants from stage HH16 chick embryos displayed neither increased endocardial sheet size nor number of invaded cells in our study (Supplemental Fig. 1). These results demonstrate that MeHA signaling alone is insufficient to cause the dramatic increases in endocardial cell outgrowth and transformed cells observed on Coll-MeHA gels.
Additionally, larger endocardial sheet sizes and more transformed cells present on Coll-MeHA gels cannot be explained by differences in explant viability, as explants demonstrate similar viability levels on differing gel compositions at all time points (Fig. 1D). Increased proliferation on Coll-MeHA gels (Fig. 1E) may produce some of the large number of invaded cells, but the proliferation rate should have little effect on the migration depths, which are also increased in Coll-MeHA gels. In the traditional collagen gel cushion assay, only 1–2% proliferation is seen at 2d post-explantation of AVCs [11]. Collagen control groups in these studies display roughly 15% proliferation at all time points. The increase in proliferation observed on these gels is likely due to effects of media supplementation which was required for longer culture times used in the studies. In a set of control experiments in which explants on 0.12wt% collagen gels did not receive this additional media, average proliferation was ~4% at 2d, similar to previously reported literature values. Moreover, as a large increase in number of transformed cells was not observed in samples with soluble or uncrosslinked MeHA, HA signaling cannot be solely responsible for the increased number of invaded cells in Coll-MeHA gels.
4.2 Contractile forces and endocardial EMT
Mechanical factors considered in this study included substrate stiffness and active contractile forces generated during explant beating, which led to gel deformation. Levels of invasion do not appear to be stiffness-dependent as 0.4wt% collagen and 0.2wt% collagen + 0.5wt% MeHA are approximately the same stiffness but only Coll-MeHA gels promote greater number of invaded cells. Moreover, 0.12wt% and 0.2wt% collagen have similar moduli (Fig. 3G) but dramatically different endocardial sheet size (Fig. 1A). This suggests that EMT is not directly related to substrate stiffness and prompted further investigation of myocardial induced deformation in this system.
Previous studies have indicated that the myocardium is required to induce EMT in the collagen gel assay [15]. We hypothesized that it was not only soluble signals but also mechanotransduction from the myocardium that affect EMT. To test this, we used a series of experiments to reduce the effective myocardial contraction rate and magnitude without removing myocardial signaling. Through both physical removal of the myocardium and pharmacological impairment via tricaine, we showed that myocardial contraction regulates EMT. Explants incubated with tricaine had slower, weaker contractions which led to significantly smaller endocardial sheets and fewer invaded cells. Experiments in which the myocardium was removed from the gel substrate, but remained unattached in the media, demonstrated the differential contributions of soluble signaling and myocardial contractions in regulating EMT; the presence of the myocardium (unattached in the media) partially rescued EMT which indicates that the role of soluble factors secreted by the myocardium are contributory but not dominant.
4.3 Mechanical network of hydrogel in EMT regulation
We observed greater regional gel deformations on Coll-MeHA gels at all time points when compared to collagen only controls (Fig. 3A). This was unexpected, but can be explained by the fact that the Coll-MeHA gel is a semi-interpenetrating network (IPN) caused by sequential MeHA crosslinking followed by collagen gelation around the MeHA crosslinks. The semi-IPN nature of the Coll-MeHA gels therefore transduces strains generated by myocardium over a larger area than a collagen only network. We believe this larger area of mechanotransduction promotes enhanced EMT observed in Coll-MeHA systems, as recent work has suggested that HA modulates integrin-mediated mechanotransduction and subsequent cell phenotype [26]. This could provide a potential intracellular mechanism describing the important cross-talk between mechanical forces and soluble signaling factors in Coll-MeHA gels that regulate EMT. This is the first report of mechanical regulation of endocardial EMT which provides insight into the behavior of a widely studied embryonic cell type.
