Abstract
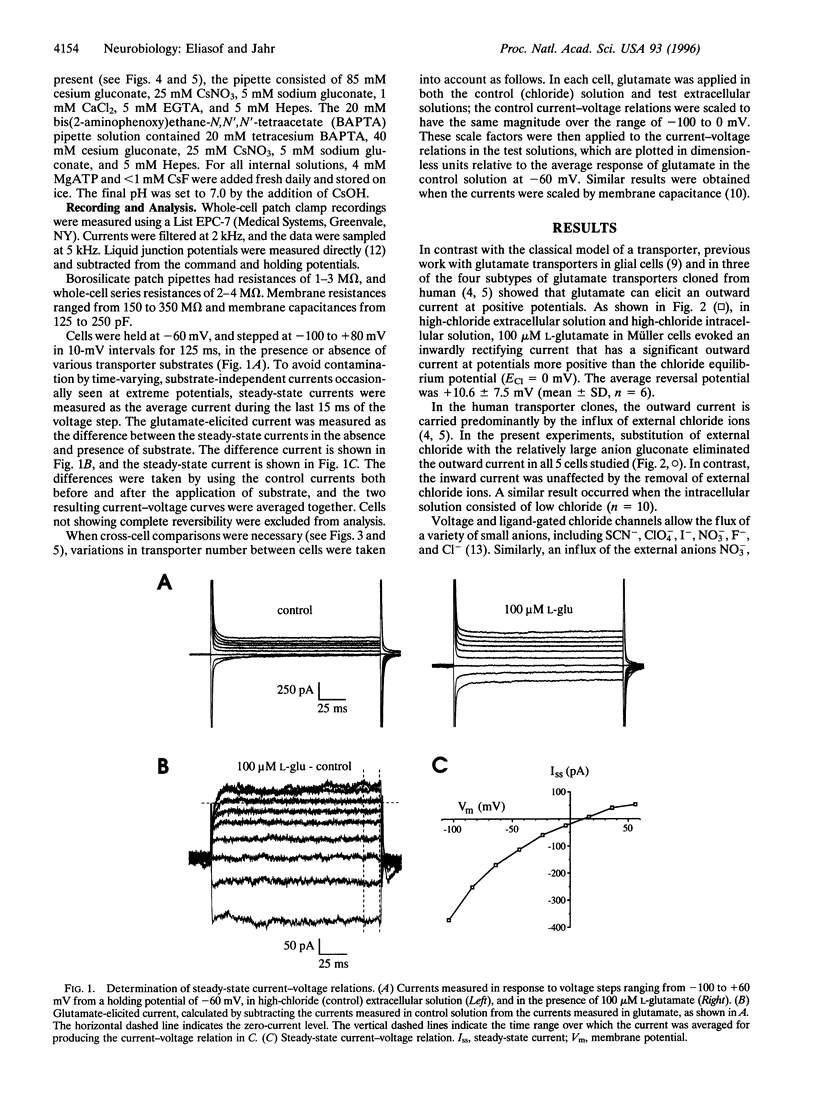
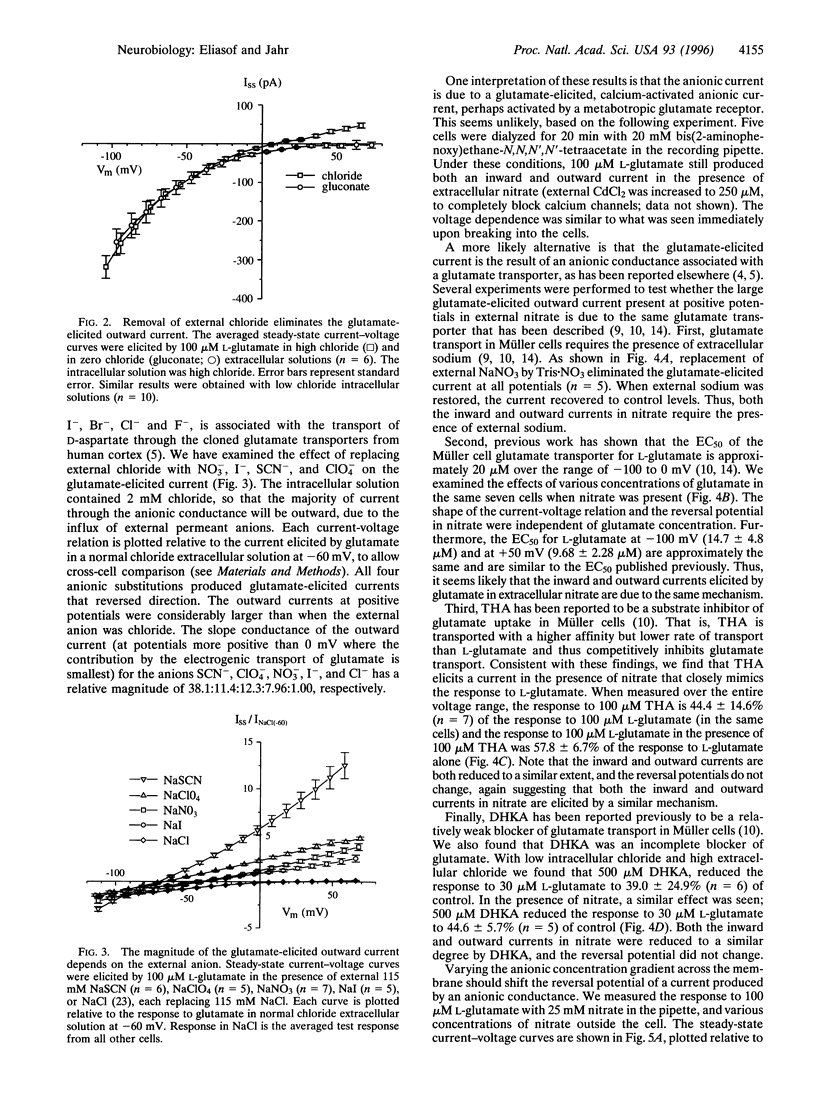
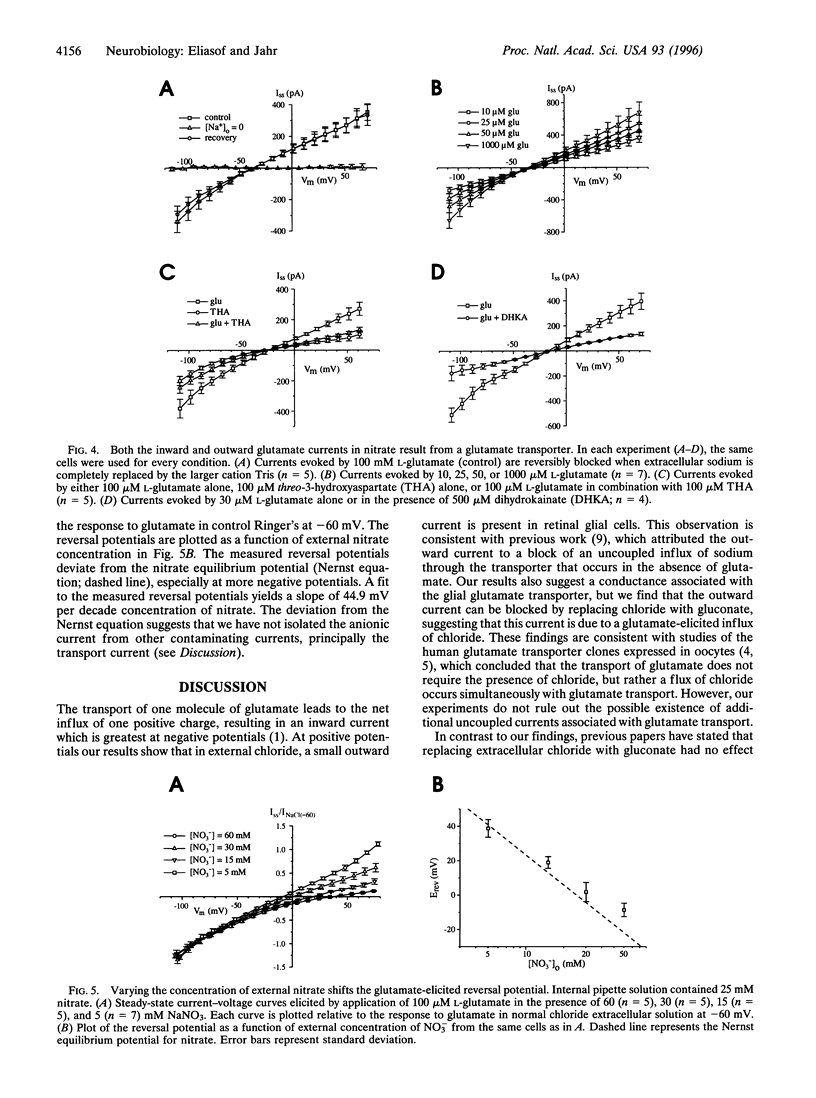
Application of L-glutamate to retinal glial (Müller) cells results in an inwardly rectifying current due to the net influx of one positive charge per molecule of glutamate transported into the cell. However, at positive potentials an outward current can be elicited by glutamate. This outward current is eliminated by removal of external chloride ions. Substitution of external chloride with the anions thiocyanate, perchlorate, nitrate, and iodide, which are known to be more permeant at other chloride channels, results in a considerably larger glutamate-elicited outward current at positive potentials. The large outward current in external nitrate has the same ionic dependence, apparent affinity for L-glutamate, and pharmacology as the glutamate transporter previously reported to exist in these cells. Varying the concentration of external nitrate shifts the reversal potential in a manner consistent with a conductance permeable to nitrate. Together, these results suggest that the glutamate transporter in retinal glial cells is associated with an anionic conductance. This anionic conductance may be important for preventing a reduction in the rate of transport due the depolarization that would otherwise occur as a result of electrogenic glutamate uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour B., Brew H., Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991 