5. Conclusion
We have shown that Coll-MeHA gels are a more robust platform for in vitro studies as they relate to endocardial EMT and subsequent heart valve formation. This system also allows for controlled mechanical properties and substrate composition for varied ECM signaling components, which yields a more physiologically relevant in vitro environment. The enhanced EMT observed on Coll-MeHA gels provides a larger population of endocardial and progenitor VICs, which represent difficult to obtain embryonic cell types. The population of VIC progenitors generated during EMT is of interest to a large body of cardiac research, from developmental etiologies of congenital heart defects to degenerative, age-associated valve disorders, and even tissue engineered heart valves. Finally, the Coll-MeHA platform has demonstrated a previously unknown connection between active mechanical forces and the regulation of endocardial EMT.
Supplementary Material
EMT behaviors of ventricular explants on Coll-MeHA gels. (A) EC sheet sizes produced by ventricular explants do not differ based on gel composition, and are significantly lower than EC sheets produced by AVC explants. (B) Number of invaded cells are fewer from ventricular explants on all gel types when compared to AVC explants. All data presented as average ± SEM and represents 3 technical replicates with n ≥ 10 biological replicates.
Tricaine does inhibit cell migration. (A) Relative wound areas of mouse embryonic fibroblasts incubated with 1.5mM tricaine compared to controls. Data presented as average ± SEM, n=2. (B) Representative images of controls (left) and cells with tricaine (right) at 0h (top row) and 24h (bottom row).
Acknowledgments
The authors wish to thank the following funding sources: American Heart Association (12PRE12070154 to M.K.S.L.), NIH (HL094707 to W.D.M. and HL092551 to J.V.B and H.S.B.), NSF (1055384 to W.D.M.). Chicken eggs were provided as a generous gift from Tyson, Inc. For use of the SEM: Vanderbilt Institute of Nanoscale Science and Engineering (NSF ARI-R2 DMR-0963361), Anglea L. Zachman, and Hak-Joon Sung. The authors wish to thank Alison K. Schroer and Meghan A. Bowler for assistance with MATLAB. Digital image correlation software developed by Christopher Eberl, Daniel Gianola, and Sven Bundschuh and downloaded from the MATLAB File Exchange program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnett JV, Desgrosellier JS. Early events in valvulogenesis: A signaling perspective. Birth Defects Res C Embryo Today. 2003;69(1):58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- 2.Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235(1):50–9. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combs MD, Yutzey KE. Heart valve development: Regulatory networks in development and disease. Circ Res. 2009;105(5):408–21. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annual Review of Physiology. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez KJ, Piechura LM, Masters KS. Regulation of valvular interstitial cell phenotype and function by hyaluronic acid in 2-d and 3-d culture environments. Matrix Biol. 2011;30(1):70–82. doi: 10.1016/j.matbio.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sewell-Loftin MK, Chun YW, Khademhosseini A, Merryman WD. Emt-inducing biomaterials for heart valve engineering: Taking cues from developmental biology. J Cardiovasc Transl Res. 2011;4(5):658–71. doi: 10.1007/s12265-011-9300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong EJ, Bischoff J. Heart valve development - endothelial cell signaling and differentiation. Circ Res. 2004;95(5):459–70. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig EA, Parker P, Austin AF, Barnett JV, Camenisch TD. Involvement of the mekk1 signaling pathway in the regulation of epicardial cell behavior by hyaluronan. Cell Signal. 2010;22(6):968–76. doi: 10.1016/j.cellsig.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill CR, Sanchez NS, Love JD, Arrieta JA, Hong CC, Brown CB, et al. Bmp2 signals loss of epithelial character in epicardial cells but requires the type iii tgfbeta receptor to promote invasion. Cell Signal. 2012;24(5):1012–22. doi: 10.1016/j.cellsig.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez NS, Barnett JV. Tgfbeta and bmp-2 regulate epicardial cell invasion via tgfbetar3 activation of the par6/smurf1/rhoa pathway. Cell Signal. 2012;24(2):539–48. doi: 10.1016/j.cellsig.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend TA, Robinson JY, How T, DeLaughter DM, Blobe GC, Barnett JV. Endocardial cell epithelial-mesenchymal transformation requires type iii tgfbeta receptor interaction with gipc. Cell Signal. 