May;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Szatkowski M., Amato A., Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992 Dec 3;360(6403):471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Eliasof S., Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci. 1993 Jan;13(1):402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M. The neurotransmitter amino acid transport systems. A fresh outlook on an old problem. Biochem Pharmacol. 1987 Nov 1;36(21):3547–3555. doi: 10.1016/0006-2952(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Fairman W. A., Vandenberg R. J., Arriza J. L., Kavanaugh M. P., Amara S. G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995 Jun 15;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F., Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987 Oct;90(4):453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. B., Dowling J. E. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J Neurosci. 1995 May;15(5 Pt 2):3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. B., Werblin F. S. A glutamate-elicited chloride current with transporter-like properties in rod photoreceptors of the tiger salamander. Vis Neurosci. 1996 Jan-Feb;13(1):135–144. doi: 10.1017/s0952523800007185. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Anion permeation in an apical membrane chloride channel of a secretory epithelial cell. J Gen Physiol. 1992 Mar;99(3):339–366. doi: 10.1085/jgp.99.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978 Sep 19;17(19):3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Welsh M. J. Apical membrane Cl- channels in airway epithelia: anion selectivity and effect of an inhibitor. Am J Physiol. 1990 Aug;259(2 Pt 1):C295–C301. doi: 10.1152/ajpcell.1990.259.2.C295. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Voltage-dependent calcium and potassium channels in retinal glial cells. 1985 Oct 31-Nov 6Nature. 317(6040):809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D., Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990 Nov;11(11):462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Picaud S. A., Larsson H. P., Grant G. B., Lecar H., Werblin F. S. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995 Oct;74(4):1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Sarantis M., Everett K., Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature. 1988 Mar 31;332(6163):451–453. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. L-glutamate conditionally modulates the K+ current of Müller glial cells. Neuron. 1993 Jun;10(6):1141–1149. doi: 10.1016/0896-6273(93)90062-v. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A., Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J Physiol. 1990 Jul;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche J. I., Amara S. G., Kavanaugh M. P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995 Sep;15(3):721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]