2012;24(1):247–56. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu YN, Norris RA, Mahler G, Recknagel A, Butcher JT. Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: Relevance for heart valve tissue engineering. Tissue Eng Part A. 2010;16(11):3375–83. doi: 10.1089/ten.tea.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens MV, Broka DM, Parker P, Rogowitz E, Vaillancourt RR, Camenisch TD. Mekk3 initiates transforming growth factor beta 2-dependent epithelial-to-mesenchymal transition during endocardial cushion morphogenesis. Circ Res. 2008;103(12):1430–40. doi: 10.1161/CIRCRESAHA.108.180752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91(2):235–45. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- 15.Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: A regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95(1):108–14. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- 16.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106(3):349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaughter DM, Saint-Jean L, Baldwin HS, Barnett JV. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2011 doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lencinas A, Tavares AL, Barnett JV, Runyan RB. Collagen gel analysis of epithelial-mesenchymal transition in the embryo heart: An in vitro model system for the analysis of tissue interaction, signal transduction, and environmental effects. Birth Defects Res C Embryo Today. 2011;93(4):298–311. doi: 10.1002/bdrc.20222. [DOI] [PubMed] [Google Scholar]
- 19.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type iii tgf-beta receptor for endocardial cell transformation in the heart. Science. 1999;283(5410):2080–2. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 20.DeLaughter DM, Saint-Jean L, Baldwin HS, Barnett JV. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2011;91(6):511–25. doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernanke DH, Markwald RR. Effects of two glycosaminoglycans on seeding of cardiac cushion tissue cells into a collagen-lattice culture system. Anat Rec. 1984;210(1):25–31. doi: 10.1002/ar.1092100105. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ Res. 1994;74(2):244–52. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- 23.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26(15):2517–25. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. Designing scaffolds for valvular interstitial cells: Cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004;71(1):172–80. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 25.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15(7):1645–53. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35(1):71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merryman WD. Mechano-potential etiologies of aortic valve disease. J Biomech. 2010;43(1):87–92. doi: 10.1016/j.jbiomech.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 29.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54(1):115–21. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Smeds KA, Pfister-Serres A, Hatchell DL, Grinstaff MW. Synthesis of a novel polysaccharide hydrogel. J Macromol Sci, Pure Appl Chem. 1999;A36(7–8):981–9. [Google Scholar]
- 31.Sewell-Loftin MK, Brown CB, Baldwin HS, Merryman WD. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J Heart Valve Dis. 2012;21:513–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Bartman T, Walsh EC, Wen KK, McKane M, Ren J, Alexander J, et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2(5):E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers LS, Lalani S, Hardy KM, Xiang X, Broka D, Antin PB, et al. Depolymerized hyaluronan induces vascular endothelial growth factor, a negative regulator of developmental epithelial-to-mesenchymal transformation. Circ Res. 2006;99(6):583–9. doi: 10.1161/01.RES.0000242561.95978.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EMT behaviors of ventricular explants on Coll-MeHA gels. (A) EC sheet sizes produced by ventricular explants do not differ based on gel composition, and are significantly lower than EC sheets produced by AVC explants. (B) Number of invaded cells are fewer from ventricular explants on all gel types when compared to AVC explants. All data presented as average ± SEM and represents 3 technical replicates with n ≥ 10 biological replicates.
Tricaine does inhibit cell migration. (A) Relative wound areas of mouse embryonic fibroblasts incubated with 1.5mM tricaine compared to controls. Data presented as average ± SEM, n=2. (B) Representative images of controls (left) and cells with tricaine (right) at 0h (top row) and 24h (bottom row).